Abstract
Background
The ACTH stimulation test is currently required for definitive diagnosis of hypoadrenocorticism. Increased cost of synthetic ACTH (cosyntropin) has prompted a search for alternative diagnostic methods.
Objective
The purpose of this study was to determine whether a cortisol‐to‐ACTH ratio (CAR) can be used to differentiate dogs with hypoadrenocorticism from normal dogs and those with nonadrenal illness.
Animals
Eight healthy dogs (H), 19 dogs with nonadrenal illness (NAI), and 15 dogs with hypoadrenocorticism (HAD).
Methods
Dogs in the HAD group were retrospectively identified from PUVTH medical records. The NAI group consisted of hospitalized dogs with clinical signs, clinicopathologic findings, or both, consistent with a diagnosis of hypoadrenocorticism, but in which hypoadrenocorticism was ruled out based on ACTH stimulation test results. Healthy dogs were recruited from hospital staff and students. Endogenous ACTH concentrations and cortisol concentrations before and after ACTH stimulation were measured in all dogs.
Results
Baseline cortisol concentration was significantly lower, and ACTH concentration was significantly higher, in the HAD group versus the H and NAI group (P < .001). However, there was overlap among groups. Cortisol‐to‐ACTH ratio was significantly lower in the HAD group versus the H and NAI groups (P < .001), and there was no overlap between the HAD group and the other 2 groups.
Conclusions and Clinical Importance
CAR can be used for definitive diagnosis of primary hypoadrenocorticism.
Keywords: Addison's, Adrenocorticotropic, Canine, Cosyntropin, Hormone
Abbreviations
- ACTH
adrenocorticotropic hormone
- CAR
cortisol‐to‐ACTH ratio
- PUVTH
Purdue University Veterinary Teaching Hospital
Hypoadrenocorticism is an uncommon disease in dogs, but can be fatal without timely diagnosis and treatment. Definitive diagnosis of hypoadrenocorticism requires an ACTH stimulation test. This requires the use of synthetic ACTH (cosyntropin, Cortrosyn). Cosyntropin is relatively expensive (approximately $630/10 vials of 250 μg/vial1 or $93/single vial2 as of 2014), and many general practitioners do not keep it in stock for this reason. Alternative methods of diagnosis are needed. The use of a less expensive, low‐dose ACTH stimulation test is effective for the definitive diagnosis of hypoadrenocorticism,1 whereas measurement of baseline cortisol concentrations in serum is helpful in ruling out the disease.2 However, the low‐dose ACTH stimulation test still requires the use of cosyntropin, and a baseline cortisol sample alone cannot be used for definitive diagnosis.
The use of a single blood sample for diagnosis of hypoadrenocorticism could potentially yield a more efficient diagnosis than an ACTH stimulation test, eliminating the need for synthetic ACTH and the 1‐hour time interval between blood samples. Measurement of baseline ACTH and cortisol concentrations and subsequent calculation of a cortisol‐to‐ACTH ratio (CAR) effectively differentiates normal dogs from those with hypoadrenocorticism,3 and dogs with hypoadrenocorticism have significantly lower CARs than healthy dogs. However, whether CARs are different between dogs with nonadrenal illness and dogs with hypoadrenocorticism is unknown.
As dogs with nonadrenal illness might respond differently to ACTH stimulation testing than normal dogs,4 it is possible that the CAR values in dogs with nonadrenal illness are also dissimilar to those in normal dogs. The purpose of this study was to determine whether a CAR can be used to differentiate dogs with hypoadrenocorticism from normal dogs and those with nonadrenal illness. Our hypothesis was that there would be overlap in the range of CARs between healthy dogs and dogs with nonadrenal illness, but not between dogs with hypoadrenocorticism and the other 2 groups.
Materials and Methods
Study Design and Animals
A retrospective study was conducted in which cases of hypoadrenocorticism were identified from medical records of the Purdue University College of Veterinary Medicine (PUVTH). Controls consisted of dogs with clinical signs consistent with hypoadrenocorticism but in which hypoadrenocorticism had been excluded, and a group of healthy dogs. Endogenous ACTH concentrations and pre‐ and post‐ACTH stimulation cortisol concentrations were measured in all dogs.
Dogs in the “healthy” control group (H) were recruited from hospital personnel and students, and had no signs of systemic illness. The “non‐adrenal illness” control (NAI) group consisted of hospitalized dogs with clinical signs, clinicopathologic findings, or both, consistent with a diagnosis of hypoadrenocorticism. Hypoadrenocorticism was ruled out in both groups based on a post‐ACTH stimulation cortisol concentration of >3 μg/dL.2
For the hypoadrenocorticism group (HAD), the PUVTH clinical pathology database was used to identify hospitalized dogs in which endogenous ACTH (ACTH) concentrations was measured between 2001 and 2013. Medical records were then evaluated, and dogs with confirmed primary hypoadrenocorticism, based on a post‐ACTH stimulation cortisol sample of <2 μg/dL, were identified.
Dogs were excluded from the study if a glucocorticoid medication had been administered within 30 days before testing. This study was approved by the Purdue University Animal Care and Use Committee.
Sample Collection
For the ACTH stimulation testing, an initial blood sample was collected and ACTH was then administered IV using either 5 μg/kg or 250 μg/dog of synthetic cosyntropin. A poststimulation blood sample was drawn 1 hour later.
In the H and NAI groups, all plasma samples for endogenous ACTH analysis were obtained before ACTH stimulation. For dogs in the HAD group, plasma samples were obtained before or at least 12 hours after ACTH stimulation testing. No glucocorticoids were administered before sample collection.
Sample Handling
Blood samples for cortisol measurement were collected before and after stimulation into room‐temperature serum clot tubes. Blood samples for endogenous ACTH analysis were collected into cold silicon‐coated EDTA tubes. Serum and plasma were removed from their respective tubes and frozen at −20°C. Care was taken to separate and freeze the plasma immediately after collection. Directly before analysis, samples were thawed at room temperature.
Cortisol concentrations were determined by the DPC‐Immulite automated chemiluminescent immunoassay. The intra‐assay variation is 13.3–14.5% and interassay variation is 7.4–8.0%. Intra‐assay variation was determined using 19 replicates for each of the 2 control samples (10.5 and 14.0 μg/dL). Interassay variation was determined using 19 replicates for each of the 3 control samples (3.6, 11.8, and 25.5 μg/dL).5 All serum samples from the H group were analyzed in the same run, and both the pre‐ and post‐ACTH stimulation serum samples from each dog in the NAI group and most dogs in the HAD group were analyzed in the same run to minimize interassay variation, but allow for timely case management.
ACTH concentrations were determined by the DPC‐Immulite automated chemiluminescent immunoassay. In our laboratory, the coefficient of variance (CV) for intra‐assay variation is 8.2%, 4.1%, and 4.3% for low, moderate, and high ACTH concentrations. The CVs for interassay precision of low, moderate, and high concentrations of ACTH are 6.0%, 4.6%, and 14.8%.6 All plasma samples from dogs in the H and NAI groups were analyzed in the same run to minimize interassay variation, but samples from dogs in the HAD group were analyzed individually to allow for timely case management.
Statistical Analysis and Presentation of Data
For dogs with cortisol values reported as “<1 μg/dL”, 0.5 μg/dL was used for statistical calculations. For ACTH values reported as “<10 pg/mL” and “>1250 pg/mL,” 5 pg/mL and 1250 pg/mL were used for calculations, respectively. The value of 5 pg/mL was chosen because the actual value could be anywhere between 0 and 9.99 pg/mL.
Histograms and qqplots were used to visually assess if ACTH, cortisol, and CAR values were normally distributed by PROC UNIVARIATE in SAS for Windows 9.3.3 No variable was found to be normally distributed. Accordingly, a nonparametric analysis, Kruskal‐Wallis, using PROC NPAR1WAY in SAS for Windows 9.33 was used to test for a group effect. The Dwass, Steel, Critchlow‐Fligner (DSCF) multiple comparison analysis was used for pair‐wise comparisons among the 3 groups if a significant group effect was determined. An alpha level of 0.05 was used to determine statistical significance for all analyses. Results are graphically represented in box and whiskers plots.
Results
Eight healthy, adult dogs were recruited for the H group in 2005, as part of another study.1 The median age of the dogs was 7.0 years (range: 1.5–11), and the median weight was 8.5 kg (range: 3.9–13.6). The group consisted of 2 neutered male and 6 spayed female dogs and included 2 mixed‐breed dogs, 2 King Charles Cavalier Spaniels, 1 Shetland Sheepdog, 1 French Bulldog, 1 Schipperke, and 1 Chinese Crested dog.
Dogs in the NAI group were also part of another study, and 19 were enrolled between 2005 and 2007.1 This group was comprised of 2 intact females, 12 spayed females, 1 intact male, and 4 neutered male dogs. The median age was 5 years (range: 0.5–15) and median weight was 10 kg (range: 1.0–28.1). The group comprised 4 mixed‐breed dogs, 4 Yorkshire Terriers, and 11 other purebred dogs. Clinical signs and clinicopathologic abnormalities that were consistent with hypoadrenocorticism included vomiting, diarrhea, weight loss, anorexia, hyponatremia, hyperkalemia, hypoalbuminemia, and hypocholesterolemia. Final diagnoses were gastrointestinal disease, 10 dogs; urinary disease, 3 dogs; pancreatic disease, 2 dogs; and other miscellaneous diagnoses, 4 dogs.
For the HAD group, 18 dogs were identified. Three of these dogs were excluded because of recent glucocorticoid administration. Fifteen dogs were included in the study, comprised of 7 spayed female and 8 neutered male dogs. The median age was 5.5 years (range: 0.7–9) and the median weight was 25 kg (range: 3.1–57). The group included 3 West Highland White Terriers, 2 Great Danes, 2 Labrador Retrievers, 2 Standard Poodles, 1 mixed‐breed dog, and 1 each of 5 other breeds. Six dogs had evidence of combined mineralocorticoid and glucocorticoid deficiency (including hyperkalemia, hyponatremia, or both, MGDH), whereas 9 dogs had glucocorticoid deficiency with normal electrolyte concentrations (“atypical” hypoadrenocorticism, GDH).
For the H, NAI, and HAD groups, median baseline cortisol concentration were 2.6 μg/dL (range: 1.4–7.7), 4.3 μg/dL (range: 0.5–20.2), and <1 μg/dL (range: 0.5–1.2) (see Fig 1). There was a significant group effect (P < .0001). The baseline cortisol concentrations were significantly lower in the HAD group than either the NAI (P < .001) or H (P < .001) group. There was no significant difference (P = .1587) between the NAI and H groups. As demonstrated in Figure 1, there was some overlap between the NAI and HAD groups.
Figure 1.

Box and whiskers plot comparing baseline cortisol concentrations among groups (H = healthy, NAI = nonadrenal illness, HAD = hyperadrenocorticism). The box represents the interquartile range from the 25th to 75th percentile, the horizontal bar through the box represents the median, the diamond in the box represents the mean, and the whiskers represent the minimum and maximum values (range).
The median ACTH concentrations were 10.4 pg/mL (range: <10–41.2), <10 pg/mL (range: 5–164), and 700 pg/mL (range: 16.2–1250) for the H, NAI, and HAD groups, respectively. There was a significant group effect (P < .001). The ACTH concentrations were significantly higher in the HAD group than either the NAI (P < .001) or H (P < .001) group. There was no significant difference (P = .9178) between the NAI and H groups. As shown in Figure 2, there was some overlap in values in the 3 groups.
Figure 2.

Box and whiskers log scale plot comparing ACTH concentrations among groups (H = healthy, NAI = nonadrenal illness, HAD = hyperadrenocorticism). The box represents the interquartile range from the 25th to 75th percentile, the horizontal bar through the box represents the median, the diamond in the box represents the mean, and the whiskers represent the minimum and maximum values (range).
CAR was calculated by dividing cortisol concentration (μg/dL) by ACTH concentration (pg/mL). The median CARs were 2.27, 2.84, and 0.000714 for the H, NAI, and HAD groups, respectively. For the CARs, there was a significant group effect (P < .001). The CARs were significantly lower in the HAD group than either the NAI (P < .001) or H (P < .001) group. There was no significant difference (P = .9938) between the NAI and H groups. As demonstrated in Figure 3, there was significant overlap between the H and NAI groups, but not between the HAD group and the H or NAI groups.
Figure 3.
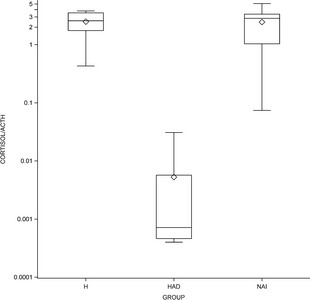
Box and whiskers log scale plot comparing cortisol:ACTH ratio (CAR) among the 3 groups (H = healthy, NAI = nonadrenal illness, HAD = hyperadrenocorticism). The box represents the interquartile range from the 25th to 75th percentile, the horizontal bar through the box represents the median, the diamond in the box represents the mean, and the whiskers represent the minimum and maximum values (range).
Discussion
Results of our study are consistent with a previously published article in which dogs with primary hypoadrenocorticism were reported to have significantly lower CARs than healthy dogs.3 In addition, our study demonstrated that the CAR in dogs with hypoadrenocorticism is also significantly lower than dogs with nonadrenal illness. [Correction made after online publication June 25, 2014: the previous sentence has been updated]
In this study, there was a higher prevalence of dogs with GDH than the 20–30% of cases that has been reported in previous publications.7, 8 However, this was explained by the retrospective nature of the study and the inclusion criteria of having an ACTH concentration measured, as measurement of ACTH is much more likely to be performed in dogs with GDH. As dogs with GDH do not have electrolyte abnormalities, an ACTH concentration is routinely measured to rule out secondary hypoadrenocorticism. Dogs with electrolyte abnormalities are assumed to have primary hypoadrenocorticism, so ACTH concentrations are infrequently measured. Although the overrepresentation of GDH dogs is a weakness of the study, the CAR range for GDH dogs (0.0004–0.010, median 0.000701) was similar to the CAR range for MGDH dogs (0.0004–0.0310, median 0.000101).
No dogs with secondary hypoadrenocorticism were included in this study. There were 2 dogs diagnosed with suspected secondary hypoadrenocorticism between 2001 and 2013. However, their ACTH stimulation cortisol values were >2 μg/dL, but <3 μg/dL, so they were excluded from the study. As secondary dogs would, by definition, have low or undetectable ACTH concentrations, their CAR values would be expected to be much higher than those of the HAD group in our study. Therefore, the CAR might be less useful for diagnosis of dogs with suspected secondary hypoadrenocorticism. In a dog with normal electrolyte concentrations, a low baseline cortisol concentration (<2 μg/dL), and a low to undetectable ACTH concentration, an ACTH stimulation test should be used to diagnose hypoadrenocorticism.
Plasma was collected for measurement of ACTH concentrations for all dogs in the H and NAI groups before ACTH stimulation testing. However, in 5/15 of the dogs in the HAD group, plasma was collected at least 12 hours after stimulation testing, but before glucocorticoid administration. In 1 dog, plasma was collected 12 hours after stimulation testing, but plasma was collected >24 hours after testing in the other 4 dogs. Given the reported cosyntropin elimination half‐life of 128 minutes in healthy dogs, 27 minutes in dogs with nonadrenal illness, and 20 minutes in dogs with hyperadrenocorticism,9 less than 2% of the administered cosyntropin would be expected to be present 12 hours after administration. If these 5 dogs were excluded from analysis, a statistically significant difference among the CARs from the 3 groups remained (P < .001). The CAR values were significantly lower in the HAD group than either the NAI (P < .001) or H (P = .001) group. There was no significant difference (P = .99) between the HAI and H groups.
The major weakness of this study is its retrospective nature. A prospective study would have allowed better standardization of sample collection, and would have likely resulted in a more typical percentage of GDH dogs. Hypoadrenocorticism was on the list of differential diagnoses for every dog in the NAI group, although it was not always the top differential. However, it is important to rule out hypoadrenocorticism in dogs that present with gastrointestinal tract signs, despite the fact that these dogs don't have electrolyte abnormalities because there are no clinical signs pathognomonic for hypoadrenocorticism and further diagnostic testing often requires anesthesia. The severity of illness in the 10 dogs with GDH was similar to that of the dogs in the NAI group.
In conclusion, our study confirmed that the CAR can be used for definitive diagnosis of primary hypoadrenocorticism. As measurement of both cortisol and ACTH can be performed on a single blood sample, this methodology is logistically simpler than an ACTH stimulation test, and does not require the availability of cosyntropin. Thus, a properly collected blood sample could be stored and used retrospectively in dogs that are subsequently given glucocorticoids, which could complicate the interpretation of an ACTH stimulation test. The disadvantage of this method is the need for careful sample collection and handling. Samples for ACTH measurement should be obtained before glucocorticoid administration, as glucocorticoids will suppress ACTH production. In addition, ACTH degrades quickly at room temperature, and falsely decreased ACTH concentrations could lead to a missed diagnosis of hypoadrenocorticism using the CAR. Laboratory recommendations regarding sample handling vary, but most recommend either freezing the sample immediately after collection, and keeping it frozen while shipping, or addition of aprotinin to the sample.6
Acknowledgments
This study was supported by a grant from the Barry and Savannah French‐Poodle Memorial Fund. The authors thank all participating clinicians, technicians, and students for their assistance.
Conflict of Interest: Authors disclose no conflict of interest.
This study was performed at the Purdue University Veterinary Teaching Hospital.
Footnotes
Henry Schein Animal Health, Dublin, OH
MWI Veterinary Supply, Boise, ID
SAS Institute, Inc, Cary, NC
References
- 1. Lathan P, Moore GE, Zambon S, Scott‐Moncrieff JC. Use of a low‐dose ACTH stimulation test for diagnosis of hypoadrenocorticism in dogs. J Vet Intern Med 2008;22:1070–1073. [DOI] [PubMed] [Google Scholar]
- 2. Lennon EM, Boyle TE, Hutchins G, et al. Use of basal serum or plasma cortisol concentrations to rule out a diagnosis of hypoadrenocorticism in dogs: 123 cases (2000–2005). J Am Vet Med Assoc 2007;231:413–416. [DOI] [PubMed] [Google Scholar]
- 3. Javadi S, Galac S, Boer P, et al. Aldosterone‐to‐renin and cortisol‐to‐adrenocorticotropic hormone ratios in healthy dogs and dogs with primary hypoadrenocorticism. J Vet Intern Med 2006;20:556–561. [DOI] [PubMed] [Google Scholar]
- 4. Burkitt JM, Haskins SC, Newlson RW, Kass PH. Relative adrenal insufficiency in dogs with sepsis. J Vet Intern Med 2007;21:226–231. [DOI] [PubMed] [Google Scholar]
- 5. Singh AK, Jiang Y, Jiang Y, et al. Validation of nonradioactive chemiluminescent immunoassaymethods for the analysis of thyroxine and cortisol in blood samples obtained from dogs, cats, and horses. J Vet Diag Invest 1997;9:261–268. [DOI] [PubMed] [Google Scholar]
- 6. Scott‐Moncrieff JC, Koshko MA, Brown JA, et al. Validation of a chemiluminescent enzyme immunometric assay for plasma adrenocorticotropic hormone in the dog. Vet Clin Path 2003;32:180–187. [DOI] [PubMed] [Google Scholar]
- 7. Thompson AL, Scott‐Moncrieff JC, Anderson JD. Comparison of classic hypoadrenocorticism with glucocorticoid‐deficient hypoadrenocorticism in dogs: 46 cases (1985–2005). J Am Vet Med 2007;230:1190–1194. [DOI] [PubMed] [Google Scholar]
- 8. Hughes AM, Nelson RW, Famula TR, Bannasch DL. Clinical features and heritability of hypoadrenocorticism in Nova Scotia Duck Tolling Retrievers: 25 cases (1994–2006). J Amer Vet Med Assoc 2007;231:407–412. [DOI] [PubMed] [Google Scholar]
- 9. Greco DS, Behrend EN, Brown SA, et al. Pharmacokinetics of exogenous corticotropin in normal dogs, hospitalized dogs with non‐adrenal illness and adrenopathic dogs. J Vet Pharmacol Therap 1998;21:369–374. [DOI] [PubMed] [Google Scholar]


