Abstract
Background
Benign urethral obstructions (BUO) in dogs result in substantial morbidity because of challenges with conventional therapies. Treatment of malignant urethral obstructions with intraluminal urethral stents is reported to successfully relieve obstructions.
Hypothesis/Objectives
To evaluate the efficacy and outcome of urethral stent placement for treatment of BUO in dogs.
Animals
Eleven client‐owned animals with urethral stents placed for treatment of BUO.
Methods
Retrospective study in which medical records were reviewed in dogs diagnosed with BUO and treated with a metallic urethral stent. Data collected included signalment, cause of benign obstruction, procedure time, size and type of stent, complications, and short‐ and long‐term outcome.
Results
Eleven dogs with 15 urethral stents were included. Intraluminal urethral stent(s) relieved the obstructions in all dogs. Four dogs had 2 stents placed in separate procedures because of incomplete patency after treatment (n = 1), inadvertent compression of the stent (n = 1), or tissue ingrowth through the stent (n = 2). The median continence score after stent placement was 10 of 10 (range 3–10) with 6 dogs being continent, 3 mildly incontinent, and 1 each moderately and severely incontinent. All owners considered their dog to have an excellent long‐term clinical outcome with long‐term urethral patency. The median follow‐up time was 24 months (range 4–48).
Conclusions and Clinical Importance
Urethral stents appear to be an effective treatment for benign urinary obstructions. Moderate to severe incontinence developed in a minority (12.5%) of dogs. Stents relieved obstructions in all dogs with an excellent long‐term outcome.
Keywords: Cystourethrogram, Hydraulic occluder, Reflex dyssynergia, Stricture, Urinary obstruction
Abbreviations
- BEMS
balloon expandable metallic stent
- BUO
benign urethral obstruction
- CSEMS
covered self‐expanding metallic stent
- SEMS
self‐expanding metallic stent
- USMI
urethral sphincter mechanism incontinence
Urethral obstructions can be associated with benign or malignant diseases. Benign urethral obstructions (BUO) are uncommonly reported in dogs and can be caused by urethral strictures, reflex dyssynergia, stone disease, and granulomatous/proliferative urethritis.1, 2, 3 Conventional treatment options, such as urethral resection and anastomosis, medical management for urethral relaxation, or urinary diversion techniques, like cystostomy tube placement, are available but are associated with failure, complications such as urethral stricture, incontinence, urine leakage, and bacterial cystitis.4, 5
Alternative therapies to surgical intervention and urinary diversion techniques include urethral balloon dilatation, with or without antifibrotic treatment, and urethral stent placement.4, 5, 6, 7, 8 Balloon dilatation of ureth‐ral strictures has been described in 3 separate case reports.6, 7, 9 In 2 of these dogs, a single treatment res‐ulted in resolution of clinical signs for up to 22 months after the procedure. The third dog required 3 separate balloon dilatation procedures and after dilatation was mild to moderately incontinent with a weak urine stream. In humans, balloon dilatation is also used to treat urethral strictures but typically requires multiple dilatations (up to 11) to result in a satisfactory outcome.10
Urethral stent placement may have an advantage over balloon dilatation in that it can provide immediate relief from the obstruction in 1 anesthetic episode, potentially saving cost and decreasing morbidity. In addition, it may be therapeutic in conditions that are not expected to respond to balloon dilatation, like urethral reflex dyssynergia and refractory proliferative urethritis. Urethral stents were first described in 2006 in dogs for malignant obstructions8 and have since been described in a larger number of dogs.11, 12 For these dogs, urethral stenting resolved urethral obstructions in 98–100% of dogs with 1 procedure. The most frequently reported complication with urethral stent placement was post‐stent urinary incontinence.
The objective of this retrospective study was to evaluate the short‐ and long‐term outcomes of dogs with BUO treated with a transluminal urethral stent. The hypothesis was two fold: (1) urethral stents would be an effective, minimally invasive means to relieve benign urethral obstructions in dogs, and (2) urinary incontinence rates would be similar to that reported for stenting for malignant urethral obstructions (~26%).
Materials and Methods
Case Selection
Medical records from the Matthew J. Ryan Veterinary Hospital of the University of Pennsylvania (2007–2009) and the Animal Medical Center (2009–2011) were reviewed for dogs with BUO treated with a transurethral metallic stent from 2007 to 2011. All dogs were diagnosed with a benign urethral obstruction based on a combination of various forms of imaging including a cystourethrogram ± cystourethroscopy (Figs 1, 2, 3), histopathology from urethral tissue biopsy when possible, a known history of trauma or surgery that would suggest a presumptive diagnosis of a benign urethral stricture, or both. All dogs required a minimum of 1‐year follow‐up time for inclusion.
Figure 1.
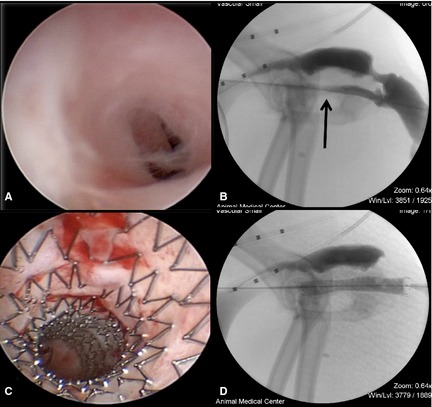
Urethral stricture with SEMS placement. (A) Cystourethroscopy demonstrated marked narrowing of the urethral with apparently fibrous tissue, consistent with a urethral stricture. (B) A cystourethrogram performed on the same dog supported the diagnosis, demonstrating narrowing of the contrast within the urethra (black arrow). (C) After SEMS placement, the urethral lumen is of the same diameter as the surrounding normal urethra. (D) Cystourethrography demonstrated appropriate SEMS placement and resolution of the urethral stricture with stent placement.
Figure 2.
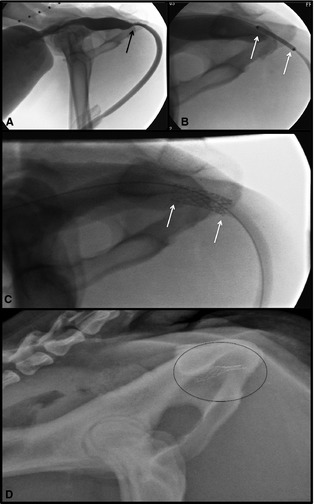
BEMS placement in a dog. (A) Cystourethrography demonstrated marked narrowing of the urethra with proximal urethra dilation, consistent with a urethral stricture (black arrow). (B) A BEMS is guided into place over the guide wire with the stent centered over the stricture and the ends of the stent (white arrows) at least 5 mm into normal urethral mucosa on either side of the stricture. (C) The BEMS was deployed within the pelvic urethra (white arrows at either end of stent). (D) Inadvertent compression of the BEMS occurred, seen as collapse of the caudal aspect of the urethral stent (circle around stent). The BEMS was removed via cystotomy and a SEMS was placed.
Figure 3.
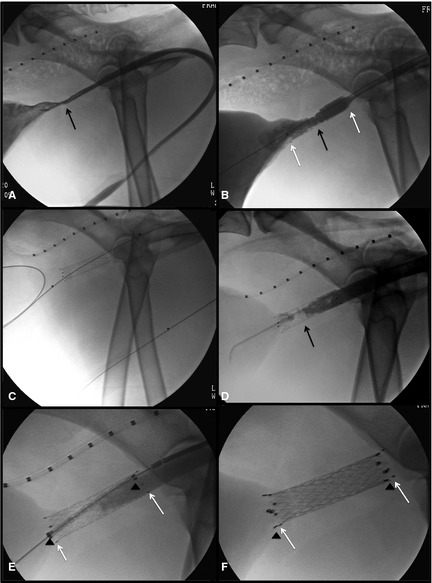
Placement of covered metallic stent (CSEMS). (A) Cystourethrography demonstrated marked narrowing of the urethra just caudal to the trigone. (B) A SEMS was placed successfully. (C) Four months later, the dog developed recurrent stranguria and a second cystourethrogram was performed, using a guide wire passed up the urethral lumen into the bladder. (D) A contrast cystourethrogram demonstrated irregular urethral narrowing, consistent with tissue ingrowth through the SEMS. (E) A second stent, a CSEMS, was placed within the lumen of the first SEMS. (F) Successful deployment of the CSEMS.
Medical Record Review
Each dog's record was reviewed and the following data were recorded: signalment, clinical signs and physical examination findings, diagnosis and underlying etiology of the obstruction, the presence of a concurrent urinary tract infection(s), continence score before stent placement, duration of obstruction, and detail of the procedure for urethral stent placement. Data were recorded regarding the type of urethral stent placed (balloon expandable metallic stent [BEMS], self‐expanding metallic stent [SEMS], or covered self‐expanding metallic stent [CSEMS]), stent length and diameter, obstruction length and location, as well as procedure and anesthesia times. Intraoperative and postoperative complications were noted, including urethral tear/trauma, stent migration, compression, re‐obstruction, or need for a 2nd procedure. Outcome data were obtained immediately after stent placement and for several months to years of follow‐up, including degree of incontinence after stent placement, any long‐term complications, and overall follow‐up time.
Urinary incontinence is medically defined as the inability of the body to control evacuative function resulting from abnormalities in both voiding and storage of urine. For this study, urinary incontinence was classified as previously reported8, 11: mild (dribbling of urine immediately before or after voluntary urination only) or severe (involuntary discharge of urine between episodes of voluntary urination). In addition, a continence score was used as previously reported on a continuous scale of 1 through 1013: 1 being completely incontinent (leakage constantly, never achieving a full bladder), 5 being moderately incontinent (leakage mainly when laying down or immediately before or after urination) with minimal leakage between urinations, 7.5 being mildly incontinent (leakage intermittently, only when sleeping, not when awake), 9 being mostly continent (urine noticed on fur 1–2 times per week, no leakage found at rest or when awake), and 10 being fully continent (no leakage at all).13 For consistency throughout the study, dogs that had urethral sphincter mechanism incontinence before urethral occlusion were excluded from the incontinence score evaluation.
Stranguria was also classified as previously reported8, 11 both pre‐ and post‐stent placement. This was categorized as obstructed (inability to void urine or empty the bladder), severe (straining sufficient to empty the bladder but unable to produce a full stream of urine), moderate (straining between voluntary urinations, in which a full stream of urine was produced), or mild (straining only apparent after voluntary urination accompanied by the ability to produce a good stream of urine).
Diagnosis
All dogs were diagnosed with a benign urethral obstruction based on a combination of various forms of imaging including a cystourethrogram ± cystourethroscopy, histopathology from urethral tissue biopsy when possible, a known history of trauma or surgery that suggested a presumptive diagnosis of a benign urethral stricture, or both. Dogs were diagnosed with presumptive reflex dyssynergia based on supportive clinical signs and a lack of findings of alternate causes of benign urethral obstruction based on cystourethroscopy, cystourethrography, urine culture, abdominal ultrasound and abdominal radiographs. In addition, dogs with suspected reflex dyssynergia demonstrated a repeatable focal narrowing within the urethral lumen during a conscious cystourethrogram. Urethral pressure profilometry and cystometrography were not performed because of the lack of availability and owner decline of further referral.
Stent Placement
Urethral stents were placed as previously described.8, 11 Briefly, the patient was anesthetized and placed in lateral recumbency. The prepuce, or vulva, was clipped, aseptically prepared, and draped. A 0.035 in hydrophilic angle‐tipped guide wire (Weasel Wire1 ) was passed retrograde up the urethral lumen, past the urethral obstruction, and into the urinary bladder. A vascular sheath (7F vascular sheath and dilator1) was inserted into the urethra over the guide wire. In male dogs an angled‐vascular catheter (4F Berenstein catheter1) was placed over the guide wire, through the sheath, and into the urinary bladder. Contrast2 was injected either through the sheath (females) or catheter (males) during a pull‐out cystourethrogram (Figs 1B, 2A, and 3A) to highlight the narrowing of the urethra and determine the exact location of the obstruction. Then, using a marker catheter in the colon to adjust for magnification, as previously reported,8, 11 the urethral obstruction length and normal urethral diameter were measured and an appropriate stent size was selected. The size of the stent was chosen extend at least 5 mm beyond the length of the obstruction both cranially and caudally. The diameter was chosen to be 10–20% larger than the normal urethral diameter in the stented region. For the 2 dogs with presumptive reflex dyssynergia, a conscious cystourethrogram was performed, and a repetitive area of focal spasm/narrowing was seen in both dogs. This narrowing was suspected to be the location of obstruction and was stented using fluoroscopic guidance as described above once the patient was placed under general anesthesia.
Results
Fifteen urethral stents were placed in 11 dogs that fulfilled the selection criteria (4 dogs had 2 stents each). Dogs ranged in age from 7 months to 11 years (median 3 years). There were varied breeds: 2 Newfoundlands, 2 Golden Retrievers, 1 Labrador Retriever, 1 English Bulldog, 1 Toy Poodle, 1 Pug, 1 Standard Poodle, 1 Maltese, and a mixed breed dog. Six dogs were castrated males, 4 were spayed females, and 1 was an intact female.
Presenting clinical signs at the time of urethral obstruction diagnosis included severe stranguria (11/11), urinary incontinence (3/11), severe pollakiuria (2/11), and hematuria (1/11). Clinical signs were present 3 days to 2 years before presentation (median 2 weeks). Eight dogs had complete urethral obstructions; the remaining 3 were partially obstructed. Five dogs had overflow incontinence secondary to urinary obstruction. Three dogs initially presented, before the development of a urethral obstruction, for refractory urethral sphincter mechanism incontinence and had a urethral hydraulic occluder placed as a treatment. The urethral obstructed then developed after urethral hydraulic occluder placement and associated urethral stenosis. On physical examination, 5/11 had a full turgid bladder, 2/11 dogs had a painful abdomen, and 3/11 dogs were noted to have urinary incontinence during physical examination with a full bladder. Nine of 11 dogs had a history of bacterial cystitis that was diagnosed before (2/9), or at the time of (7/9) stent placement. The culture results included 5 dogs with Staphylococcus spp., 2 of which were multidrug resistant. One dog had a positive culture for multidrug resistant Enterococcus spp. and another dog had a positive culture for multidrug resistant Escherichia coli. All dogs were started on appropriate antibiotic treatment.
Underlying conditions that resulted in the urethral obstruction(s) included urethral stone removal (3), extraluminal stenosis after urethral hydraulic occluder placement for severe urinary incontinence (3), suspected urethral reflex dyssynergia (2), postsurgical urethral resection and anastomosis after inadvertent prostatectomy during a cryptorchid castration (1), proliferative urethritis with urethral fibrosis (1), and urethral tear with secondary stricture from vehicular trauma (1). Of the dogs with urethral strictures, all dogs had an identifiable cause of the stricture. Of the 3 dogs with HO placement that developed urethral obstructions, 1 had the occluder device surgically removed and the stenosis persisted. For the remaining 2 dogs, the owners declined a second surgical procedure for removal and elected stent placement because of financial constraints. Both dogs with presumptive reflex dyssynergia had failed medical management that included administration of diazepam, tamsulosin, phenoxybenzamine, prazosin, or some combination thereof. Dogs with proliferative urethritis were treated with antibiotics, nonsteroidal anti‐inflammatory drugs or corticosteroids, azathioprine, or both and remained refractory to medical treatment alone.
Five of the 9 dogs that had been diagnosed with a urethral stricture as a cause for their obstruction were treated with balloon dilatation before stent placement. These 5 dogs received 1–4 balloon dilatation procedures before attempting urethral stent placement (median 1 procedure). Two dogs with an intraluminal urethral stricture initially had a BEMS placed. The remaining dogs were initially treated with self‐expanding metallic stents (SEMS; 1 covered [CSEMS]; 9 uncovered). One dog received 2 SEMS to appropriately span the occluded region. Stents ranged in diameter from 4 to 12 mm (median 10 mm) and in length from 18 to 80 mm (median 40 mm). Four dogs required 2 stents; a second stent was required because of compression of a BEMS (n = 1) (Fig 2D), tissue ingrowth through the open mesh of an uncovered SEMS (n = 1) (Fig 3D), an additional region of obstruction in a dog with reflex dyssynergia (n = 1), and the development of proliferative urethritis with an associated urinary tract infection resulting in tissue re‐obstruction (n = 1) of the first SEMS. All 4 dogs were re‐stented successfully (exchanging the BEMS for an SEMS, placing a covered stent [CSEMS] inside the uncovered stent for the tissue ingrowth, and adding an additional SEMS for the reflex dyssynergia). Procedure time for stent placement was recorded in 9/11 dogs and ranged from 50 to 135 minutes (median 100 minutes).
Perioperative (operative and postoperative <1 week) complications occurred in 3/15 stent placements. In 1 dog, there was compression of the initial BEMS stent within 24 hours that potentially occurred during rectal temperature assessment (Fig 2). A cystotomy was performed; the collapsed BEMS was removed and replaced transurethrally with a SEMS. In 1 dog with reflex dyssynergia, there was persistent stranguria after SEMS placement so a second SEMS was placed 24 hours later after determining an additional area of urethral spasm on a urethrogram. After the second stent, the dog's stranguria resolved. In the third dog, a guide wire could not be passed retrograde because of significant narrowing of a urethral stricture and was inadvertently passed into the periurethral tissue. An antegrade catheterization was required, which was successful and allowed for normal stent deployment. No adverse effect occurred with this complication. All 3 dogs had normal micturition after their procedures. Major postoperative (>1 week) complications were seen in 2 dogs requiring the placement of a covered stent (CSEMS). Tissue in‐growth into the first stent, causing a recurrent urethral stricture, occurred 2 weeks after stent placement in 1 dog. In a second dog, proliferative tissue developed throughout the entire urethra within the stent lumen 4 months post‐stent placement. This proliferative tissue was biopsied and the histopathology suggested proliferative urethritis, mainly of lymphoplasmacystic inflammation, fibrosis and transitional epithelial cells. A CSEMS was placed in this dog.
When continence was determined for the 8 dogs that did not have prestent concurrent urethral sphincter mechanism incontinence (USMI), 2/8 developed incontinence after stent placement, one being mild (continence score 8), and the other being mild to moderate (continence score 7). In addition, 1 dog had mild incontinence associated with bladder atony before stent placement that remained unchanged after stent placement (continence score 7.5). Of the 3 dogs with concurrent USMI and hydraulic occluder placement, 2 dogs had improvement of their continence scores (score of 4 prestent and hydraulic occluder to 10 post‐stent, and score of 3 prestent and hydraulic occluder to 8 post‐stent, respectively) and 1 dog had an unchanged continence score of 3. Incontinent dogs received phenylpropanolamine and either diethylstilbestrol (females; 1.0–1.5 mg/kg q12h–q8h PO) or methyltestosterone (males; 0.5 mg/kg/d PO) once urethral patency was reestablished.
Information was available on serial urine cultures after stent placement in 9 of 11 dogs and at least 1 positive culture was documented in 2 of 9 dogs after stent placement at 2 and 5 months post‐stent placement. These dogs were successfully treated with appropriate antibiotic treatment.
Urethral stent placement resulted in resolution of the urethral obstruction in all dogs. Long‐term follow‐up data were available for all dogs. Duration of follow‐up ranged from 4 months (1 dog was euthanized for lymphoma 4 months after stent placement) to 4 years, with a median of 24 months. All dogs maintained a patent urethra.
Discussion
This series describes the successful treatment of benign urethral obstruction in dogs using metallic urethral stents. Long‐term follow‐up (median 2 years) suggested this was a safe, efficacious, and durable treatment option, as most of these dogs will be alive far longer than those with malignant obstructions. Most dogs presented with signs and physical examination abnormalities consistent with a partial or complete urethral obstruction. The majority (82%) of dogs had a bacterial cystitis at the time of diagnosis, emphasizing the need for urinalysis and bacterial culture and sensitivity testing in dogs diagnosed with BUO. For these dogs, stents were placed in the face of an active infection when necessary and subsequently treated with an appropriate antibiotic for 4–6 weeks duration; there was no apparent adverse outcome in dogs that had stents placed while bacterial cystitis was present.
Five of the 9 dogs with urethral strictures as a cause for the obstruction were treated with balloon dilatation before stent placement and it was unsuccessful in all patients. Most dogs received 1 dilatation procedure before stent placement, but some received up to 4. It could be that with multiple balloon dilatation procedures, these cases could have achieved a similar positive outcome as occurred with stent placement, however, the owners of these dogs elected stent placement rather than repeat dilatation, typically because of financial constraints or specialist availability to perform the procedure. As this was not a study comparing dogs that were successfully treated with balloon dilatation versus urethral stent placement, no conclusion can be made regarding the superiority of urethral stents over balloon dilatation for BUO. In all circumstances, serial balloon dilatation was discussed as a potential treatment option before considering stent placement. Because of the potential need for numerous balloon dilatation procedures and the associated cost, many owners elected urethral stenting as a primary treatment modality. Others opted for urethral stent placement after failure of 1 or more balloon dilatation procedures.
In this report, perioperative complications were uncommon (3 events in 15 stent placements), and all were successfully managed. Four dogs had a second stent placed; in 3 dogs, it was caused by failure of the first stent (1 case of BEMS compression, 2 cases of tissue in‐growth through a SEMS). The authors' now consider using SEMS over BEMS for urethral obstructions because of the compression seen with BEMS. The benefits of a BEMS are that they are shorter (<2 cm versus 3–6 cm) and easier to place than the SEMS, so that if the lesion is very short in length than these might still be considered. In contrast to BEMS, the SEMS have recoil and constant outward radial force that maintains patency, regardless of external compression. With intraluminal urethral strictures, the risk of tissue in‐growth through the interstices of a noncovered urethral stent is a potential complication; placement of a CSEMS could be considered to reduce the risk of re‐stricture. In 1 dog in this study, the owner elected to have a CSEMS placed initially because of risk of re‐stricture with SEMS. Because of the excessive cost associated with CSEMS, however, a standard SEMS is typically placed first.
Urinary incontinence has been reported as a complication with urethral stenting for malignant urethral obstructions, with 11/42 (26%), 4/29 (14%), and 2/12 (16%) of dogs developing severe incontinence.8, 11, 12 When all levels of continence are considered (mild to severe) in these reports on malignant obstructions, the prevalence of incontinence is upward of 50%. Incontinence was the main complication, affecting 3/8 dogs without concurrent USMI that were evaluated; however, only one was moderately incontinent (12.5%), whereas the others were considered mild. One of these dogs was incontinent before stent placement because of bladder atony with overflow incontinence and this dog's incontinence score was unchanged by placement of the stent, suggesting the stent did not make the incontinence worse. This incontinence rate compares similarly, or better, than that reported for malignant obstructions, but the case numbers are too low to accurately compare.
There are several limitations of this study. Its retrospective nature precluded comparison of different stent types for different underlying disease processes, as well as the effect of concurrent conditions such as bacterial cystitis on outcome. Furthermore, only 11 dogs with multiple causes of benign urinary obstructions were seen over the 5‐year study period. As such, a comprehensive analysis of outcome to the underlying cause of the benign urinary obstruction, as well as concurrent conditions, was not possible. Furthermore, each client elected different primary procedures, making it difficult to compare or assess failure and success rates of balloon dilatation alone, or the placement of uncovered versus covered stents.
In conclusion, 11 dogs in this report were successfully treated using 15 urethral stents, with a low major complication rate. Stents were used to treat a variety of etiologies, including intraluminal strictures, extraluminal stenosis, proliferative urethritis, and presumptive reflex dyssynergia. A good short‐ and long‐term outcome with resolution of urethral obstruction was seen in all dogs. Intraluminal urethral stent placement appears to be a safe and effective treatment for the relief of BUO. Further study would be needed to determine whether intraluminal stents provide a treatment advantage over other minimally invasive modalities such as balloon dilatation alone, with and without antifibrotic treatment.
Acknowledgment
Conflict of Interest Declaration: The authors disclose no conflict of interest.
This is a retrospective study performed at the Animal Medical Center and the Matthew J. Ryan Veterinary Hospital of the University of Pennsylvania.
This work was presented in abstract form at ECVIM in Liverpool in September 2013.
Footnotes
Infiniti Medical LLC, Menlo Park, CA
Omnipaque iohexol injection; GE Healthcare, Wauwatosa, WI
References
- 1. Diaz Espineira MM, Viehoff FW, Nickel RF. Idiopathic detrusor‐urethral dyssynergia in dogs: A retrospective analysis of 22 cases. J Small Anim Pract 1998;39:264–270. [DOI] [PubMed] [Google Scholar]
- 2. Moroff SD, Brown BA, Matthiesen DT, Scott RC. Infiltrative urethral disease in female dogs: 41 cases (1980–1987). J Am Vet Med Assoc 1991;199:247–251. [PubMed] [Google Scholar]
- 3. Hostutler RA, Chew DJ, Eaton KA, DiBartola SP. Cystoscopic appearance of proliferative urethritis in 2 dogs before and after treatment. J Vet Intern Med 2004;18:113–116. [DOI] [PubMed] [Google Scholar]
- 4. Salinardi BJ, Marks SL, Davidson JR, Senior DF. The use of a low‐profile cystostomy tube to relieve urethral obstruction in a dog. J Am Anim Hosp Assoc 2003;39:403–405. [DOI] [PubMed] [Google Scholar]
- 5. Anderson RB, Aronson LR, Drobatz KJ, Atilla A. Prognostic factors for successful outcome following urethral rupture in dogs and cats. J Am Anim Hosp Assoc 2006;42:136–146. [DOI] [PubMed] [Google Scholar]
- 6. Powers MY, Campbell BG, Weisse C. Porcine small intestinal submucosa augmentation urethroplasty and balloon dilatation of a urethral stricture secondary to inadvertent prostatectomy in a dog. J Am Anim Hosp Assoc 2010;46:358–365. [DOI] [PubMed] [Google Scholar]
- 7. Bennett SL, Edwards GE, Tyrrell D. Balloon dilation of a urethral stricture in a dog. Aust Vet J 2005;83:552–554. [DOI] [PubMed] [Google Scholar]
- 8. Weisse C, Berent A, Todd K, et al. Evaluation of palliative stenting for management of malignant urethral obstructions in dogs. J Am Vet Med Assoc 2006;229:226–234. [DOI] [PubMed] [Google Scholar]
- 9. Wood MW, Vaden S, Cerda‐Gonzalez S, Keene B. Cystoscopic‐guided balloon dilation of a urethral stricture in a female dog. Can Vet J 2007;48:731–733. [PMC free article] [PubMed] [Google Scholar]
- 10. Dewan PA, Gotov E, Chiang D. Guide wire‐assisted urethral dilatation for urethral strictures in pediatric urology. J Pediatr Surg 2003;38:1790–1792. [DOI] [PubMed] [Google Scholar]
- 11. Blackburn AL, Berent AC, Weisse CW, Brown DC. Evaluation of outcome following urethral stent placement for the treatment of obstructive carcinoma of the urethra in dogs: 42 cases (2004–2008). J Am Vet Med Assoc 2013;242:59–68. [DOI] [PubMed] [Google Scholar]
- 12. McMillan SK, Knapp DW, Ramos‐Vara JA, et al. Outcome of urethral stent placement for management of urethral obstruction secondary to transitional cell carcinoma in dogs: 19 cases (2007–2010). J Am Vet Med Assoc 2012;241:1627–1632. [DOI] [PubMed] [Google Scholar]
- 13. Berent AC, Weisse C, Mayhew PD, et al. Evaluation of cystoscopic‐guided laser ablation of intramural ectopic ureters in female dogs. J Am Vet Med Assoc 2012;240:716–725. [DOI] [PubMed] [Google Scholar]


