Abstract
Background
Brachycephalic dogs are at risk for arterial hypertension and obstructive sleep apnea, which are both associated with chronic magnesium (Mg) depletion.
Hypothesis/Objectives
To compare the period prevalence of hypomagnesemia between Boxers and Bulldogs presented to a referral teaching hospital. To screen a group of Bulldogs for evidence of hypomagnesemia, and to obtain pilot data regarding the utility of parenteral Mg tolerance testing (PMgTT) in the diagnosis of whole‐body Mg deficiency.
Animals
Chemistry laboratory submissions were retrospectively analyzed for serum total Mg (tMg) in Boxers and Bulldogs. Prospectively, 16 healthy client‐owned Bulldogs were enrolled.
Methods
Retrospective case study. tMg concentrations were compared between Boxers and Bulldogs. Dogs with low serum albumin or high serum creatinine concentrations were excluded. Prospectively, ionized Mg (iMg), tMg, and arterial blood pressure were measured and iMg‐to‐tMg ratio (iMg : tMg) was calculated. Parenteral Mg tolerance testing (PMgTT) was performed in 3/16 dogs.
Results
In the retrospective study, period prevalence of hypomagnesemia was 4.7% in Boxers and 15% in Bulldogs (P = .02). The risk ratio for hypomagnesemia in Bulldogs was 1.8 when compared to Boxers (CI: 1.3–2.7). In the prospective study, iMg was [median (interquartile)] 0.43 (0.42–0.46) mmol/L (reference range 0.4–0.52), tMg was 1.9 (1.8–1.9) mg/dL (reference range 1.9–2.5). iMg : tMg was [mean (±SD)] 0.59 ± 0.04. Percentage retention after PMgTT were 55%, 95%, and 67%, respectively.
Conclusions and Clinical Importance
Mg deficiency is common in Bulldogs and could contribute to comorbidities often observed in this breed. iMg : tMg and PMgTT might prove helpful in detecting chronic subclinical Mg deficiency.
Keywords: Boxer, Bulldog, Hypercapnea, Intracellular magnesium, Parenteral magnesium tolerance testing, Sleep apnea‐hypopnea syndrome
Abbreviations
- CLMD
chronic latent magnesium deficiency
- DAP
diastolic arterial blood pressure
- iMg : tMg
ionized‐to‐total magnesium ratio
- iMg
ionized magnesium
- MAP
mean arterial blood pressure
- Mg
magnesium
- PMgTT
parenteral magnesium tolerance testing
- SAP
systolic arterial blood pressure
- SD
standard deviation
- tMg
total magnesium
Magnesium (Mg) is an essential divalent cation that serves as a cofactor for hundreds of enzymatic reactions, influences cell membrane stability, and contributes to the stable tertiary structure of nucleic acids and proteins, among other functions.1, 2, 3 The majority of a mammal's readily exchangeable Mg is stored within cells, although cells are not uniform in their Mg concentrations. After rapid intravenous administration to dogs, the peak tissue Mg concentration and rate of exchange between intracellular and extracellular compartments vary widely among different organs.4 This wide variation in exchange rates (and abrupt translocation in some disease states) limits the precision with which extracellular concentrations can be used to evaluate whole‐body or intracellular Mg status.
Chronic Mg deficiency and intracellular Mg depletion are associated with hypertension in many species.5, 6, 7, 8, 9 It has recently been reported that systemic blood pressures are often significantly higher in brachycephalic breeds relative to other breed types.10 Obstructive sleep apnea associated with recurrent Mg deficiency has recently been reported in a human patient.11 As both obstructive sleep apnea and systemic hypertension are thought to be common in the Bulldog,12 we hypothesized that this breed is more likely to have unrecognized chronic Mg deficiency than is a closely related breed in which obstructive sleep apnea is rarely, if ever, reported (Boxer dogs).13
The specific aims of this study were as follows: (1) to retrospectively compare the period prevalence of hypomagnesemia in a large group of Boxers and Bulldogs presented to an academic veterinary specialty hospital, (2) to prospectively screen a group of young, healthy Bulldogs for hypomagnesemia, and (3) to explore the utility of parenteral magnesium tolerance testing (PMgTT) in the diagnosis of whole‐body Mg deficiency in dogs.
Materials and Methods
Design and Study Population
Dogs were identified for the retrospective portion of this study using clinical pathology data stored as part of the Veterinary Medical and Administrative Computer System at the University of California, Davis. Extracted data were migrated to a master file constructed within a commercially available spreadsheet software tool.1 Dogs for the prospective portion of this study were recruited from two large local breed enthusiast clubs. The Clinical Trials Review Board of the Pritchard Veterinary Medical Teaching Hospital at the University of California, Davis, approved both owner consent forms and the study.
Inclusion and Exclusion Criteria
All dogs coded as Boxers, Bulldogs, or English Bulldogs within the clinical pathology data archives that had at least 1 tMg measurement were included in the initial data set. All chemistry panels reported between September of 2008 and June of 2012 were extracted to create the uncensored data set. The tMg, creatinine, and albumin concentrations were retained for further analysis. Cases were excluded based on hypoalbuminemia or azotemia being present. Remaining dogs were identified as hypomagnesemic or nonhypomagnesemic based on a tMg cutoff value of 1.9 mg/dL (institutional canine reference interval = 1.9–2.5 mg/dL). For dogs in which multiple samples were submitted in a single visit or across multiple visits, only the earliest sample submitted from that dog was included in the analysis.
All dogs for the prospective arm of this study were deemed to be in good health by their owners and after evaluation of data from a physical examination, complete blood count, and a serum chemistry profile. Dietary history was obtained from all dogs by the investigators and recorded. All dogs were fasted overnight and all blood sampling occurred between 0900 and 1200 hours the next day. Blood was drawn from a peripheral venous site without sedation and transferred to anticoagulant‐free vacuum tubes2 for subsequent serum separation by centrifugation after clot formation. Serum total (tMg) and ionized (iMg) concentrations were determined using commercially available equipment designed for this purpose.3, 4 Arterial blood pressure was recorded from 9 of 16 dogs as previously described.10 In brief, after a period of rest, 5 oscillometric systolic (SAP), mean (MAP), and diastolic (DAP) arterial blood pressure measurements were performed.5 The cuff size was approximately 40% of the circumference of the leg above the tibiotarsal joint.14 The readings with the highest and lowest MAP were rejected, and the average of the 3 remaining values then was calculated.
For the PMgTT, all dogs identified as hypomagnesemic during the prospective prevalence study were encouraged to return for additional testing. Three owners elected to enroll their pets in this more invasive portion of the project. All 3 dogs were intact males and received a standardized sedation protocol of methadone (10 mg/dog IV) and dexmedetomidine (100 μg/dog IV) to facilitate aseptic placement of a male Foley urinary catheter, which was connected to a sterile closed collection system. Dexmedetomidine and methadone were subsequently reversed with atipamezole (1 mg/dog IM) and naloxone (0.25 mg/dog IM). The dogs were allowed to recover until the next morning while remaining fasted overnight. On Day 2, urine was collected for baseline urine Mg and creatinine concentrations. After complete emptying of the bladder, a PMgTT was performed as described elsewhere.15 In brief, 0.1 mmol/kg of elemental Mg was infused IV over 4 hours (starting at t 0) and 24‐hour urine output was recorded. The pooled output was subsequently saved for later analysis. Every 4 hours, the urine collection system was emptied into a glass reservoir to which 100 mL of glacial acetic acid6 had been added to further acidify the urine and prevent Mg precipitates from forming ex vivo. After 24 hours, the pooled urine sample was thoroughly mixed and an aliquot submitted for repeat Mg and creatinine determination. A previously published standard formula [% Mg retention = 1 − {(24 hour Mg) − ([Preinfusion urine Mg : Cr] ×[24 hour Cr])}/Mg dose infused*100] was used to determine the percent Mg dose retention after adjusting for dilution by the acetic acid.15 Human criteria were used to classify the results of the PMgTT in the absence of established canine standards. Up to 20% retention was considered to be not supportive of whole‐body Mg deficiency, values from 20 to 50% retention were considered suggestive of whole‐body Mg deficiency, and >50% retention was considered diagnostic for whole‐body deficiency of Mg.
Statistical Methods
All statistical analyses and data plotting were performed with 2 commercially available software programs.7, 8 Shapiro‐Wilk testing was used to test for normal distribution of the data in each group. Data not fitting the predictions of a Gaussian distribution were analyzed with nonparametric methods and are presented as median and interquartile range hereafter. Mann‐Whitney U‐tests were used to compare median tMg between Boxers and Bulldogs within the censored data sets. Fisher's exact testing was used to compare the proportion of Boxers and Bulldogs categorized as hypomagnesemic. Risk ratios (relative risk) and odds ratios were calculated and confidence intervals estimated using commercial analysis software.7 For all tests, two‐tailed P values were used where applicable and P < .05 was considered significant.
Results
The uncensored data set consisted of 314 and 244 chemistry lab accessions from 208 and 128 Boxers and Bulldogs, respectively. Exclusion of samples from dogs that were hypoalbuminemic, azotemic, or had multiple samples submitted left 126 Boxer and 80 Bulldog samples for subsequent analysis. tMg concentrations for both groups were not normally distributed (Fig 1). The median (interquartile range; IQR) tMg for Boxers and Bulldogs was 2.1 (2.0–2.3) mg/dL and 2.1 (1.9–2.2) mg/dL, respectively. This difference was found to be statistically significant with a two‐tailed P = .0069. The period prevalence of hypomagnesemia was 4.7% for Boxers (6/126) and 15% for Bulldogs (12/80) during the study interval. Fisher's Exact testing found the proportions of hypomagnesemic dogs to be significantly different between the Boxer and Bulldog groups (P = .02). Bulldogs in this population had a relative risk (RR) of hypomagnesemia of 1.8 relative to Boxers (95% confidence interval = 1.3–2.7). The odds ratio of hypomagnesemia being identified in a sample from a Bulldog relative to a sample from a Boxer was 3.5 (95% confidence interval = 1.3–9.8).
Figure 1.
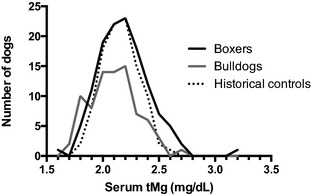
Histogram of serum total magnesium concentrations in Boxers (n = 126), Bulldogs (n = 80), and the historical control dogs used to establish the canine serum total magnesium reference interval at the authors' institution (n = 101). Bins are 0.1 mg/dL in width. The historical controls were a group of clinically healthy dogs between 1 and 8 years of age that are enrolled in our practice's community blood donor program.
Sixteen healthy juvenile or adult Bulldogs with a median age of 131 (range 44–281) weeks were enrolled in the prospective study. There were 4 castrated males, 7 intact males, 1 spayed female, and 4 intact females in this cohort. All dogs were fed commercially available canine diets with reported Mg content well in excess of National Research Council (NRC) mandates. No dogs were receiving diuretics, proton pump inhibitors, or H2‐receptor blockers at the time of blood sampling or within the prior 3 months.
The median (IQR) iMg for the 16 Bulldogs was 0.43 (range 0.42–0.46) mmol/L, which is within our laboratory's canine reference interval of 0.40–0.52 mmol/L. No dogs had iMg concentrations that fell below this reference interval; however, 19% of the dogs (3 of 16) had concentrations recorded as precisely 0.40 mmol/L.
Serum tMg concentrations for the 16 Bulldogs had a median (IQR) value of 1.9 (1.8–1.9) mg/dL, which is at the lower limit of our laboratory's canine reference interval of 1.9–2.5 mg/dL. Thus, 50% of the Bulldog samples were at or below the lower limit of the reference interval (Fig 1). All Bulldogs except for one had a serum tMg of less than 2.0 mg/dL. That dog had the highest recorded value (2.0 mg/dL) for tMg obtained from any dog in the group. The owner subsequently revealed that this dog received a nutritional supplement containing Mg oxide.9 No other dogs were receiving Mg supplementation.
The ionized‐to‐total magnesium ratio (iMg : tMg) was normally distributed with a mean (±standard deviation, SD) of 0.59 (±0.04). At present, there is no established canine reference interval for iMg : tMg. In rats, feeding a Mg‐deficient diet (70 ppm versus 1000 ppm controls) leads to significant, progressive increases in the iMg : tMg (from 0.60 to 0.72).16 Similarly, Norris and colleagues examined serum tMg and iMg concentrations in groups (n = 18 each) of healthy cats fed either a Mg‐deficient or Mg‐replete diet.17 Their raw data were extracted by the authors using graphic design software with spatial mapping tools10 and independent posthoc calculation performed. The mean (±SD) iMg : tMg was found to be 0.56 (±0.05) and 0.59 (±0.05) in cats fed Mg‐deficient or Mg‐replete diets, respectively, and these values were statistically significantly different.
Percent retention of parenteral Mg dose administered for 3 dogs was 55%, 95%, and 67%. Based on criteria presented above, all 3 dogs were classified as moderately to severely whole‐body Mg depleted. Alternatively, application of the Mg‐deficiency staging approach of Mansmann classifies each of these 3 dogs as having either (Type I, Stage 2) or (Type II, Stage 4) Mg deficiency.18 In this approach, Mg deficiency is subcategorized based on intracellular (Type 1; normal serum concentrations) or extracellular (Type 2; decreased serum concentration) Mg deficiency. Each of these 2 types is then further categorized into stages 1–4 based on erythrocyte Mg concentrations, baseline renal excretion concentrations, and PMgTT results. Higher numeric values for staging represent more severe deficiency within that type.
SAP, MAP, and DAP were 147 (range 100–234) mmHg, 110 (range 59–180) mmHg, and 85 (range 56–132) mmHg, respectively. Using the categorization scheme recently put forward in an American College of Veterinary Internal Medicine consensus statement, each dog was assigned a target‐organ damage risk category based on SAP or DAP values.19 Based on these criteria, 3 dogs were assigned to risk category I, 3 to risk category II, 1 to risk category III, and 2 to risk category IV. Thus, 67% of the apparently healthy Bulldogs in this cohort had blood pressure measurements that would lead to the assignment of a risk of target‐organ damage category of II or higher. These findings are comparable to those reported in previous work with a cohort of brachycephalic dogs from western Europe.10
Discussion
The principal findings of the present study are that the prevalence of hypomagnesemia is significantly higher in Bulldogs than in Boxers within a population of dogs presented to a veterinary academic referral hospital. Furthermore, prospective investigation of the prevalence of hypomagnesemia in apparently healthy Bulldogs revealed that hypomagnesemia is even more likely to be identified in this breed than the data from hospitalized dogs would suggest. PMgTT in a subset of hypomagnesemic Bulldogs strongly suggests that low serum tMg concentrations are indicative of whole‐body Mg deficiency in these dogs.
A previous study of hospitalized canine populations has reported a period prevalence of hypomagnesemia of approximately 6%, which closely matches the prevalence in our Boxer control group (4.7%).20 Defining hypomagnesemia by iMg versus tMg has been a somewhat controversial topic in both human and veterinary medicine. The greater biologic activity of iMg and an early report linking iMg to outcomes in critically ill human patients led to the widespread misconception that iMg is the more important marker in all settings in regard to clinical monitoring.21 However, several lines of evidence suggest that iMg should not necessarily serve as the preferred monitoring tool for the routine assessment of Mg status in clinical practice (human or veterinary).17, 22, 23, 24, 25
Current recommendations are that there should be evaluation of tMg or iMg : tMg when screening for chronic Mg imbalance. Interestingly, it has been suggested that “the evaluation of total Mg in plasma or serum appears as a better marker than ionized Mg in Mg imbalance; it should be privileged as the initial investigation in clinical practice.”26 This inverse relationship between Mg balance and iMg : tMg, wherein the ratio decreases in Mg excess and rises in Mg deficiency, was discussed in the same report.26 This relationship is supported by empiric data from dietary Mg restriction in both rodents and felines.16, 17 Alteration in the iMg : tMg might be the result of a homeostatic mechanism, which serves to maintain iMg within the reference range while allowing total serum concentrations to decrease.
In the present study, hypomagnesemia was far more frequently identified in Bulldogs than in Boxers. The Boxer was selected as a control group for several reasons. First, the Boxer and Bulldog are highly related breeds.13 However, it is the authors' opinion that the Boxer is rarely afflicted with brachycephalic syndrome. This opinion is based, in part, on the finding that only two staphylectomies have been performed on Boxers in the past 10 years at the authors' institution and upper airway obstruction is a rare presenting complaint in Boxers presented to our practice (unreported data). Furthermore, the Boxer has not been reported to have a breed predisposition to either systemic hypertension or glomerular disease. Lastly, the Boxer had not been identified in a prior study as a breed at increased or decreased risk of hypomagnesemia.20
The prevalence of hypomagnesemia was even greater in the group of Bulldogs that were assessed prospectively. While the Mg content of the diets being fed to the 16 Bulldogs was not analyzed in the present study, the authors feel that simple dietary Mg deficiency is highly unlikely as an underlying mechanism for our findings. No dogs were fed a homemade diet. All were fed commercial diets whose dietary Mg content is reported to conform to the minimum requirements put forward by the National Research Council (NRC) of the National Academies. The dogs were from over a dozen different households spread over two large states. The majority (75%) of dogs were receiving mixtures of more than one commercial diet (kibble of one type and canned food of another).
PMgTT has not yet been validated in dogs, but is currently considered the gold standard in the diagnosis of chronic Mg deficiency in humans.5, 15, 27 Human patients with serum Mg concentrations within the reference range, yet with abnormal PMgTT results, have been described as having chronic latent magnesium deficiency (CLMD).28, 29, 30, 31 CLMD patients are described as individuals who have a small, chronic, negative Mg balance, but whose serum Mg concentration is within the lower part of the reference interval (latent), and from a clinical standpoint are incorrectly viewed as having normal Mg status.30
Among the 3 dogs that underwent PMgTT, none would be classified as having CLMD because all had tMg below our reference interval. They instead have chronic Mg deficiency (not a latent form). However, many of the Bulldogs in our study that had tMg concentrations in the 1.9–2.0 mg/dL range might be classified as CLMD patients if PMgTT results indicated whole‐body deficiency and one applies the human criteria. The authors suspect that CLMD may be highly prevalent in Bulldogs, at least in our region. Many of the manifestations of obstructive sleep apnea syndrome and chronic Mg deficiency are similar or identical (eg, hypertension, catecholamine excess, metabolic syndrome, chronic low‐grade systemic inflammation, hypercoagulability, adverse cardiac events) and in some cases clinical signs that have routinely been attributed to brachycephalic syndrome in predisposed dogs may be exacerbated by coexistent Mg deficiency.
CLMD is caused by 3 factors leading to a chronic, ongoing, negative Mg balance: (1) inadequate intake, (2) decreased gastrointestinal absorption, or (3) increased excretion by the kidneys.29 While insufficient dietary intake may contribute to Mg deficiency in Bulldogs, it seems likely that other factors are involved as discussed previously. Decreased gastrointestinal absorption seems a likely contributing mechanism based on the evidence currently at hand. If excessive, obligatory renal losses were the main predisposing factor, then the PMgTT testing results would have demonstrated markedly lower retention rates. Clearly the kidneys of these 3 dogs can actively retain large quantities of filtered Mg.
“Spot” PaCO2 tensions are higher in brachycephalic dogs than in nonbrachycephalic controls.10 However, while the mean PaCO2 was higher in brachycephalic dogs, it was still within a range that would be considered normal and appropriate for healthy canines. Moreover, the bicarbonate concentrations did not differ between the groups in that study. These data suggest that brachycephalic dogs do not typically exist in a state of chronic, compensated respiratory acidosis while awake and alert. These findings, and those of earlier studies, suggest that brachycephalic syndrome, like sleep apnea in humans, is better characterized as frequent recurrent, transient, self‐resolving episodes of hypercapnea while at rest.12 Acute increases in PaCO2 induced by carbon dioxide inhalation are associated with marked increases in urinary Mg excretion in a sheep model.32 Thus, it is possible that some or many Bulldogs enter a vicious cycle of hypercapnia‐induced Mg deficiency, which leads to respiratory and pharyngeal muscle weakness and further hypercapnia. This scenario might be further amplified by the normal circadian pattern of increased nocturnal renal Mg excretion that has been observed in many species.33, 34 Moreover, these circadian rhythms may be disrupted in the early stages of hypertension, but become re‐established and then exacerbated as hypertensive syndromes progress.35 If obstructive sleep apnea in the form of brachycephalic syndrome is leading to worsening hypercapnia at night, then renal Mg excretion might be further amplified in excess of that observed because of the normal circadian rhythms in electrolyte excretion. The expression of renal Mg transporters is influenced by acid‐base balance in animal models and alterations in either (or both) PaCO2 and bicarbonate concentrations may alter expression of renal Mg transport proteins in Bulldogs as well.36
In addition to periodic excessive urinary Mg losses, the possibility of reduced gastrointestinal uptake must be considered as a possible mechanism for hypomagnesemia in Bulldogs. While subclinical proximal bowel inflammatory lesions are highly prevalent in brachycephalic dogs,37 the proximal bowel is not the site of Mg absorption in this species.35, 38 However, agents that alter proximal bowel function and the luminal environment such as proton pump inhibitors can lead to hypocalcemia and hypomagnesemia.39, 40 Thus, it is not implausible that proximal bowel lesions might lead to alterations in distal bowel absorptive processes in Bulldogs. The transient receptor potential melastatins 6 (TRPM6) and 7 (TRPM7) are the predominant intestinal Mg channels involved in active colonic Mg uptake in humans41 and mutations in these channels are associated with familial hypomagnesemia in humans.42
Sixty‐seven percent of the Bulldogs in the prospective arm of this study could be classified as mildly to severely hypertensive. The potential mechanisms linking brachycephalic conformation and systemic hypertension have been described elsewhere.10 Mg deficiency has long been implicated as a risk factor for systemic hypertension as well.43 Reduced Mg intake (or enteral Mg absorption) is a significant independent risk factor for hypertension independent of plasma Mg concentrations.44 The antihypertensive effects of Mg are largely ascribed to its innate function as an endogenous calcium channel blocker45, 46, 47, 48, 49 and its ability to reduce arterial stiffness.50
There are several limitations to the present study that bear mentioning. The vast majority of whole‐body Mg content resides in bone and the intracellular compartment. Neither of these Mg pools was ever directly assessed at any point in our work. Dietary analysis was not performed in this pilot study nor was fecal Mg content evaluated, both of which would have added valuable information. In addition, the number of dogs that were enrolled in the PMgTT investigation was quite limited (n = 3) and these data should be considered in light of that fact. Follow‐up work to increase sample size and prospectively investigate the effects of Mg supplementation on arterial blood pressure is underway at present.
In summary, the present study identified a high prevalence of hypomagnesemia in Bulldogs relative to Boxers. A prospective pilot study of the prevalence of hypomagnesemia in a group of young, clinically healthy Bulldogs identified the near‐uniform presence of hypomagnesemia in this cohort. Follow‐up investigations of 3 dogs yielded PMgTT results that would be consistent with moderate to severe cellular Mg depletion in humans. Hypomagnesemia and cellular Mg deficiency may play an important and progressive role in the development of systemic hypertension and in the worsening of airway obstructive episodes in Bulldogs.
Acknowledgment
The prospective portion of this project was supported by the Center for Companion Animal Health, School of Veterinary Medicine, University of California, Davis.
Conflict of Interest: Authors disclose no conflict of interest.
This study was performed at the School of Veterinary Medicine, University of California ‐ Davis, Davis, CA.
Footnotes
Excel, Microsoft Inc, Redmond, WA
3 mL anticoagulant free glass Vacutainer tubes, BD Medical, Franklin Lakes, NJ
Cobas 6000 C501 Chemistry Analyzer, ROCHE Diagnostics, Pleasanton, CA
Nova 8, Nova Biomedical, Waltham, MA
Cardell 9401 Diagnostic Monitor, Midmark International, Versailles, OH
Sigma‐Aldrich, cat#537020, St. Louis, MO
Prism 6, Graphpad Software, San Diego, CA
Sigmaplot 11, Systat Software Inc, Chicago, IL
Show Stopper, Animal Naturals, Inc, Benicia, CA
Photoshop for MacOS, Adobe Software, San Jose, CA
References
- 1. Bara M, Guiet‐Bara A, Durlach J. Modification of human amniotic membrane stability after addition of magnesium salts. Magnes Res 1988;1:23–27. [PubMed] [Google Scholar]
- 2. Bara M, Guiet‐Bara A, Durlach J. A qualitative theory of the screening‐binding effects of magnesium salts on epithelial cell membranes: A new hypothesis. Magnes Res 1989;2:243–248. [PubMed] [Google Scholar]
- 3. Saris NE, Mervaala E, Karppanen H, et al. Magnesium. An update on physiological, clinical and analytical aspects. Clin Chim Acta 2000;294:1–26. [DOI] [PubMed] [Google Scholar]
- 4. Lazzara R, Hyatt K, Love WD, et al. Tissue distribution, kinetics, and biologic half‐life of Mg28 in the dog. Am J Physiol 1963;204:1086–1094. [DOI] [PubMed] [Google Scholar]
- 5. Ayuk J, Gittoes NJ. How should hypomagnesaemia be investigated and treated? Clin Endocrinol 2011;75:743–746. [DOI] [PubMed] [Google Scholar]
- 6. Touyz RM, Schiffrin EL. The effect of angiotensin II on platelet intracellular free magnesium and calcium ionic concentrations in essential hypertension. J Hypertens 1993;11:551–558. [DOI] [PubMed] [Google Scholar]
- 7. Resnick LM, Gupta RK, Laragh JH. Intracellular free magnesium in erythrocytes of essential hypertension: Relation to blood pressure and serum divalent cations. Proc Natl Acad Sci USA 1984;81:6511–6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rude R, Manoogian C, Ehrlich L, et al. Mechanisms of blood pressure regulation by magnesium in man. Magnesium 1989;8:266–273. [PubMed] [Google Scholar]
- 9. Guerrero‐Romero F, Rodriguez‐Moran M. The effect of lowering blood pressure by magnesium supplementation in diabetic hypertensive adults with low serum magnesium levels: A randomized, double‐blind, placebo‐controlled clinical trial. J Hum Hypertens 2009;23:245–251. [DOI] [PubMed] [Google Scholar]
- 10. Hoareau GL, Jourdan G, Mellema M, et al. Evaluation of arterial blood gases and arterial blood pressures in brachycephalic dogs. J Vet Intern Med 2012;26:897–904. [DOI] [PubMed] [Google Scholar]
- 11. Blasco LM, Novo F, Gonzalez‐Fernandez CR. Chronic cyclic nonnephrogenic magnesium depletion without losses. N Engl J Med 2012;366:1845–1846. [DOI] [PubMed] [Google Scholar]
- 12. Hendricks JC, Kline LR, Kovalski RJ, et al. The English Bulldog: A natural model of sleep‐disordered breathing. J Appl Physiol 1987;63:1344–1350. [DOI] [PubMed] [Google Scholar]
- 13. Vaysse A, Ratnakumar A, Derrien T, et al. Identification of genomic regions associated with phenotypic variation between dog breeds using selection mapping. PLoS Genet 2011;7:e1002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Valtonen MH, Eriksson LM. The effect of cuff width on accuracy of indirect measurement of blood pressure in dogs. Res Vet Sci 1970;11:358–362. [PubMed] [Google Scholar]
- 15. Ryzen E, Elbaum N, Singer FR, et al. Parenteral magnesium tolerance testing in the evaluation of magnesium deficiency. Magnesium 1985;4:137–147. [PubMed] [Google Scholar]
- 16. Zimmermann P, Weiss U, Classen HG, et al. The impact of diets with different magnesium contents on magnesium and calcium in serum and tissues of the rat. Life Sci 2000;67:949–958. [DOI] [PubMed] [Google Scholar]
- 17. Norris CR, Christopher MM, Howard KA, Nelson RW. Effect of magnesium‐deficient diet on serum and urine magnesium concentrations in healthy cats. Am J Vet Res 1999;60:1159–1163. [PubMed] [Google Scholar]
- 18. Mansmann HC. Consider magnesium homeostasis. 2. Staging of magnesium deficiencies. Pediatr Asthma Allergy Immunol 1993;7:211–215. [Google Scholar]
- 19. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007;21:542–558. [DOI] [PubMed] [Google Scholar]
- 20. Khanna C, Lund EM, Raffe M, et al. Hypomagnesemia in 188 dogs: a hospital population‐based prevalence study. J Vet Intern Med 1998;12:304–309. [DOI] [PubMed] [Google Scholar]
- 21. Chernow B, Bamberger S, Stoiko M, et al. Hypomagnesemia in patients in postoperative intensive care. Chest 1989;95:391–397. [DOI] [PubMed] [Google Scholar]
- 22. Toll J, Erb H, Birnbaum N, et al. Prevalence and incidence of serum magnesium abnormalities in hospitalized cats. J Vet Intern Med 2002;16:217–221. [DOI] [PubMed] [Google Scholar]
- 23. Huijgen HJ, Soesan M, Sanders R, et al. Magnesium levels in critically ill patients. What should we measure? Am J Clin Pathol 2000;114:688–695. [DOI] [PubMed] [Google Scholar]
- 24. Koch SM, Warters RD, Mehlhorn U. The simultaneous measurement of ionized and total calcium and ionized and total magnesium in intensive care unit patients. J Crit Care 2002;17:203–205. [DOI] [PubMed] [Google Scholar]
- 25. Norris CR, Nelson RW, Christopher MM. Serum total and ionized magnesium concentrations and urinary fractional excretion of magnesium in cats with diabetes mellitus and diabetic ketoacidosis. J Am Vet Med Assoc 1999;215:1455–1459. [PubMed] [Google Scholar]
- 26. Durlach J, Pages N, Bac P, et al. Importance of the ratio between ionized and total Mg in serum or plasma: New data on the regulation of Mg status and practical importance of total Mg concentration in the investigation of Mg imbalance. Magnes Res 2002;15:203–205. [PubMed] [Google Scholar]
- 27. Agus ZS. Hypomagnesemia. J Am Soc Nephrol 1999;10:1616–1622. [DOI] [PubMed] [Google Scholar]
- 28. Elin RJ. Magnesium metabolism in health and disease. Dis Mon 1988;34:161–218. [DOI] [PubMed] [Google Scholar]
- 29. Elin RJ. Assessment of magnesium status for diagnosis and therapy. Magnes Res 2010;23:S194–S198. [DOI] [PubMed] [Google Scholar]
- 30. Elin RJ. Re‐evaluation of the concept of chronic, latent, magnesium deficiency. Magnes Res 2011;24:225–227. [DOI] [PubMed] [Google Scholar]
- 31. Gunther T. Magnesium in bone and the magnesium load test. Magnes Res 2011;24:223–224. [DOI] [PubMed] [Google Scholar]
- 32. Stacy BD, Wilson BW. Acidosis and hypercalciuria: Renal mechanisms affecting calcium, magnesium and sodium excretion in the sheep. J Physiol 1970;210:549–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roelfsema F, van der Heide D, Smeenk D. Circadian rhythms of urinary electrolyte excretion in freely moving rats. Life Sci 1980;27:2303–2309. [DOI] [PubMed] [Google Scholar]
- 34. Touitou Y, Auzeby A, Camus F, et al. Twenty‐four‐hour profiles of urinary excretion of calcium, magnesium, phosphorus, urea, and creatinine in healthy prepubertal boys. Clin Biochem 2010;43:102–105. [DOI] [PubMed] [Google Scholar]
- 35. Aslanian NL, Assatrian DG, Bagdassarian RA, et al. Circadian rhythms of electrolyte excretion in hypertensive patients and healthy subjects. Chronobiologia 1978;5:251–262. [PubMed] [Google Scholar]
- 36. Nijenhuis T, Renkema KY, Hoenderop JG, et al. Acid‐base status determines the renal expression of Ca2+ and Mg2+ transport proteins. J Am Soc Nephrol 2006;17:617–626. [DOI] [PubMed] [Google Scholar]
- 37. Poncet CM, Dupre GP, Freiche VG, et al. Prevalence of gastrointestinal tract lesions in 73 brachycephalic dogs with upper respiratory syndrome. J Small Anim Pract 2005;46:273–279. [DOI] [PubMed] [Google Scholar]
- 38. Schweigel M, Martens H. Magnesium transport in the gastrointestinal tract. Front Biosci 2000;5:D666–D677. [DOI] [PubMed] [Google Scholar]
- 39. Ito T, Jensen RT. Association of long‐term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, iron, and magnesium. Curr Gastroenterol Rep 2010;12:448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Danziger J, William JH, Scott DJ, et al. Proton‐pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int 2013;83:692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Quamme GA. Recent developments in intestinal magnesium absorption. Curr Opin Gastroenterol 2008;24:230–235. [DOI] [PubMed] [Google Scholar]
- 42. Knoers NV. Inherited forms of renal hypomagnesemia: An update. Pediatr Nephrol 2009;24:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Berthelot A, Esposito J. Effects of dietary magnesium on the development of hypertension in the spontaneously hypertensive rat. J Am Coll Nutr 1983;2:343–353. [DOI] [PubMed] [Google Scholar]
- 44. Joosten MM, Gansevoort RT, Mukamal KJ, et al. Urinary magnesium excretion and risk of hypertension: The prevention of renal and vascular end‐stage disease study. Hypertension 2013;61:1161–1167. [DOI] [PubMed] [Google Scholar]
- 45. Teragawa H, Matsuura H, Chayama K, et al. Mechanisms responsible for vasodilation upon magnesium infusion in vivo: Clinical evidence. Magnes Res 2002;15:241–246. [PubMed] [Google Scholar]
- 46. Yogi A, Callera GE, Antunes TT, et al. Vascular biology of magnesium and its transporters in hypertension. Magnes Res 2010;23:S207–S215. [DOI] [PubMed] [Google Scholar]
- 47. Touyz RM. Transient receptor potential melastatin 6 and 7 channels, magnesium transport, and vascular biology: Implications in hypertension. Am J Physiol Heart Circ Physiol 2008;294:H1103–H1118. [DOI] [PubMed] [Google Scholar]
- 48. Yogi A, Callera GE, Antunes TT, et al. Transient receptor potential melastatin 7 (TRPM7) cation channels, magnesium and the vascular system in hypertension. Circ J 2011;75:237–245. [DOI] [PubMed] [Google Scholar]
- 49. Zholos A, Johnson C, Burdyga T, et al. TRPM channels in the vasculature. Adv Exp Med Biol 2011;704:707–729. [DOI] [PubMed] [Google Scholar]
- 50. Kisters K, Gremmler B, Hausberg M. Magnesium and arterial stiffness. Hypertension 2006;47:e3. [DOI] [PubMed] [Google Scholar]


