Abstract
Background
Myocardial injury, detected by cardiac troponin I and T (cTnI and cTnT), has been associated with long‐term death in the noncardiac human intensive care unit (ICU).
Hypothesis
Presence of myocardial injury predicts 1‐year case fatality in critically ill dogs with systemic inflammation.
Animals
Thirty‐eight dogs with evidence of systemic inflammation and no primary cardiac disease.
Methods
Prospective cohort study. In dogs admitted to the ICU with evidence of systemic inflammation, blood samples were obtained at ICU admission for measurement of cTnI and cTnT, and cTnI was measured once daily during ICU hospitalization. Receiver operating characteristic (ROC) curves were used to examine prognostic capacity of admission cTnI, admission cTnT, and peak cTnI concentrations.
Results
One‐year case fatality rate was 47% (18/38 dogs). Admission cTnI concentrations were (median [range]) 0.48 [0.004–141.50] ng/mL, and peak cTnI concentrations were 1.21 [0.021–141.50] ng/mL. Admission cTnT concentrations were 15 [<13–3744] ng/L. For each marker, non‐survivors had significantly higher concentrations than survivors (P = .0082–.038). ROC analyses revealed areas under curves [95% CI] of 0.707 [0.537–0.843] for peak cTnI and 0.739 [0.571–0.867] for admission cTnT, respectively. At the optimal cut‐off, concentrations were 1.17 ng/mL (peak cTnI) and 23 ng/L (admission cTnT), sensitivities were 72% and 72%, and specificities were 70% and 80%, respectively.
Conclusions and Clinical Importance
While peak cTnI and admission cTnT are significantly related to 1‐year case fatality in critically ill dogs with systemic inflammation, low sensitivities and specificities prevent their prediction of long‐term outcome in individual patients. Troponins might play a role in identification of dogs at long‐term risk of death.
Keywords: Biomarker, Companion animals, Intensive care unit, Myocardial injury, Prognostic significance
Abbreviations
- AUC
area under the curve
- cTnI
cardiac troponin I
- cTnT
cardiac troponin T
- ICU
intensive care unit
- ROC
receiver operating characteristic
- VHUP
Veterinary Hospital of the University of Pennsylvania
Myocardial injury as detected by cardiac troponins in critical disease is gaining an increasing amount of attention in emergency and critical care in both human and veterinary medicine. Increased concentrations of cardiac troponins have been detected in a variety of cardiac as well as noncardiac critical diseases.1, 2, 3, 4, 5, 6 Elevated cardiac troponins are, therefore, evolving from being thought of as synonymous with acute myocardial infarction, for which they are diagnostic gold standard biomarkers in human medicine,7 to representing myocardial injury in a broader context.8 When excluding patients with cardiac disease (ischemic or nonischemic), myocardial injury has been detected in up to 80% of critically ill human patients9 with the highest percentage in those with inflammatory diseases and shock.10, 11, 12, 13 Myocardial injury in patients with systemic inflammation is suspected to have a multifactorial cause14 involving especially hemodynamic changes and toxic effects of cytokines.6, 11, 15, 16
Many human studies investigating myocardial injury in noncardiac critically ill patients have found that an increased concentration of cardiac troponin is significantly associated with short‐term death,6, 11, 17, 18, 19 and recently this association was confirmed in critically ill dogs as well.20 In fact, human patients with myocardial injury due to noncardiac critical disease have up to 4 times higher case fatality rate compared with patients with normal troponin concentrations.6, 11, 17, 19 Additionally, in both humans and dogs, the cardiac troponins have been shown to provide short‐term prognostic information beyond conventional risk scoring.6, 20, 21 As critically ill human patients with and without myocardial injury generally have similar clinical characteristics,17, 19 measurement of cardiac troponin may, therefore, be of high importance in the ICU.21 It has been speculated that daily measurements of troponins in human ICU patients could be of further value, as peak concentrations could possibly provide stronger associations with death,14, 22 and changes (increase or decrease) could be an indicator of outcome. Conflicting evidence exists. One study found no meaningful temporal changes in troponin concentrations in relation to death or survival,16 whereas another study found that a rise in troponin concentrations indicated a poor prognosis and a higher risk of multiple organ failure.23
Few studies have investigated troponins as predictors of long‐term case fatality in critically ill patients. Stein et al. (2008) concluded that, in humans, even borderline elevations were associated with in‐hospital death, but not with death at 6 months.24 However, other studies have demonstrated an independent association of troponin with death even at 2–3 years of follow‐up.6, 14, 21, 22 No studies have examined cardiac troponin as a predictor of long‐term outcome in noncardiac critical disease in veterinary medicine.
The objective of this study was to investigate the long‐term prognostic potential of cardiac troponins I and T (cTnI and cTnT) in critically ill dogs with systemic inflammation and to evaluate the importance of daily troponin measurement in the ICU during hospitalization. It was hypothesized that admission as well as peak troponin concentrations during ICU hospitalization would be predictive of case fatality 1 year postadmission.
Materials and Methods
Study Population
The study population was drawn from a population of critically ill client‐owned dogs with systemic inflammation included in a previously reported study of the short‐term prognostic importance of myocardial injury in the summer of 2011 at the ICU of the Veterinary Hospital of the University of Pennsylvania (VHUP).20 Dogs with primary structural heart disease or recent treatment with cardiotoxic drugs (eg, doxorubicin) at the time of ICU admission had been excluded from the primary study to avoid noninflammatory causes of myocardial injury.20 Dogs were included in this follow‐up study if 1 year outcome information could be obtained. Clinical outcome was defined as survival or death 1 year postadmission. Nonsurvivors were excluded if the cause of death was unrelated to the cause of hospitalization in the summer 2011. Outcome and, for nonsurvivors, time and cause of death or euthanasia were determined through telephone contact with the owner.
Blood Sampling and Analyses
Serum for analysis of cTnI and cTnT was obtained at the time of ICU admission. A follow‐up serum sample was collected every day for as long as the animal was hospitalized in the ICU to allow for serial determination of cTnI. Serial determination of cTnT was not possible as ethical regulations necessitated serum volume restrictions. Serum was collected into gel separator tubes, allowed to clot for 30 minutes at room temperature, centrifuged for 10 minutes at 1300× g, separated, and stored in cryovials at −70°C within 2 hours of blood collection. Samples were stored for a maximum of 8 months until batch analysis. Cardiac troponin I and cTnT were analyzed by commercially available high‐sensitivity immunoassays.1 , 2 The cTnI assay has recently been validated for use in companion animals,25 and the cTnT assay has been used previously for assessment of myocardial injury in dogs.26
Statistical Analyses
Data were assessed for normality by the D'Agostino‐Pearson omnibus test. Logarithmic transformation was applied where this assured a Gaussian distribution of otherwise nonparametric data. A two‐tailed t‐test was used to compare Gaussian data, and the Mann‐Whitney U‐test was used to compare non‐Gaussian data. The prognostic capability for 1‐year case fatality of admission cTnI and cTnT as well as peak cTnI was assessed using receiver operating characteristic (ROC) curve analysis. A significant prognosticator was defined as having an area under the curve (AUC) significantly >0.5, ie, with 0.5 not included in the 95% CI. Statistical significance was defined as P < .05. All statistical analyses were conducted by commercial statistical software (Normality, t‐test, Mann‐Whitney U‐test,3 ROC curve analysis4).
Results
Study Population Characteristics
Forty‐two dogs entered the primary study20 with 4 dogs lost to long‐term follow‐up. One‐year outcome information was available for the remaining 38 dogs. These dogs were 1 female intact, 19 female spayed, and 18 male castrated dogs with an age span of 1–14 years (mean 7.5 years). Seven dogs were mixed breeds; all other dogs were purebreds of 21 different breeds, the most frequent being Labrador Retriever (n = 6) and French Bulldog (n = 3). Dogs presented with a primary diagnosis of trauma (n = 7, 3 dogs with poly trauma and 4 dogs with localized trauma, 1 of which was later discovered to be due to neoplasia), neoplasia (n = 8), gastrointestinal disease (n = 8), respiratory disease (n = 4), neurologic disease (n = 4), hematologic disease (n = 2), and various (n = 5: hemoabdomen due to splenic rupture (n = 2) and 1 of each of biliary mucocoele, peritonitis, and gastric dilatation volvulus) diseases.
Clinical Outcome
The 1‐year case fatality rate was 47% (18/38 dogs). Eleven dogs died within the first 28 days of admission.20 Seven additional dogs died between that time and 1 year postadmission, all of causes believed to be related to the cause of hospitalization in 2011. On original presentation, 4 of these dogs were diagnosed with neoplasia, 1 with localized bone trauma which was later discovered to be due to neoplasia, 1 with respiratory disease, and 1 with peritonitis. Two dogs died at home after rapid clinical deterioration, and 5 dogs were euthanized because of progression or recurrence of clinical signs and perceived poor prognosis. Four of the dogs died within 4 months of ICU admission, and the last 3 dogs died within 11 months.
Cardiac Troponins
Admission cTnI concentrations were (median [range]) 0.48 [0.004–141.50] ng/mL. Nonsurvivors (1.74 [0.054–141.50] ng/mL) had significantly higher cTnI concentrations than survivors (0.22 [0.004–15.86] ng/mL) (P = .038) (Fig 1A).
Figure 1.
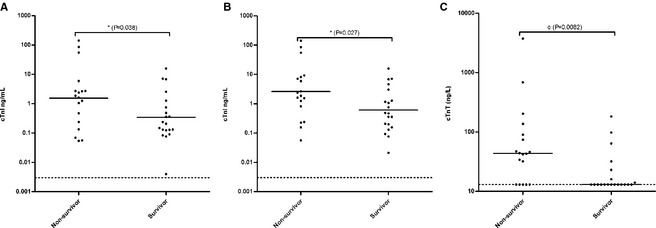
Serum admission cardiac troponin I (cTnI) (A), peak cTnI (B), and admission cardiac troponin T (cTnT) (C) concentrations of 38 critically ill dogs with systemic inflammation (nonsurvivors and survivors). Geometric mean concentrations (μ) for Gaussian and median concentrations (m) for non‐Gaussian data are shown as horizontal lines. Significant differences among groups are symbolized with * (two‐tailed t‐test) and o (Mann‐Whitney U‐test). The detection limit of the assay is shown as a grid line.
The median number of days of ICU hospitalization was 2 [1–8]. For 3 dogs that died during their first day in the ICU, only admission concentrations were available. For these and for an additional 16 dogs that had peak cTnI concentrations at admission, admission concentrations were, therefore, used in the peak analysis. The median day of ICU hospitalization that the cTnI concentration peaked was day 1.5 [1–4]. Peak cTnI concentrations were 1.21 [0.021–141.50] ng/mL, but were not significantly higher than admission concentrations (P = .27). Nonsurvivors (2.14 [0.056–141.50] ng/mL) had significantly higher peak cTnI concentrations than survivors (0.48 [0.021–15.86] ng/mL) (P = .027) (Fig 1B). Upon visual inspection of serial cTnI concentrations, there was no consistent trend toward a continuing rise among nonsurvivors or decrease among survivors (data not shown).
Admission cTnT concentrations were 15 [<13–3744] ng/L. Nonsurvivors (43.5 [<13–3744] ng/L) had significantly higher cTnT concentrations than survivors (<13 [<13–183] ng/L) (P = .0082) (Fig 1C).
When comparing short‐term (died within 28 days, n = 11) with long‐term (died within 1–12 months, n = 7) nonsurvivors, there was no significant difference in admission cTnI (2.64 [0.24–85.2] ng/mL versus 0.13 [0.054–141.50] ng/mL, P = .085), peak cTnI (5.92 [0.24–85.2] ng/mL versus 1.25 [0.056–141.50] ng/mL, P = .085), or admission cTnT (47 [13–686] ng/L versus 13 [13–3744] ng/L, P = .23).
Peak cTnI and admission cTnT, but not admission cTnI, were found to be significant prognosticators for 1‐year case fatality (Table 1, Fig 2). For dogs with peak cTnI concentrations above 1.17 ng/mL, the optimal discriminative cut‐off identified by the ROC curve analysis, the case fatality rate was 65% (13/20), whereas it was 28% (5/18) for dogs with concentrations below this cut‐off. For cTnT, the optimal cut‐off was 23 ng/L. Dogs with admission concentrations above this value had a 1‐year case fatality rate of 72% (13/18), whereas it was 25% (5/20) for dogs with lower cTnT concentrations.
Table 1.
Prognostic capacity of the cardiac troponins in 38 critically ill dogs with systemic inflammation evaluated by analysis of receiver operating characteristic curves (ROC).
| Marker | AUC‐ROC | 95% CI of AUC‐ROC | Prognostic Sensitivity | Prognostic Specificity | Optimal Cut‐off (ROC) |
|---|---|---|---|---|---|
| Admission cTnI | 0.669 | [0.498–0.813] | – | – | – |
| Peak cTnIa | 0.707 | [0.537–0.843] | 72% | 70% | 1.17 ng/mL |
| Admission cTnTa | 0.739 | [0.571–0.867] | 72% | 80% | 23 ng/L |
AUC‐ROC, area under the receiver operating characteristic curve; CI, confidence interval; cTnI, cardiac troponin I; cTnT, cardiac troponin T.
Indicates significant prognostic capacity.
Figure 2.
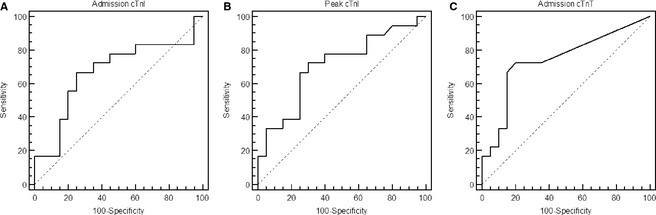
ROC analyses of serum admission cardiac troponin I (cTnI) (A), peak cTnI (B), and admission cardiac troponin T (cTnT) (C) concentrations as predictors of case fatality 1‐year postadmission of 38 critically ill dogs with systemic inflammation.
Discussion
In this study, the cardiac troponins were shown to be predictors of long‐term case fatality in critically ill dogs with systemic inflammation as has been previously shown in humans.6, 14, 21, 22 Daily measurements of troponins in the ICU were of value in the prognostication of the dogs as peak cTnI, but not admission cTnI concentrations were associated with 1‐year case fatality.
Admission cTnI was not a significant long‐term prognosticator, but a trend toward significance was noticed, and it is possible that a larger cohort would have revealed a long‐term prognostic potential. Interestingly, although peak cTnI concentrations were not significantly higher than admission concentrations, peak cTnI predicted 1‐year case fatality in this cohort of dogs. Temporal changes of circulating cTnI did not, however, distinguish nonsurvivors from survivors. It has been reported that cardiac troponin concentrations in critically ill humans often peak at or shortly after ICU admission.15, 17 This is in line with our findings and suggests that myocardial injury has been initiated by events preceding ICU admission and could be a partial cause of the patient's worsening condition, leading to ICU hospitalization. Larger studies are necessary to elucidate whether serial monitoring of troponins is of value in monitoring the individual patient.
The best long‐term prognostic marker in this study was admission cTnT. This was a surprising finding because admission cTnI was the best predictor of short‐term death in critically ill dogs.20 Cardiac troponin I had an excellent negative predictive value as all dogs with admission cTnI concentrations below the optimal discriminative cut‐off (0.24 ng/mL) were alive 28 days postadmission, but a less optimal positive predictive value as only 46% of dogs with higher concentrations had died with several survivors among those with the highest concentrations.20 However, cTnT is known to be released with more severe cardiac injury than cTnI, possibly because of its larger size or a closer binding to the cardiomyocyte contractile apparatus.3, 27 Therefore, based on the findings of the present study, a greater amount of cardiac injury may be a marker of more severe systemic illness, leading to a higher risk of death also in the long term. As inflammatory myocardial injury is believed to be reversible in many cases, it is not considered likely that permanent myocardial injury results from the initial inflammatory condition, and that the dogs later on succumb to a cardiac problem. Rather we are suggesting that high cardiac troponin concentrations reveal a subset of patients with a higher risk of death, likely due to progression of their original underlying disease, and with a need for close follow‐up after hospital discharge. However, further studies, measuring troponins and performing EKGs and echocardiography 1–6 months after the initial presentation, are needed to assess whether permanent myocardial injury could also be a contributor to death in these cases.
As with all outcome prediction systems, caution must be exercised in application of these measurements to individual patients, and decisions of euthanasia should not be based on the concentrations of cardiac troponins, but rather on an educated overall assessment of the patient. This is even more apparent when noticing the overlap of troponin concentrations between survivors and nonsurvivors and the resultant low prognostic sensitivities and specificities provided by the markers for long‐term prediction. Based on our findings, troponins may be valuable in risk stratification of critically ill dogs in clinical research. It has also been discussed whether patients with evidence of myocardial injury might benefit from more aggressive treatment in the ICU,17, 28 in which case normalizing of troponin concentrations after treatment might be associated with an improved outcome. This appears to be the case in human patients with myocardial injury in relation to primary cardiac disease29; however, the causes of myocardial injury in cardiac and noncardiac disease are most likely different,30, 31 and comparisons can, therefore, not be directly made. In inflammatory disease, cytokines and reactive oxygen species produced by activated leukocytes are thought to be important mediators of cardiac injury with a direct toxic, but possibly reversible effect on the cardiomyocytes.10, 30, 32 Further studies are necessary to examine whether serial measurements of cardiac troponins may provide a tool for monitoring patients and titrating treatment accordingly.
This study contains several limitations. First of all, as admission cTnI came very close to significant prognostic capacity, a larger cohort of dogs is necessary to further elucidate whether admission cTnI may be used for this purpose. Secondly, because of limited available serum, serial measurements of cTnT were not available. As peak cTnI was superior to admission cTnI in this study, peak cTnT could possibly be an even better long‐term prognosticator in dogs with systemic inflammation. Thirdly, in this study, as in the primary study, euthanized animals were included in the group of nonsurvivors, and this may provide a source of error, although we attempted to minimize it by exclusion of dogs euthanized for causes other than a grave prognosis.
In conclusion, peak cTnI and admission cTnT concentrations were predictive of 1‐year case fatality in a cohort of critically ill dogs with systemic inflammation. Low prognostic sensitivities and specificities of these markers prevent them from being used to predict long‐term outcome in individual patients, but they may play an important role in identification of long‐term risk patients in the ICU, especially in patients not initially judged at high risk by conventional risk scoring systems.20, 21
Acknowledgments
The authors thank Professor Jens Peter Goetze, Department of Clinical Biochemistry, Rigshospitalet, University of Copenhagen, Denmark, for assistance with cardiac troponin T measurements and the staff at Matthew J. Ryan Veterinary Hospital of the University of Pennsylvania for help with inclusion of patients for the study.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
This study is a 1‐year follow‐up of a study carried out at the Matthew J. Ryan Veterinary Hospital of the University of Pennsylvania, Philadelphia, PA in the summer 2011.
Footnotes
ADVIA Centaur CP TnI‐ultra; Siemens Healthcare Diagnostics Inc, Tarrytown, NY
Elecsys hs‐TnT; Roche Diagnostic Corporation, Indianapolis, IN
GraphPad Prism 5.02 for Windows; GraphPad Software, San Diego, CA
MedCalc 6.00.012; MedCalc Software, Mariakerke, Belgium
References
- 1. Horwich TB, Patel J, MacLellan WR, Fonarow GC. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation 2003;108:833–838. [DOI] [PubMed] [Google Scholar]
- 2. Linklater AKJ, Lichtenberger MK, Thamm DH, et al. Serum concentrations of cardiac troponin I and cardiac troponin T in dogs with class IV congestive heart failure due to mitral valve disease. J Vet Emerg Crit Care 2007;17:243–249. [Google Scholar]
- 3. Schober KE, Cornand C, Kirbach B, et al. Serum cardiac troponin I and cardiac troponin T concentrations in dogs with gastric dilatation‐volvulus. J Am Vet Med Assoc 2002;221:381–388. [DOI] [PubMed] [Google Scholar]
- 4. Porciello F, Rishniw M, Herndon WE, et al. Cardiac troponin I is elevated in dogs and cats with azotaemia renal failure and in dogs with non‐cardiac systemic disease. Aust Vet J 2008;86:390–394. [DOI] [PubMed] [Google Scholar]
- 5. Pelander L, Hagman R, Haggstrom J. Concentrations of cardiac troponin I before and after ovariohysterectomy in 46 female dogs with pyometra. Acta Vet Scand 2008;50:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu TT, Yuan A, Chen CY, et al. Cardiac troponin I levels are a risk factor for mortality and multiple organ failure in noncardiac critically ill patients and have an additive effect to the APACHE II score in outcome prediction. Shock 2004;22:95–101. [DOI] [PubMed] [Google Scholar]
- 7. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581–1598. [DOI] [PubMed] [Google Scholar]
- 8. Markou N, Gregorakos L, Myrianthefs P. Increased blood troponin levels in ICU patients. Curr Opin Crit Care 2011;17:454–463. [DOI] [PubMed] [Google Scholar]
- 9. Rosjo H, Varpula M, Hagve T, et al. Circulating high sensitivity troponin T in severe sepsis and septic shock: Distribution, associated factors, and relation to outcome. Intensive Care Med 2011;37:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ammann P, Fehr T, Minder EI, et al. Elevation of troponin I in sepsis and septic shock. Intensive Care Med 2001;27:965–969. [DOI] [PubMed] [Google Scholar]
- 11. Ammann P, Maggiorini M, Bertel O, et al. Troponin as a risk factor for mortality in critically ill patients without acute coronary syndromes. J Am Coll Cardiol 2003;41:2004–2009. [DOI] [PubMed] [Google Scholar]
- 12. Arlati S, Brenna S, Prencipe L, et al. Myocardial necrosis in ICU Patients with acute non‐cardiac disease: A prospective study. Intensive Care Med 2000;26:31–37. [DOI] [PubMed] [Google Scholar]
- 13. Turner A, Tsamitros M, Bellomo R. Myocardial cell injury in septic shock. Crit Care Med 1999;27:1775–1780. [DOI] [PubMed] [Google Scholar]
- 14. Vasile VC, Babuin L, Perez JAR, et al. Long‐term prognostic significance of elevated cardiac troponin levels in critically ill patients with acute gastrointestinal bleeding. Crit Care Med 2009;37:140–147. [DOI] [PubMed] [Google Scholar]
- 15. Noble JS, Reid AM, Jordan LVM, et al. Troponin I and myocardial injury in the ICU. Br J Anaesth 1999;82:41–46. [DOI] [PubMed] [Google Scholar]
- 16. ver Elst KM, Spapen HD, Nguyen DN, et al. Cardiac troponins I and T are biological markers of left ventricular dysfunction in septic Shock. Clin Chem 2000;46:650–657. [PubMed] [Google Scholar]
- 17. Guest T, Ramanathan A, Tuteur P, et al. Myocardial injury in critically ill patients ‐ a frequently unrecognized complication. J Am Med Assoc 1995;273:1945–1949. [PubMed] [Google Scholar]
- 18. John J, Woodward DB, Wang Y, et al. Troponin‐I as a prognosticator of mortality in severe sepsis patients. J Crit Care 2010;25:270–275. [DOI] [PubMed] [Google Scholar]
- 19. Wright RS, Williams BA, Cramner H, et al. Elevations of cardiac troponin I are associated with increased short‐term mortality in noncardiac critically ill emergency department patients. Am J Cardiol 2002;90:634–636. [DOI] [PubMed] [Google Scholar]
- 20. Langhorn R, Oyama MA, King LG, et al. Prognostic importance of myocardial injury in critically ill dogs with systemic inflammation. J Vet Intern Med 2013;27:895–903. [DOI] [PubMed] [Google Scholar]
- 21. Vasile VC, Chai H, Khambatta S, et al. Significance of elevated cardiac troponin t levels in critically ill patients with acute respiratory disease. Am J Med 2010;123:1049–1058. [DOI] [PubMed] [Google Scholar]
- 22. Babuin L, Vasile VC, Perez JAR, et al. Elevated cardiac troponin is an independent risk factor for short‐ and long‐term mortality in medical intensive care unit patients. Crit Care Med 2008;36:759–765. [DOI] [PubMed] [Google Scholar]
- 23. Spies C, Haude V, Fitzner R, et al. Serum cardiac troponin t as a prognostic marker in early sepsis. Chest 1998;113:1055–1063. [DOI] [PubMed] [Google Scholar]
- 24. Stein R, Gupta B, Agarwal S, et al. Prognostic implications of normal (< 0.10 ng/ml) and borderline (0.10 to 1.49 ng/ml) troponin elevation levels in critically ill patients without acute coronary syndrome. Am J Cardiol 2008;102:509–512. [DOI] [PubMed] [Google Scholar]
- 25. Langhorn R, Willesen J, Tarnow I, Kjelgaard‐Hansen M. Evaluation of a high sensitivity assay for measurement of canine and feline serum cardiac troponin I. Vet Clin Pathol 2013;42:490–498. [DOI] [PubMed] [Google Scholar]
- 26. DeFrancesco TC, Atkins CE, Keene BW, et al. Prospective clinical evaluation of serum cardiac troponin T in dogs admitted to a veterinary teaching hospital. J Vet Intern Med 2002;16:553–557. [DOI] [PubMed] [Google Scholar]
- 27. Shaw SP, Rozanski EA, Rush JE. Cardiac troponins I and T in dogs with pericardial effusion. J Vet Intern Med 2004;18:322–324. [DOI] [PubMed] [Google Scholar]
- 28. Krishnagopalan S, Kumar A, Parrillo JE, Kumar A. Myocardial dysfunction in the patient with sepsis. Curr Opin Crit Care 2002;8:376–388. [DOI] [PubMed] [Google Scholar]
- 29. Miller WL, Hartman KA, Burritt MF, et al. Profiles of serial changes in cardiac troponin T concentrations and outcome in ambulatory patients with chronic heart failure. J Am Coll Cardiol 2009;54:1715–1721. [DOI] [PubMed] [Google Scholar]
- 30. Wu AHB. Increased troponin in patients with sepsis and septic shock: Myocardial necrosis or reversible myocardial depression? Intensive Care Med 2001;27:959–961. [DOI] [PubMed] [Google Scholar]
- 31. White HD. Pathobiology of troponin elevations: Do elevations occur with myocardial ischemia as well as necrosis? J Am Coll Cardiol 2011;57:2406–2408. [DOI] [PubMed] [Google Scholar]
- 32. Quenot JP, Le Teuff G, Quantin C, et al. Myocardial injury in critically ill patients ‐ Relation to increased cardiac troponin I and hospital mortality. Chest 2005;128:2758–2764. [DOI] [PubMed] [Google Scholar]


