Abstract
Background
Portable blood glucose meters (PBGMs) allow easy glucose measurements. As animal‐specific PBGMs are not available everywhere, those for humans are widely used.
Objectives
To assess the accuracy and precision of 9 PBGMs in canine whole blood (WB) and plasma, based on the ISO 15197:2013.
Animals
Fifty‐nine client‐owned dogs attending the Veterinary Teaching Hospital.
Methods
Analytical evaluation of 100 blood samples was performed for accuracy and 23 for precision (glucose 29–579 mg/dL) following ISO recommendations. A PBGM was considered accurate if 95% of the measurements were within ±15 mg/dL from the reference when glucose was <100 mg/dL and within ±15% when it was ≥100 mg/dL, and if 99% of them were within zones A and B in error grid analysis (EG). A hexokinase‐based analyzer was used as reference. Ninety samples were assessed for hematocrit interferences.
Results
Accuracy requirements were not fulfilled by any PBGM in WB (74% of measurements within the limits for the most accurate) and by 1 only in plasma. However, the EG analysis in WB was passed by 6 PBGM and by all in plasma. The most accurate were also the most precise, with coefficients of variation <5% in WB and <3% in plasma. Hematocrit correlated with bias against the reference method in 4 PBGM (r = −0.243 − [−0.371]; P < .021).
Conclusions and Clinical Importance
This disparity among PBGM suggests that meters approved for humans need to be evaluated before use in other species.
Keywords: Diabetes, Dog, ISO 15197:2003, ISO 15197:2013
Abbreviations
- CEN
European Committee for Standardization
- GDH
glucose dehydrogenase
- GO
glucose oxidase
- PBGM
portable blood glucose meter
- WB
whole blood
In humans, self‐monitoring of blood glucose is crucial for the management of insulin‐treated diabetes and has allowed more accurate treatment dosing and has contributed to a reduction in disease complications.1 In veterinary medicine, portable blood glucose meters (PBGM) allow owners and practitioners to obtain glucose measurements easily and make immediate therapeutic decisions. Although specific PBGM have been developed for small animals, they are not available everywhere and those developed for humans are widely used.
Many studies have been published where these “human” devices have been tested in dogs and other animals,2, 3, 4, 5, 6, 7, 8, 9 and at the time this study was performed, the previous study in dogs had been published in 20099 and new PBGM had been developed and become available since then. In published studies, not all PBGM were accurate enough to be used in dogs and even those specifically developed for animals led to inappropriate clinical interpretation.3
Our aim was to assess the analytical accuracy and precision of 9 available PBGM in canine whole blood (WB) and plasma samples, based on a standardized systematic evaluation approved by the European Committee for Standardization (CEN), the recommendations of the ISO 15197:2003.10 However, during the review of this manuscript the ISO 15197:2013 was released and, therefore, results were reanalyzed based on the current standard.11
Both ISO 15197:2003 and 2013 are international standards utilized to evaluate accuracy and precision of these devices for human blood.10, 11, 12, 13 They establish minimum performance criteria for blood glucose monitoring systems, based on analytical precision and accuracy and specify the procedures to demonstrate the systems’ conformity to these standards.10, 11
Materials and Methods
Setting and Design
A total of 9 PBGM were assessed for accuracy and precision in canine blood samples based on ISO 15197:2003 guidelines. The samples were obtained in the Veterinary Teaching Hospital and all measurements were performed in its clinical laboratory.
Glucose Monitoring Systems and Reference Method
The following PBGM were assessed by using a single meter of each brand: AccuChek Aviva Nano1 (Aviva), FreeStyle Freedom Lite2 (Freestyle), Glucocard G+ meter (GT 1820)3 (Glucocard), Hemocue Glucose 201+ 4 (Hemocue), OneTouch UltraEasy5 (Ultra), OneTouch VerioPro5 (Verio) and OneTouch Vita5 (Vita), Optium Xceed2 (Optium), and StatStrip Xpress Glucose Hospital Meter6 (StatStrip). Their main features are summarized in Table 1. The different devices and strips or cuvettes were donated by their manufacturers. All are currently widely available and used in human medicine and were the most recent models at the time this study was performed (April 2011–April 2012).
Table 1.
Main features of the evaluated devices according to their manufacturers.
| PBGM | Sample (μL) | Measurement Range (mg/dL) | Measurement Time (seconds) | Measurement Method |
|---|---|---|---|---|
| AccuChek Aviva Nano | 0.6 | 10–600 | 5 | GDH |
| Freestyle Freedom Lite | 0.3 | 20–500 | 5 | GDH |
| Glucocard G+ meter (GT 1820) | 0.6 | 10–600 | 5.5 | GDH |
| Hemocue Glucose 201+ | 5 | 0–400 | 40–240 | GDH |
| OneTouch Ultra | 1 | 20–600 | 5 | GO |
| OneTouch VerioPro | 0.4 | 20–600 | 5 | GDH |
| OneTouch Vita | 0.4 | 20–600 | 5 | GO |
| Optium Xceed | 1.5 | 20–500 | 5 | GDH |
| Statstrip Xpress Glucose H. M. | 0.6 | 10–600 | 6 | GO |
PBGM, portable blood glucose meters; GDH, glucose dehydrogenase; GO, glucose oxidase.
As a reference method, the hospital's automated laboratory analyzer (Catalyst7), based on a multilayered, dry‐slide technology, hexokinase method, was employed on plasma samples, as in routine clinical practice.8Quality controls for the reference method and each device were performed following manufacturer′s instructions. The reference method typically shows coefficients of variation below 5% for most measurements and less than 1% for glucose.8
Samples and Protocol
Whole blood samples were collected from 59 client‐owned dogs with diabetes, insulinoma and other unrelated diseases, attending the Veterinary Teaching Hospital between May 2011 and April 2012. Most samples were obtained for clinical purposes and opportunistically included in this assessment. The protocol was approved by the Animal Welfare Ethics Committee (Comité Ético de Bienestar Animal, ULPGC; Reference number 007/2011). They were extracted from the jugular, cephalic, or saphenous vein, with a syringe (22G‐needle). Immediately after sampling, 100 μL of WB was separated to be used on all of the PBGM and the rest was poured into lithium‐heparin tubes10, 11 and centrifuged. After plasma separation, 300 μL were used in the automated laboratory analyzer, to measure glucose and other routine analytes, and the rest, to measure plasma glucose with all the PBGM. To avoid glucose consumption in the sample, all measurements were performed consecutively, with a maximum delay of 20 minutes between sampling and testing. To avoid systematic bias because of sample processing, PBGM were randomly and blindly extracted from an opaque container and measurements were performed in the initial order and then shifted several times during the assay. All devices were operated and calibrated according to the manufacturers’ instructions.
Samples were classified according to their glucose concentration, measured by the reference method, into normoglycemic (73–143 mg/dL), hypoglycemic, and hyperglycemic. In addition, for each ISO‐established interval samples were collected as recommended: 5 <50 mg/dL, 15 between 51 and 80 mg/dL, 20 between 81 and 120 mg/dL, 30 between 121 and 200 mg/dL, 15 between 201 and 300 mg/dL, 10 between 301 and 400 mg/dL, and 5 >400 mg/dL. Following ISO recommendations, when patient samples were not available for a specific range, euglycemic WB samples were either incubated at room temperature to allow for erythrocyte glucose consumption8, 10, 11 or Glucose G75289 was added.10, 11 After direct addition of glucose, one of the meters (Glucocard) was used as a preliminary estimation that the sample was within the aimed glucose range. In that case, if this range was confirmed by the reference method, the rest of the procedure was completed.
When analytical errors were warned by a device, the measurement was repeated attempting to correct the error, until it was obtained. If, after a third attempt, the error persisted, the value was defined as missing. When values outside the measurable range were obtained (LO or HI), the value immediately below or above the limit, respectively, was entered.
A total of 100 (WB and plasma) samples were used to assess accuracy: glucose was measured in each sample with the 9 PBGM and the reference method. According to ISO 15197:2003 requirements for human use, a PBGM is considered accurate if 95% of the measurements are within ±15 mg/dL from the reference when glucose is <75 mg/dL, and within ±20% when glucose is ≥75 mg/dL.10 The results were also analyzed following the ISO 15197:2013 requirements11 that establish narrower accuracy limits (±15 mg/dL from the reference when glucose is <100 mg/dL, and within ±15% when glucose is ≥100 mg/dL).
Error grid analysis assessment for type 1 diabetes11 was performed to asses clinical risk for each measurement (see Fig 2).14 To define a PBGM as accurate, the ISO 15197:2013 requires 99% of the values to be within zones A and B.11
Figure 2.
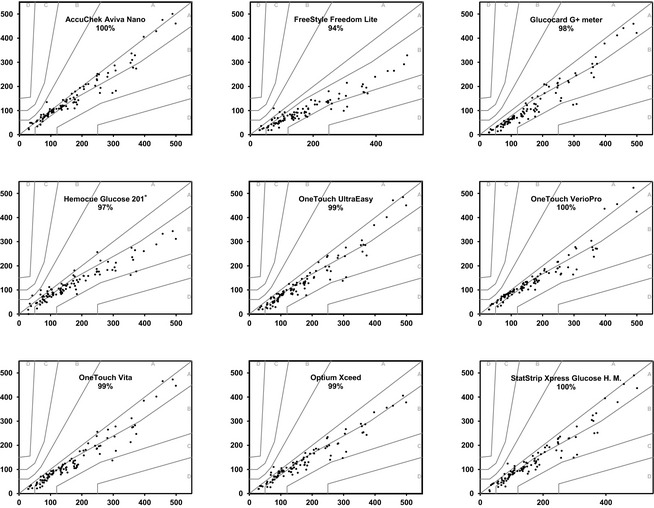
Error grid analysis representation for whole blood for each device with the percentage of values within A–B zones. The reference glucose values (“true” glucose value), on the x axis, are plotted against the blood glucose by the glucose meter (y axis). The different zones designate the magnitude of risk derived from the determination: no effect on clinical action (zone A), altered clinical action – little or no effect on clinical outcome (zone B), altered clinical action – likely to affect clinical outcome (zone C), altered clinical action – could have significant medical risk (zone D) and altered clinical action – could have dangerous consequences (zone E).14 ISO 15197:2013 requires that 99% of the values fall within zones A–B for a device to be considered accurate.
Precision was assessed on 23 samples, 8 in the hypoglycemic and hyperglycemic ranges, and 7 in the normoglycemic range. On each sample, 3 measurements were performed with each PBGM.
Hematocrit was assessed in 90 samples, either by a microhematocrit centrifuge (StatSpin VT7), or by the hospital's automated analyzer (Lasercyte Dx7), and was classified as low (<37%), normal (37–55%), or high (>55%), following the reference of the automated analyzer.
Statistical Analysis
To assess accuracy, PBGM values and the reference method were compared using paired Student's t or Wilcoxon's test. The differences between the PBGM and the reference method were plotted against the reference values in Bland‐Altman plots. Passing‐Bablok linear regression analysis was performed to detect constant and proportional bias. If the 95% CI for the slope did not include 1, this was considered evidence of proportional bias. If the 95% CI for the y intercept did not include 0, this was considered evidence of constant bias.15 To assess precision, mean, standard deviation, and coefficient of variation were calculated for each device. Interference by hematocrit was assessed comparing the differences between PBGM and the reference method in low, normal, and high hematocrit samples (Kruskall‐Wallis test) and evaluating their correlation with hematocrit values (Spearman tests).
Statistical analyses were performed by a commercial statistical software package.10 Differences were considered significant when two‐tailed P was below .05.
Results
One hundred samples from 57 dogs with glucose concentrations ranging from 29 to 579 mg/dL were included in the study and analyzed for accuracy and 23 of them (same range) were also analyzed for precision. A total of 43 samples were treated to complete the required number for hypo‐ and hyperglycemic ranges: 6 were incubated at room temperature and to 27, glucose was added, respectively. The other 10 were included in the normoglycemic range, as they did not fall within the expected limits. Analytical errors, warned by Aviva, Ultra, Verio, Optium, and StatStrip, included insufficient volume in the strip chamber, incorrect application of the sample, and defective strips. Verio and Hemocue failed to measure 1 hyper‐ and 1 hypoglycemic sample, respectively.
Accuracy
Mean differences in glucose concentrations (mean and SD) obtained with the reference method and the 9 PBGM assessed (both for WB and plasma) are displayed in Table 2. WB glucose concentration was lower for all PBGMs compared with the reference method (175.30 [SD 115.74] mg/dL), though the Aviva PBGM was the most accurate (155.98 [SD 105.79] mg/dL) (P < .005). Regarding plasma samples, the most exact were Freestyle (174.41 [SD 111.70] mg/dL) (P = .665) and StatStrip (179.22 [SD 129.16] mg/dL) (P = .148).
Table 2.
Deviation from “trueness”: Reference mean values and devices’ mean differences from reference [mg/dL (SD)] for WB and P, for the whole range and per glycemic interval.
| Device | Blood Source | Mean difference with reference (SD) | |||
|---|---|---|---|---|---|
| Whole Range (N = 100) | Hypoglycemia (N = 15) | Normoglycemia (N = 38) | Hyperglycemia (N = 47) | ||
| Reference value Mean (SD) | 175.30 (115.74) | 51.38 (14.98) | 106.15 (22.30) | 265.04 (106.39) | |
| Aviva | WB | 19.32 (28.19) | 1.38 (12.61)d | 8.87 (13.05) | 32.67 (33.67)d |
| P | −10.90 (15.20) | −6.54 (3.48) | −5.69 (6.40) | −16.31 (19.82) | |
| FreeStyle | WB | 74.15 (55.24) | 18.69 (13.60) | 40.38 (21.09) | 116.60 (48.83) |
| P | 0.89 (20.48)b | −2.00 (4.95)d | 1.49 (9.85)d | 1.19 (28.21)d | |
| Glucocard | WB | 48.18 (30.84) | 25.54 (10.62) | 40.64 (11.60) | 60.44 (39.02) |
| P | 28.10 (20.21) | 15.46 (5.97) | 24.97 (6.62) | 34.06 (26.97) | |
| Hemocue | WB | 47.83 (49.15)a | 2.75 (20.93)d | 22.08 (15.92) | 80.02 (51.00) |
| P | −10.79 (32.66) | −19.15 (9.61) | −18.49 (7.85) | −2.27 (45.07)d | |
| Ultra | WB | 38.7 (30.99) | 16.92 (13.16) | 27.15 (13.53) | 54.23 (36.56) |
| P | −64.71 (43.32) | −6.62 (11.41)d | −40.87 (17.16) | −99.81 (31.91) | |
| Verio | WB | 29.96 (32.45)a | 6.77 (11.19)d | 14.44 (10.09) | 49.25 (37.28) |
| P | −13.19 (14.42)a | −5.42 (5.47) | −8.38 (7.16) | −19.17 (17.81) | |
| Vita | WB | 38.82 (30.95) | 16.46 (12.69) | 25.15 (11.56) | 54.58 (36.25) |
| P | −59.61 (40.45) | −3.61 (9.61)d | −35.80 (13.71) | −94.13 (26.76) | |
| Optium | WB | 38.57 (34.76) | 10.85 (14.96) | 19.03 (15.32) | 61.96 (34.78) |
| P | −48.56 (31.95) | −13.46 (9.67) | −39.00 (11.99) | −65.83 (35.90) | |
| StatStrip | WB | 33.28 (30.28) | 12.62 (11.09) | 20.44 (15.21) | 49.26 (34.57) |
| P | −3.92 (26.87)c | 4.85 (4.78) | 5.62 (7.32) | −14.04 (35.64) | |
WB, whole blood; P, plasma; PBGMs, portable blood glucose meters.
a One hundred determinations were obtained for all devices, except Verio (99 WB and 98 P samples) and Hemocue (99 WB samples). Normoglycemia is defined between 73 and 143 mg/dL.
b, cAll PBGMs showed significant differences with the reference in both whole blood and plasma, for the whole glucose range, with the exception of bFreestyle (P = .665) and cStatStrip (P = .148) in plasma samples, and Vita and Ultra for the same samples in the hypoglycemic range (P = .200 and P = .059, respectively) and Hemocue (P = .729), in hyperglycemia.
d P > .05 (nonsignificant) compared with reference by glycemic ranges.
When evaluating the different glycemic intervals in WB (Table 2), in the hypoglycemic range, only 2 devices showed similar values to the hexokinase method (51.38 [SD 14.98] mg/dL), Aviva (P = .678) and Hemocue (P = .605), whereas Verio reached the limit of statistical significance (P = .05). For the normoglycemic and hyperglycemic intervals, all devices showed significantly lower values than the reference (P < .005).
For the corresponding plasma samples (Table 2), Freestyle and StatStrip were similar to the reference (175.30 [SD 115.74] mg/dL) for all glycemic intervals (P = .686 and P = .148, respectively), Vita and Ultra, in the hypoglycemic range (P = .200 and P = .059, respectively) and Hemocue (P = .729), in the hyperglycemic range.
None of the devices fulfilled ISO 15197:2013 accuracy requirements for WB (95% of values within the global glycemic range) (Fig 1A). The most accurate PBGM was Aviva with 74% of total measurements within the limits. For plasma, standards were only achieved by Aviva, and approached by FreeStyle and Verio with 97%, 92%, and 91.92% of values within limits, respectively (Fig 1B).
Figure 1.
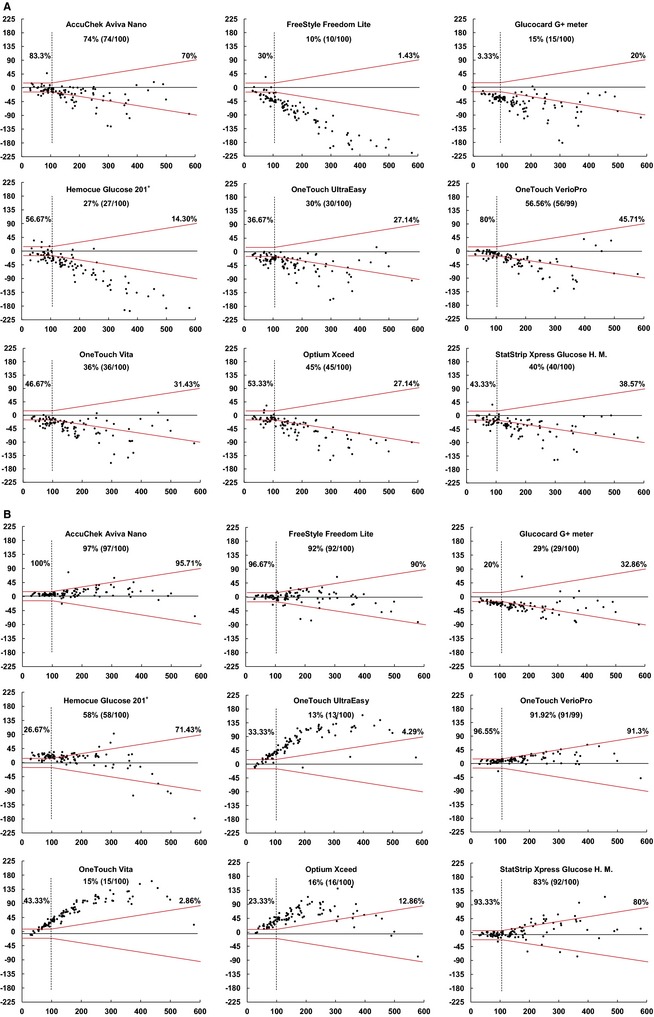
Bland‐Altman plots representing accuracy of portable blood glucose meters for whole blood (A) and plasma (B) for ISO 15197:2013. On the x axis, are the reference glucose values, plotted against the absolute errors for each corresponding value. The standard required limits defined by the red symmetric lines: at ±15 mg/dL from the reference value for glucose determinations <100 mg/dL and at ±15% from the reference for glucose ≥100 mg/dL. Percentages express the number of samples within limits when reference was < or ≥100 mg/dL and for the total number of measurements (central% value).
Even the previous, somewhat laxer, ISO 15197:2003 accuracy requirements, were not fulfilled by any device. The 2 most accurate, Aviva and Verio, showed 82% and 64% of total measurements within the limits, respectively. For plasma, the requirements were achieved by Aviva, FreeStyle, and Verio with 99%, 95%, and 99% of values within limits, respectively, and were approached by StatStrip, with 92% of the values within the limits.
Regarding the EG analysis for WB (Fig 2), most satisfied the requirements, with all (Aviva, Verio, and StatStrip) or 99% of the values (Optium, Ultra, and Vita) falling within zones A and B. The rest of the devices approached the EG requirements (Fig 2). In plasma, all of the PBGM passed the analysis with 99% of values in zones A–B for Optium and Hemocue and 100% for the rest (data not shown).
According to Passing‐Bablok linear regression analysis, 6 devices showed both proportional and constant errors (Glucocard, Hemocue, Ultra, Verio, Vita, and StatStrip) in WB and in plasma (Aviva, Glucocard, Hemocue, Vita, Optium, and StatStrip). Constant errors alone were shown in WB for Aviva, Freestyle, and Optium, and in plasma for Freestyle, Ultra, and Verio.
Additional accuracy analyses were performed and similar results were obtained regardless of whether the samples were pretreated (incubation at room temperature or addition of glucose) or not (data not shown).
Precision
Figure 3 summarizes the results of precision assessment. Aviva and Verio had the smallest coefficient of variation (CV) both in WB (CV = 4.5% [SD 2.7%] and CV = 4.8% [SD 2.3%] respectively, range 0.8–59.9%) and in plasma (CV = 2.7% [SD 1.2%] and CV = 2.9% [SD 2.4%], range 0.47–27.8%).
Figure 3.
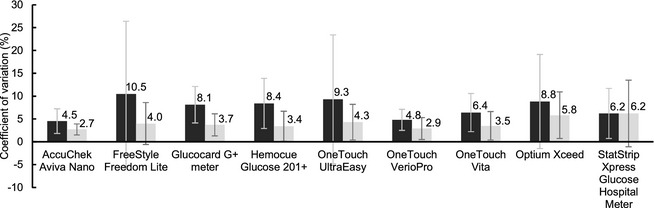
Precision evaluation: Coefficients of variation (%) for all devices in whole blood (dark) and plasma (light) with bars showing respective standard deviations.
Hematocrit Interference
Hematocrit was assessed in 90 samples (glucose 34–489 mg/dL), with 17 in the low (35 [20–40]%), 71 in the normal (45 [37–54]%), and 2 in the high hematocrit range (59.5 [59–60]%). When mean differences were compared, only Hemocue showed smaller errors in the low hematocrit range (−29.53 [SD 54.92] mg/dL) when compared with the normal (−49.34 [SD 41.91] mg/dL) and high intervals (−64.5 mg/dL [SD 20.51]) (P = .018). Negative correlations of the error with hematocrit were found for Freestyle, Hemocue, Ultra, and Vita (r = −0.243 − [−0.371]; P < .021).
Discussion
In this assessment of PBGM in dogs, all devices showed significantly lower average WB glucose values than the reference and none fulfilled previous or current ISO accuracy requirements. Only Aviva achieved the requirements in plasma and was the most accurate of the devices tested. The EG analysis in WB was passed by 6 of the devices and, in plasma, by all. Likewise, Aviva was the most precise PBGM, followed by Verio, both with CV below 5% in WB. CV was reduced in most of the glucose meters by the use of plasma samples (Fig 3). Constant error, a type of bias that could potentially be corrected by calibration, was shown in WB for Aviva, Freestyle, and Optium, and in plasma for Freestyle, Ultra, and Verio.
These results, in agreement with previous studies, support the need to assess accuracy and precision in every PBGM before it is used in dogs for clinical or research purposes.3, 9 Not only inappropriate therapeutic decisions could be made when using inaccurate PBGM, but, when used for research, results might also lead to false conclusions. Indeed, most often, no information is given on the accuracy or precision of the PBGM in animal research studies.16, 17, 18
Portable blood glucose meters are often selected based on their brand or series of models, assuming that there are no differences in accuracy among them.16, 19 However, as interpreted by previous results,2, 3, 4, 5, 6, 8, 9, 20 and in accordance with this study, there is not a preferred brand to recommend and every PBGM should be evaluated independently (Fig 1a,b).
This study also assesses the effect of hematocrit on PBGM accuracy. Previous studies have shown that PBGM yield higher glucose concentrations than the reference method in euglycemic, anemic dogs and cats.5, 7, 8, 20 In this study, 90 samples with a wide glucose (34–489 mg/dL) and hematocrit range (20–60%) were included. Four PBGM (Freestyle, Hemocue, Ultra, and Vita) showed interferences by hematocrit.
Although clinical glucose control may not be as tight in dogs as in humans, the accuracy requirements for glucose meters should not be laxer.7 This perception of permissive glucose control can lead PBGM to be judged as “acceptable” for clinical use,7, 8, 9, 20 especially if evaluations are mostly based on less stringent approaches, such as error grid analysis. The current 15197:2013 standard requires both analytical and EG criteria to be fulfilled, but the latter seem to be redundant in this study. Indeed, most of the devices fulfilled the EG criteria for WB without satisfying the analytical accuracy demands and, if a device fulfilled the analytical ISO requirements, it also passed the EG analysis. In fact, clinical consequences depend on the specific needs of patients and their circumstances. Indeed, the amplitude of the acceptable limits can be crucial in some cases, such as in tight glycemic control protocols, where an imprecision and bias below 2% have been estimated to be needed to achieve 95% accuracy in insulin administration.21 Thus, although optimal glucose control is less feasible in animals, accuracy should be strictly evaluated with rigorous analytical criteria.
The ISO 15197:2003 and 15197:2013 are standardized systematic evaluations approved by the CEN and followed by manufacturers that get the Conformité Européenne (CE) mark for their products.12, 13 The methodology and statistical tests in previous studies have been essentially the same2, 3, 4, 5, 6, 7, 8, 9 as those used here. In fact, the ISO includes these accepted analytical tests with tightening standards.22 Its detailed methodology makes it a reliable tool also in veterinary medicine and allows direct comparison among studies. Of the 9 devices assessed in this study, only Optium had been previously evaluated in dogs,5 with similar results to those here reported.
Most of the devices analyzed in this study fulfill the ISO 15197:2003 and 2013 for human capillary blood.12, 13 We are not aware of a known explanation for this interspecies gap in accuracy, but given the difference in performance between WB and plasma samples, we suggest that the cellular fraction must contain the source of error in canine samples. Furthermore, some interference with hematocrit was detected in 4 of the devices. Overall, when assessed in dogs, Aviva is the best option, regarding both accuracy and precision. Freestyle, Verio, and StatStrip were accurate in plasma samples, but were less precise.
There were several limitations in this study: the use of a single device of each PBGM, the use of venous (instead of capillary) blood, the incubation of or glucose addition to samples, the use of lithium‐heparin anticoagulant for many samples and the lack of specific PBGM for animals.
To assess more than 1 device of the 9 different PBGM would not have been feasible with the resources available. Indeed, assessing all the devices simultaneously was already a challenge. Multiple potential sources of error such as temperature, altitude, humidity, sample volume, hemolysis, or pharmacological factors have been described to interfere with the measurements.23, 24 Although not all can be accounted for, in this study, we obtained and processed the samples in relatively stable conditions and in very similar conditions to those of routine clinical practice.4 Finally, incubation of or glucose addition to the samples, despite being accepted by the ISO, might also be considered as sources of error. Nevertheless, after performing the additional accuracy evaluations for the untreated and treated samples, the similarity in the results obtained after stratification support that this was not the fact in this study.
In humans, small but significant differences have been found between capillary and venous glucose.21, 25 In fact, PBGM are calibrated to show venous‐equivalent concentrations. Thus, some additional inaccuracy should be expected when venous samples are used instead of capillary blood.25 Different ways to obtain capillary blood have been developed in small animals,7, 19 but venous sampling remains the most usual way to obtain blood in veterinary practice. This fact allowed us to obtain the necessary number of samples from routine clinical practice. The OneTouch devices were the only ones specifically recommended for capillary blood only (Table 1). Despite this, (OneTouch) Verio showed the second most accurate results for whole venous blood. Differences between capillary and venous samples in dogs have been evaluated before, proving negligible for most of the devices and glucose concentrations assessed (2–6 mg/dL).5, 7
Blood collection with sodium‐fluoride as a glycolysis inhibitor is considered the gold standard for glucose determinations.26 However, we intended to reproduce what is most frequently done in our routine veterinary practice, where samples often are collected into lithium‐heparin tubes before analysis. Early processing limited potential glucose consumption by the blood cells. In fact, according to the manufacturers, lithium heparin is an appropriate anticoagulant for all the devices27, 28, 29, 30, 31, 32, 33, 34, 35 and, in previous studies, it did not interfere with the results.3, 4, 8
Conclusions
When glycemic control is assessed, advantages of PBGM are to obtain easy, fast, and relatively cheap measurements with minimal volumes. However, even for humans, important variability in accuracy has been demonstrated. The disparity among devices in this study confirms the need of accuracy evaluations before its use in dogs and the ISO 15197:2013 is an excellent tool for this purpose in animals, as it is in humans. Although none of the PBGM fulfills the ISO requirements for whole venous blood, overall, AccuChek Aviva Nano is the best option among those evaluated, given its accuracy, precision, and lack of interference by hematocrit.
Acknowledgments
During the performance of this study, the authors were supported by a predoctoral fellowship (FUNCIS ID41/2008) (YBC) and grants EFSD/JDRF/Novo Nordisk Programme for Type 1 Diabetes 2008 (AMW, CM, JCW) and PI08/01113 and PI11/02441 (YBC, AMW, JCW, CM), from the Spanish National Research Program.
We are grateful to Natalia Navarro and Fidela González (Menarini Diagnostics), Vanessa Fernández (Roche Diagnostics), Álvaro Ladra (Abbott Científica), Juani Perera and Yeray Hernández (Izasa‐Cultek), and Celio Falcón (Lifescan Johnson and Johnson) for providing the glucose meters and test strips.
Conflict of Interest Declaration: The authors disclose no conflict of interest. The manufacturers had no role in the funding, design, analysis, or reporting of the results.
All work was performed at the Hospital Clínico Veterinario, ULPGC, Trasmontaña s/n, Arucas, Gran Canaria, Spain.
Two posters containing preliminary results were presented at the 22nd Congress of the European College of Veterinary Internal Medicine, Maastricht, 6–8th September 2012, and the 12th Congress of the Federation for European Laboratory Animal Science Associations, Barcelona, 10–13th June 2013.
Footnotes
Roche Diagnostics, Mannheim, Germany
Abbott Diabetes Care Ltd., Witney, Oxon, UK
Arkray‐Menarini Diagnostics, Shiga, Japan
Hemocue AB, Ängelholm, Sweden
LifeScan Inc., Milpitas, CA
Nova Biomedical, Waltham, MA
IDEXX Laboratories, ME
Siska WD, Rosen NK, Christian JA, Taddeo DA, DeNicola DB. Vet Clin Pathol. Proceedings of the 13th Annual Congress of the European Society for Veterinary Clinical Pathology; 2011 September 1–3; Dublin, Ireland. American Society for Veterinary Clinical Pathology, 2011
Sigma‐Aldrich Chemie Gmbh, Steinheim, Germany
IBM SPSS Statistics Version 20; SPSS Inc., Chicago, IL
References
- 1. The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin‐dependent diabetes mellitus. The Diabetes Control and Complications Trial. Arch Ophthalmol 1995;113:36–51. [DOI] [PubMed] [Google Scholar]
- 2. Bluwol K, Duarte R, Lustoza MD, et al. Avaliação de dois sensores portáteis para mensuração da glicemia em cães. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 2007;59:1408–1411. [Google Scholar]
- 3. Cohen TA, Nelson RW, Kass PH, et al. Evaluation of six portable blood glucose meters for measuring blood glucose concentration in dogs. J Am Vet Med Assoc 2009;235:276–280. [DOI] [PubMed] [Google Scholar]
- 4. Cohn LA, McCaw DL, Tate DJ, et al. Assessment of five portable blood glucose meters, a point‐of‐care analyzer, and color test strips for measuring blood glucose concentration in dogs. J Am Vet Med Assoc 2000;216:198–202. [DOI] [PubMed] [Google Scholar]
- 5. Fracassi FH, Hadar GS, Pietra M, Famigli BP. Assessment of two portable blood glucose meters for use in cats and dogs. J Vet Clin Sci 2009;2:108–121. [Google Scholar]
- 6. Joseph RJ, Allyson K, Graves TK, et al. Evaluation of two reagent strips and three reflectance meters for rapid determination of blood glucose concentrations. J Vet Intern Med 1987;1:170–174. [DOI] [PubMed] [Google Scholar]
- 7. Wess G, Reusch C. Capillary blood sampling from the ear of dogs and cats and use of portable meters to measure glucose concentration. J Small Anim Pract 2000;41:60–66. [DOI] [PubMed] [Google Scholar]
- 8. Wess G, Reusch C. Evaluation of five portable blood glucose meters for use in dogs. J Am Vet Med Assoc 2000;216:203–209. [DOI] [PubMed] [Google Scholar]
- 9. Johnson BM, Fry MM, Flatland B, et al. Comparison of a human portable blood glucose meter, veterinary portable blood glucose meter, and automated chemistry analyzer for measurement of blood glucose concentrations in dogs. J Am Vet Med Assoc 2009;235:1309–1313. [DOI] [PubMed] [Google Scholar]
- 10. International Organization for Standardization . In Vitro Diagnostic Test Systems—Requirements for Blood‐glucose Monitoring Systems for Self‐testing in Managing Diabetes Mellitus. European Committee for Standardization (CEN): Brussels; 2003. DIN EN ISO 15197:2003. [Google Scholar]
- 11. International Organization for Standardization . In Vitro Diagnostic Test Systems—Requirements for Blood‐glucose Monitoring Systems for Self‐testing in Managing Diabetes Mellitus. European Committee for Standardization (CEN): Brussels; 2013. DIN EN ISO 15197:2013. [Google Scholar]
- 12. Freckmann G, Schmid C, Baumstark A, et al. System accuracy evaluation of 43 blood glucose monitoring systems for self‐monitoring of blood glucose according to DIN EN ISO 15197. J Diabetes Sci Technol 2012;6:1060–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freckmann G, Baumstark A, Jendrike N, et al. System accuracy evaluation of 27 blood glucose monitoring systems according to DIN EN ISO 15197. Diabetes Technol Ther 2010;12:221–231. [DOI] [PubMed] [Google Scholar]
- 14. Parkes JL, Slatin SL, Pardo S, et al. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care 2000;23:1143–1148. [DOI] [PubMed] [Google Scholar]
- 15. Passing H, Bablok W. Comparison of several regression procedures for method comparison studies and determination of sample sizes. Application of linear regression procedures for method comparison studies in Clinical Chemistry, Part II. J Clin Chem Clin Biochem 1984;22:431–445. [DOI] [PubMed] [Google Scholar]
- 16. Morales AP, Conde EG, Lopez MG, et al. An improved method of 90% pancreatectomy using a low dose of streptozotocin at the pancreaticoduodenal artery results in a rapid diabetic stage in dogs. Acta Diabetol 2005;42:153–155. [DOI] [PubMed] [Google Scholar]
- 17. Palm CA, Boston RC, Refsal KR, et al. An investigation of the action of Neutral Protamine Hagedorn human analogue insulin in dogs with naturally occurring diabetes mellitus. J Vet Intern Med 2009;23:50–55. [DOI] [PubMed] [Google Scholar]
- 18. Piccione G, Casella S, Panzera M, et al. Effect of moderate treadmill exercise on some physiological parameters in untrained beagle dogs. Exp Anim 2012;61:511–515. [DOI] [PubMed] [Google Scholar]
- 19. Borin‐Crivellenti S, Crivellenti LZ, Tinucci‐Costa M. The carpal pad as an alternative sampling site for blood glucose testing in dogs. J Small Anim Pract 2012;53:684–686. [DOI] [PubMed] [Google Scholar]
- 20. Wess G, Reusch C. Assessment of five portable blood glucose meters for use in cats. Am J Vet Res 2000;61:1587–1592. [DOI] [PubMed] [Google Scholar]
- 21. Boyd R, Leigh B, Stuart P. Capillary versus venous bedside blood glucose estimations. Emerg Med J 2005;22:177–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heinemann L, Lodwig V, Freckmann G. Accuracy in blood glucose measurement: What will a tightening of requirements yield? J Diabetes Sci Technol 2012;6:435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boren SA, Clarke WL. Analytical and clinical performance of blood glucose monitors. J Diabetes Sci Technol 2010;4:84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ginsberg BH. Factors affecting blood glucose monitoring: Sources of errors in measurement. J Diabetes Sci Technol 2009;3:903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karon BS, Gandhi GY, Nuttall GA, et al. Accuracy of roche accu‐chek inform whole blood capillary, arterial, and venous glucose values in patients receiving intensive intravenous insulin therapy after cardiac surgery. Am J Clin Pathol 2007;127:919–926. [DOI] [PubMed] [Google Scholar]
- 26. Paul AE, Shiel RE, Juvet F, et al. Effect of hematocrit on accuracy of two point‐of‐care glucometers for use in dogs. Am J Vet Res 2011;72:1204–1208. [DOI] [PubMed] [Google Scholar]
- 27. Abbott‐Laboratories . FreeStyle Lite Blood Glucose Test Strips‐Product Information. Chicago, IL: Abbott Diabetes Care Inc.; 2007/2009. [Google Scholar]
- 28. Abbott‐Laboratories . Optium Plus Blood Glucose Test Strips‐Product Information. Chicago, IL: Abbott Diabetes Care Inc.; 2006, 2007–2010. [Google Scholar]
- 29. Nova‐Biomedical . Nova StatStrip Glucose Test Strips. Nova Biomedical Corp.: Waltham, MA; 2010. [Google Scholar]
- 30. Hemocue‐AB . HemoCue Glucose 201+, User′s Manual. Ängelholm, Sweden: Hemocue AB; 1990, 1992, 1995. [Google Scholar]
- 31. LifeScan . OneTouch Ultra Test Strips, Owner′s Booklet. LifeScan Inc., Milpitas, CA; 2006. [Google Scholar]
- 32. LifeScan . OneTouch Verio Test Strips, Owner′s Booklet. LifeScan Inc., Milpitas, CA; 2010. [Google Scholar]
- 33. LifeScan . OneTouch Vita Test Strips, Owner′s Booklet. LifeScan Inc., Milpitas, CA; 2009. [Google Scholar]
- 34. Menarini‐Diagnostics A . Glucocard Sensor, Owner′s Booklet. Shiga, Japan: A. Menarini Diagnostics/Arkray TM; 2009. [Google Scholar]
- 35. Roche‐Diagnostics . Accu‐chek Aviva Tests, Owner′s Booklet. Mannheim, Germany: Roche Diagnostics GmbH; 2010. [Google Scholar]


