Abstract
Background
Endoscopy is performed for direct inspection of the mucosa and acquisition of biopsies in dogs with inflammatory bowel disease (IBD).
Aim
To evaluate the interobserver agreement in the endoscopic assessment of duodenal mucosa in dogs with IBD.
Methods
Thirty‐five archived endoscopic images of grossly normal (n = 6) and inflamed (n = 29) duodenal mucosa were displayed to 3 expert and 5 trainee endoscopists. Each image was assessed independently by endoscopists for mucosal abnormalities using established indices (of hyperemia, granularity, friability, lymphatic dilatation, and erosions) or interpreted as normal mucosa (trial 1). A repeated trial (trial 2) was performed with the same images presented in random order 1 month later, and accompanied by a visual template.
Results
There was slight interobserver agreement in initial mucosal assessment for expert and trainee endoscopists in trial 1 (kappa ≤ 0.02, P > .05). Interobserver agreement improved in trial 2 for both expert and trainee endoscopists (kappa = 0.2, P > .05) for experts and (P < .05) for trainees. There was a significant (P < .01) improvement in trainee endoscopy scores of lesions from trial 1 to trial 2. Regression analysis showed a significant (P < .01) difference between expert versus trainee endoscopy scores in trial 1. Repeat lesion assessment aided by use of a visual template (trial 2) improved the overall scores of trainee endoscopists to near that of expert endoscopists (P = .06).
Conclusions and Clinical Importance
Interobserver agreement of IBD mucosal appearance from endoscopic findings benefitted from operator experience.
Keywords: Endoscopy, Inflammatory bowel disease, Mucosal assessment
Abbreviations
- CCECAI
canine chronic enteropathy clinical activity index
- CD
Crohn's disease
- CIBDAI
canine IBD activity index
- CRP
C‐reactive protein
- IBD
inflammatory bowel disease
- pANCA
peri‐nuclear antineutrophil cytoplasmic antibodies
- UC
ulcerative colitis
Different indices have been proposed to measure the activity, severity, or both of inflammatory bowel disease (IBD) in dogs to evaluate efficacy of treatment in clinical trials.1, 2, 3 All of these indices are based on clinical signs, biologic data, or both. Gastrointestinal (GI) endoscopy is a well‐established technique to directly visualize the mucosa and acquire targeted biopsy specimens for histopathologic examination. Previously, abnormal endoscopic mucosal observations in dogs with signs of chronic GI have been associated with detectable histopathologic lesions, including inflammatory and neoplastic disorders.4 As endoscopy is routinely performed for diagnosis of IBD in dogs, endoscopic findings could be used to measure disease activity.
Several endoscopic indices for evaluation of inflammatory activity in IBD in humans (ie, Crohn's disease [CD]5, 6 and ulcerative colitis [UC]7, 8, 9, 10) have been designed. All of these scoring systems were based on the severity/extent of mucosal granularity, vascular pattern, vulnerability of mucosa, and mucosal damage (mucus, fibrin, exudates, erosions, and ulcer) observed during colonoscopy. However, no standardized model has been established. Separate studies in dogs with small intestinal IBD have yielded conflicting results on the utility of endoscopic scoring as a measure of disease activity.1, 11 One potential reason for this discrepancy could be interobserver variation in identifying endoscopic abnormalities based on operator experience and the lack of systematic endoscopic assessment. The aim of this study was to evaluate the interobserver agreement in the assessment of duodenal appearance in dogs with IBD.
Materials and Methods
Selection of Images
Two hundred archived endoscopic images from consecutive duodenoscopy procedures performed in dogs with IBD between 2004 and 2012 at Iowa State University were retrieved from a computerized database and reviewed. A total of 35 endoscopic images of grossly normal (some images obtained after biopsy) and inflamed duodenal mucosa from 25 IBD dogs were selected based on the authors' experience of characteristic lesions, for study enrollment. Image selection was determined by joint agreement of authors JES and AEJ. A diagnosis of IBD was based on previously established clinicopathologic and histopathologic criteria.1, 2, 11, 12, 13 Endoscopic interpretation of intestinal lymphangiectasia included observation of multifocal to diffuse white foci within the mucosa suggestive of lymphatic distension.16 Duodenoscopy procedures were performed with a commercial video endoscope (Olympus GIF‐160)1 with still images of normal and abnormal mucosa captured by the endoscopist. The file size of the down‐loaded images was approximately 100 kb, with a pixel array of 640 × 480 and 24‐bit color. These still images were then arranged in a Powerpoint2 presentation for testing purposes.
Assessment of Images
Endoscopic still images in Powerpoint2 format were assessed by 3 expert and 5 trainee endoscopists (none were JES or AEJ), for inflammatory activity. Expert endoscopists were defined as individuals with advanced clinical training and active and consistent operator participation in a minimum of 50 GI endoscopy procedures over the preceding 24 months. The experts were experienced and familiar with mucosal lesions as identified with GI endoscopy. Trainee endoscopists had minimal endoscopic training and lacked consistent endoscopic operator experience with participation in less than 5 procedures over the same 24‐month period.
The selected images were randomized by means of a Research report randomizer3 program and assessed independently by each endoscopist for mucosal appearance. Neither the clinical data nor the date on which the image was taken was made known to the endoscopists. The endoscopic variables evaluated included hyperemia, erosions, granularity, friability, lymphatic dilatation, or the mucosal appearance was interpreted as normal (Table 1). Written definitions of each variable were made available to all endoscopists. If an individual image contained more than 1 mucosal abnormality, the endoscopist was asked to identify the salient lesion (trial 1). Each endoscopist had 2 weeks to complete the trial. The assessment of mucosal appearance was repeated 1 month after the first assessment (trial 2), although the endoscopists were not informed that they were going to assess the same images (order rerandomized) a second time. In addition, each endoscopist was instructed to review a template of representative mucosal appearances (Fig 1) before image reassessment (trial 2) to see whether this exercise improved endoscopy scores.
Table 1.
Description of endoscopic variables used in the study.
| Endoscopic Criteria for Duodenal Mucosal Assessment | |
|---|---|
| Hyperemia | Gradations of mucosal redness (pale → red) |
| Friability | Mucosal bleeding on contact with endoscope or biopsy forceps |
| Granularity | Alteration in the texture of the mucosal surface |
| Erosions | Superficial linear mucosal defect(s) with hemorrhage |
| Lymphatic dilatation | Multifocal to diffuse white foci within the mucosa |
Figure 1.
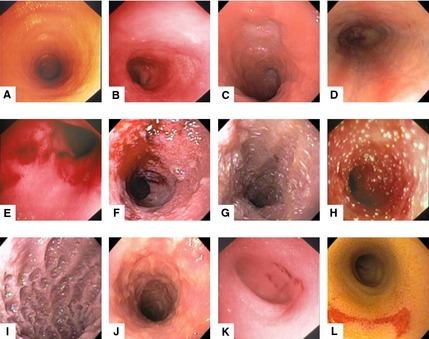
Representative still images used in the test template. These images demonstrate the endoscopic appearance of normal mucosa (A,B); hyperemia (C,D); friability (E,F); lymphatic dilatation (G,H); increased granularity (I,J); and erosions (K,L).
Analysis of Data
Data were collected from each operator using predesigned Excel4 spread sheets for statistical analysis. Fleiss kappa coefficients were calculated to assess agreement among multiple raters within expert and trainee groups and tested against null value 0 by using the “irr” package in R.5 Fleiss kappa interpretation is similar to that of Cohen's kappa. A mixed‐effects logistic regression model was used to analyze descriptive appearance agreement with gold standard for comparison of assessment accuracy by the Glimmix procedure in SAS. Group (trainee versus expert), trial, and their interaction were the fixed effects in model, whereas endoscopist was the random effect. A P value <.05 was considered statistically significant.
Results
The affected dogs were predominantly middle‐aged (age range 1–11 years) mean age of 6.8 years, exhibited chronic gastrointestinal signs (4.8 month duration), CIBDAI score of 5.9, and had a histologic biopsy grade of mild IBD 24% and moderate‐severe IBD 76%. There were 11 spayed females and 14 neutered males included in the study. Dogs with IBD included: 4 West Highland White Terriers, 3 Golden Retrievers, 2 mixed breed dogs, 2 Boxers, 2 Labrador Retrievers, 2 Shih Tzus, 2 Yorkshire terriers, and 1 each of Wheaton Terrier, German Shepherd dog, Viszla, English Bulldog, Cocker Spaniel, Gordon Setter, Beagle, and Miniature Poodle. None of the dogs had evidence of inflammation in other body systems, based on results obtained from diagnostic testing, and each dog had failed to respond fully to previous dietary and antibiotic interventions.
Among the 35 images of the test set obtained during duodenoscopic examination in dogs with IBD, 6 were of grossly normal mucosa (some images obtained postbiopsy), 6 friable, 5 hyperemic, 6 increased granularity, 7 erosions, and 5 lymphatic dilatation. All endoscopic images of abnormal mucosal appearances were associated with histologically inflamed mucosa of varying severity. The mucosal appearance of lymphatic dilatation was associated with variable distention of the supravillus, subvillus, or both lymphatic vessels microscopically in all 5 dogs. Of the 6 normal endoscopic images, 1 dog had no abnormalities observed on histopathologic examination of tissue samples, whereas biopsy specimens obtained from the other 5 dogs contained some individual biopsy specimens with histopathologic evidence of mild enteritis.
Based on Fleiss kappa statistics evaluation, the interobserver agreement within expert and trainee groups improved among experts from trial 1 kappa < 0.01 (slight agreement), P > .05, to trial 2 kappa = 0.2 (fair agreement), P > .05 and among trainees, from trial 1 kappa = 0.02 (slight agreement), P > .05 to trial 2 kappa = 0.2 (fair agreement), P < .05. By the Glimmix procedure, showing comparison within the groups, there was a significant (P < .01) improvement of 17% in the trainee endoscopists lesion assessment scores from trial 1 to trial 2. The expert endoscopists showed no statistical significant improvement between trial 1 and trial 2 (P = .19), although there was an 8% improvement. Regression analysis showed a significant (P < .01) difference among operator groups regarding trial 1 lesion assessment. Repeat duodenal image evaluation aided by use of a visual template (trial 2) improved the overall scores of trainee endoscopists to near that of expert endoscopists (P = .06).
Discussion
The results of this study indicate that operator experience matters when making endoscopic mucosal assessments. Operator experience can be gained from performing numerous endoscopic procedures, and the provision of a written/pictorial template to aide identification of mucosal lesions, or both.
Gastrointestinal endoscopy is an important tool in the diagnosis of IBD in dogs. Gastroscopy, enteroscopy, and colonoscopy are of value in the assessment of specific organ involvement in IBD and to differentiate IBD from other causes of chronic enteropathy. Recent advances in patient preparation and instrumentation,15, 17, 18 mucosal examination techniques,14, 19 and the development of forceps biopsy standards19, 20, 21 have made GI endoscopy the preferred method for diagnosis of small and large intestinal inflammation.
Inflammatory bowel disease in dogs is often characterized by a relapsing and remitting clinical course. Determination of inflammatory activity is important for assessing disease severity and for potentially tailoring treatment. Different indices for assessment of disease activity have been proposed. Clinical indices utilize scoring systems derived from GI signs alone (CIBDAI)2 or in combination with laboratory testing (CCECAI)1 to quantify intestinal activity. Noninvasive serologic markers including perinuclear antineutrophil cytoplasmic antibodies (pANCA)22, 23, 24 and C‐reactive protein2, 3, 25 provide only indirect assessment of disease activity. Histopathologic examination, while required for diagnosis of IBD, is hindered by poorly standardized grading criteria and disagreement among pathologists in defining mucosal inflammation.26, 27
As endoscopy provides immediate and direct assessment of intestinal mucosal damage, endoscopic findings might be used to measure inflammatory activity. Several endoscopic activity indices for CD5, 6 and UC7, 8, 9, 10 are in use. Salient lesions of IBD in humans (ie, CD and UC) range from erythema, loss of vascularity, friability, and granularity of the mucosa to erosions/ulceration. Similar endoscopic indices have been used in IBD in dogs including erythema, friability, erosions/ulceration, cobble‐stone appearance (granularity), white speckling on the surface, and difficulty in insufflating (stenosis).1, 11, 12 Endoscopic and histopathologic evaluations of the GI tract in dogs and cats reveal that between 48 and 83% of animals having abnormal duodenal endoscopic examination results will have histopathologic lesions.4 Clinical trials utilizing endoscopic scoring for dogs with IBD are limited and have provided conflicting results on the utility of endoscopic scoring as a measure of disease activity.1, 11 A reason for this discrepancy could be interobserver variation in identifying endoscopic abnormalities based on operator experience and the lack of systematic endoscopic assessment.
This study investigated interobserver agreement in the assessment of endoscopic activity in dogs with IBD using defined descriptors of mucosal appearance. Both written descriptions and a visual template of mucosal lesions were used to assess the role of operator experience in defining duodenal appearance in dogs. Our results indicated that there was slight to fair interobserver group agreement in lesion identification in expert and trainee endoscopists for either trial 1 or trial 2. However, there was significant interobserver difference in lesion assessment when still images of IBD were evaluated by the 3 experienced versus 5 trainee endoscopists in trial 1. Analysis of interobserver agreement showed a significant difference among operator groups regarding lesion assessment with expert endoscopists having less chance of disagreement regarding the identification of endoscopic abnormalities. This observation of better interobserver agreement of experienced versus trainee endoscopists emphasizes the value of operator experience in companion animal GI endoscopy4 and is similar to results in humans with IBD.9, 28
The association between endoscopic scores of the duodenum and colon with other inflammatory indices (ie, clinical activity [CIBDAI] and histopathology) as partial assessment of long‐term outcome in dogs with chronic enteropathies has previously been evaluated.1 The numerical endoscopic scores (range 0–3; normal to severe mucosal inflammation) were assigned by 1 of 2 operators using mucosal assessment criteria of erythema, friability, white speckling, granularity, and luminal stenosis.1 No correlation was found between endoscopy scores and histology scores pre‐ versus posttreatment; however, an endoscopy score of 3 in the duodenum, indicative of severe inflammation, was significantly associated with negative outcome.1 In a separate study, Garcia‐Sancho et al. performed endoscopic examination in 16 dogs diagnosed with lymphocytic‐plasmacytic enteritis and evaluated gastric/duodenal lesions of mucosal erythema, granularity, friability, erosions, and luminal distension before and after IBD treatment.11 While the number and relative experience of endoscopists were not noted in this report, these investigators showed significant differences between before and after treatment of macroscopic endoscopic lesions in the stomach and duodenum.
Our choice of endoscopic mucosal characteristics to evaluate was based on the personal experiences of AEJ in performance of duodenoscopic procedures over 30 years. Observer variation for graded characteristics (ie, mucosal hyperemia—is it pale, pink, or red?) is quite high, whereas that for discontinuous variables (ie, presence or absence of erosions) is generally low.8 More importantly, operator experience plays an important role in endoscopic assessment with trainee endoscopists more likely to miss mucosal lesions or misinterpret normal versus abnormal mucosa. The results of this study confirm these previous anecdotal observations. While hyperemia and luminal distensibility have been used in previous endoscopic indices for dogs, we have not found them useful in the past or in this study.1, 11
The use of the Fleiss Kappa coefficients was calculated to assess agreement among multiple observers versus Cohen's kappa which only compares interobserver agreement between 2 observers. Although the kappa scores within the expert and trainee groups for trial 1 showed only slight agreement, they both improved to fair agreement for both operator groups in trial 2. The P value for the experts in both trials were greater than .05 and could have been because of lack of power.
There were several potential limitations of our study. First, we utilized a single center for our study and focused only on duodenal endoscopic assessment of dogs with IBD. Whether the same results for interobserver variability across different study centers, endoscopic interpretation by nonspecialist clinicians, or evaluation of other alimentary tract organs in dogs having different diseases might yield similar results was not assessed. Second, we used still images of endoscopic lesions versus video streams to evaluate variation among operator cohorts. Our rationale was that still images in texts or continuing education events are routinely utilized for endoscopic training purposes. In addition, a manageable number of still images could be more easily evaluated twice by the same clinicians in this study which assured good compliance. In humans, accurate assessment of UC endoscopic activity can be achieved from archived still images.9 Lastly, one could argue that the modest improvement in endoscopy scores posttemplate (trial 2) was influenced by the designation of novice versus experienced endoscopists based on the advanced training status and duration of active clinical GI endoscopic procedure experience of individual operators. In this regard, it is possible that outliers in either group (ie, a novice endoscopist having greater expertise in recognition of mucosal appearances, and an experienced endoscopist having lesser expertise in recognition of mucosal abnormalities, or both) may have falsely reduced interobserver agreement within an operator group. It is also possible that gross mucosal observations alone are insufficient for defining mucosal appearance of IBD in dogs but aided by considering other indices as well, including clinical scores, serologic markers, and the severity of inflammation.1, 2, 22, 23, 24, 25, 26
In summary, a simple compilation of the variable mucosal appearances in the duodenum of dogs with IBD is described. According to the results from this study, accurate assessment of IBD activity from duodenal endoscopic appearance benefitted from operator experience. Acceptable agreement rates might be obtained by endoscopists under training using well‐defined endoscopic appearances.
Acknowledgment
The authors thank Amy Hodnefield and Carrie Schwake for technical assistance.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
A portion of this study was previously presented at the 2013 ACVIM Forum, Seattle, WA.
The study was performed at the Iowa State University, Department of Veterinary Clinical Sciences, Ames, IA.
Footnotes
Olympus Optical, Tokyo, Japan
Research report randomizer http://www.randomizer.org
Powerpoint, Microsoft, Redmond, WA
Excel, Microsoft, Redmond, WA
R Core Team, Vienna, Austria
References
- 1. Allenspach K, Wieland B, Grone A, Gaschen F. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J Vet Intern Med 2007;21:700–708. [DOI] [PubMed] [Google Scholar]
- 2. Jergens AE, Schreiner CA, Frank DE, et al. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med 2003;17:291–297. [DOI] [PubMed] [Google Scholar]
- 3. Jergens AE, Crandell J, Morrison JA, et al. Comparison of oral prednisone and prednisone combined with metronidazole for induction therapy of canine inflammatory bowel disease: A randomized‐controlled trial. J Vet Intern Med 2010;24:269–277. [DOI] [PubMed] [Google Scholar]
- 4. Roth L, Leib MS, Davenport DJ, Monroe WE. Comparisons between endoscopic and histologic evaluation of the gastrointestinal tract in dogs and cats: 75 cases (1984–1987). J Am Vet Med Assoc 1990;4:635–638. [PubMed] [Google Scholar]
- 5. Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn's disease: A prospective multicentre study. Groupe d'Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut 1989;30:983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daperno M, D'Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: The SES‐CD. Gastrointest Endosc 2004;60:505–512. [DOI] [PubMed] [Google Scholar]
- 7. Matts SG. The value of rectal biopsy in the diagnosis of ulcerative colitis. J Med 1961;30:393–407. [PubMed] [Google Scholar]
- 8. Baron JH, Connell AM, Lenard‐Jones JE. Variation between observers in describing mucosal appearances in proctocolitis. Br Med J 1964;1:89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Osada T, Ohkusa T, Yokoyama T, et al. Comparison of several activity indices for the evaluation of endoscopic activity in UC: Inter‐ and intraobserver consistency. Inflam Bowel Dis 2010;16:192–197. [DOI] [PubMed] [Google Scholar]
- 10. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5‐aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. N Engl J Med 1987;317:1625–1629. [DOI] [PubMed] [Google Scholar]
- 11. García‐Sancho M, Rodríguez‐Franco F, Sainz A, et al. Evaluation of clinical, macroscopic, and histopathologic response to treatment in nonhypoproteinemic dogs with lymphocytic‐plasmacytic enteritis. J Vet Intern Med 2007;21:11–17. [DOI] [PubMed] [Google Scholar]
- 12. Jergens AE, Moore FM, Haynes JS, Miles KG. Idiopathic inflammatory bowel disease in dogs and cats: 84 cases (1987–1990). J Am Vet Med Assoc 1992;201:1603–1608. [PubMed] [Google Scholar]
- 13. Craven M, Simpson JW, Ridyard AE, Chandler ML. Canine inflammatory bowel disease: Retrospective analysis of diagnosis and outcome in 80 cases (1995–2002). J Small Anim Pract 2004;45:336–342. [DOI] [PubMed] [Google Scholar]
- 14. Casamian‐Sorrosal D, Willard MD, Murray JK. Comparison of histopathologic findings in biopsies from the duodenum and ileum of dogs with enteropathy. J Vet Intern Med 2010;24:80–83. [DOI] [PubMed] [Google Scholar]
- 15. Jergens AE, Willard M, Day MJ. Endoscopic biopsy specimen collection and histopathologic considerations In: Tams TR, ed. Small Animal Endoscopy. Philadelphia, PA: C.V. Mosby Co; 2010:293–309. [Google Scholar]
- 16. Larson RN, Ginn JA, Bell CM, et al. Duodenal endoscopic findings and histopathologic confirmation of intestinal lymphangiectasia in dogs. J Vet Intern Med 2012;26:1087–1092. [DOI] [PubMed] [Google Scholar]
- 17. Burrows CF. Evaluation of a colonic lavage solution to prepare the colon of the dog for colonoscopy. J Am Vet Med Assoc 1989;195:1719–1721. [PubMed] [Google Scholar]
- 18. Richter KP, Cleveland MB. Comparison of an orally administered gastrointestinal lavage solution with traditional enema administration as preparation for colonoscopy in dogs. J Am Vet Med Assoc 1989;195:1727–1731. [PubMed] [Google Scholar]
- 19. Procoli F, Mõtsküla PF, Keyte SV, et al. Comparison of histopathologic findings in duodenal and ileal endoscopic biopsies in dogs with chronic small intestinal enteropathies. J Vet Intern Med 2013;27:268–274. [DOI] [PubMed] [Google Scholar]
- 20. Washabau RJ, Day MJ, Willard MD, et al.; WSAVA International Gastrointestinal Standardization Group . Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med 2010;24:10–26. [DOI] [PubMed] [Google Scholar]
- 21. Goutal‐Landry CM, Mansell J, Ryan KA, Gaschen FP. Effect of endoscopic forceps on quality of duodenal mucosal biopsy in healthy dogs. J Vet Intern Med 2013;27:456–461. [DOI] [PubMed] [Google Scholar]
- 22. Mancho C, Sainz Á, García‐Sancho M, et al. Evaluation of perinuclear antineutrophilic cytoplasmic antibodies in sera from dogs with inflammatory bowel disease or intestinal lymphoma. Am J Vet Res 2011;72:1333–1337. [DOI] [PubMed] [Google Scholar]
- 23. Luckschander N, Allenspach K, Hall J, et al. Perinuclear antineutrophilic cytoplasmic antibody and response to treatment in diarrheic dogs with food responsive disease or inflammatory bowel disease. J Vet Intern Med 2006;20:221–227. [DOI] [PubMed] [Google Scholar]
- 24. Allenspach K, Luckschander N, Styner M, et al. Evaluation of assays for perinuclear antineutrophilic cytoplasmic antibodies and antibodies to Saccharomyces cerevisiae in dogs with inflammatory bowel disease. Am J Vet Res 2004;65:1279–1283. [DOI] [PubMed] [Google Scholar]
- 25. Heilmann RM, Jergens AE, Ackermann MR, et al. Serum calprotectin concentrations in dogs with idiopathic inflammatory bowel disease. Am J Vet Res 2012;73:1900–1907. [DOI] [PubMed] [Google Scholar]
- 26. Day MJ, Bilzer T, Mansell J, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: A report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol 2010;138:S1–S43. [DOI] [PubMed] [Google Scholar]
- 27. Willard MD, Jergens AE, Duncan RB, et al. Inter‐observer variation among histopathologic evaluations of intestinal tissues from dogs and cats. J Am Vet Med Assoc 2002;220:1177–1182. [DOI] [PubMed] [Google Scholar]
- 28. Orlandi F, Brunelli E, Feliciangeli G, et al. Observer agreement in endoscopic assessment of ulcerative colitis. Ital J Gastroenterol Hepatol 1998;30:539–541. [PubMed] [Google Scholar]


