Abstract
Background
Left atrial size is prognostically important in dogs with myxomatous mitral valve disease (MMVD).
Hypothesis/Objectives
To compare the level of agreement in identification of left atrial enlargement (LAE) between the left atrial‐to‐aortic root ratio (LA : Ao) and left atrial volume using the biplane area‐length method indexed to body weight (LA Vol/BW).
Animals
Sixty dogs with MMVD and 22 normal dogs were prospectively studied with 2‐dimensional echocardiography.
Methods
The upper limit of normal for LA Vol/BW was defined as 1.1 mL/kg. LA : Ao was deemed normal if ≤1.5. To define overall disease severity, each dog was assigned a mitral regurgitation severity score (MRSS) based on echocardiographic parameters that did not include left atrial size. ACVIM staging also was utilized.
Results
Of 60 affected dogs, 20 were ACVIM Stage B1, 25 were Stage B2, and 15 were Stage C. LA Vol/BW identified LAE in 12 cases in which LA : Ao was normal; 7 of these were Stage B1 and 5 were Stage B2. This diagnostic disagreement was significant (P = .00012). Of the 12 cases in which diagnostic discrepancies were identified, 5/5 of the B2 dogs and 3/7 B1 dogs had a moderate MRSS, whereas 4/7 B1 dogs had a mild MRSS. No diagnostic discrepancies between LA : Ao and LA Vol/BW were apparent in dogs with a severe MRSS.
Conclusions and Clinical Importance
This study shows evidence of diagnostic disagreement between LA : Ao and LA Vol/BW for assessment of LAE. LA Vol/BW may be superior to LA : Ao for identification of mild LAE.
Keywords: Biplane area‐length method, Canine, Echocardiography, Myxomytous mitral valve disease
Abbreviations
- 2‐D
two‐dimensional
- CMR
cardiac magnetic resonance imaging
- LA Vol/BSA
left atrial volume indexed to body surface area
- LA Vol/BW
left atrial volume indexed to body weight
- LA : Ao
left atrial to aortic root ratio
- LAE
left atrial enlargement
- MMVD
myxomytous mitral valve disease
- MR
mitral regurgitation
- MRSS
mitral regurgitation severity score
- MSCT
multi‐slice computed tomography
- RT3‐D
real‐time three‐dimensional
- VHS
vertebral heart score
Myxomatous mitral valve disease (MMVD) is the most common acquired heart disease in dogs.1, 2 Typically, MMVD is slowly progressive, and many affected dogs never develop congestive heart failure.3 One of the most important predictors of progression of MMVD is increased left atrial size.3, 4 The degree of left atrial enlargement (LAE) is reflective of both the chronicity and severity of mitral regurgitation (MR).3
Given the prognostic relevance of LAE, it is important to determine which of methods used to assess left atrial size is most useful in the clinical evaluation of patients with MMVD. Currently, the most commonly used method to quantify left atrial size in veterinary medicine is the 2‐dimensional (2‐D) left atrial‐to‐aortic root ratio (LA : Ao), which provides a body weight‐independent measurement of left atrial size.5, 6 An alternative method for assessing left atrial size is measurement of left atrial volume. Volumetric assessments are based on dimensions obtained from multiple planes and may detect chamber enlargement with greater sensitivity than the LA : Ao, which is determined by a single linear atrial dimension. In human medicine, there has been a paradigm shift in the assessment of the left atrium, such that left atrial volume has become the standard for assessment of left atrial size, whereas linear dimensions used for quantification of left atrial size have fallen out of favor.7
In addition to 2‐D echocardiography, real‐time 3‐dimensional (RT3‐D) echocardiography, cardiac magnetic resonance imaging (CMR), and multi‐slice computed tomography (MSCT) also have been investigated for their utility in measuring left atrial volume. Although there appears to be good correlation among the various imaging techniques, absolute volume differs by technique, with volumes measured by echocardiographic methods generally being smaller than volumes obtained with CMR or MSCT.7, 8, 9, 10, 11, 12, 13 In veterinary pati‐ents, the requirement of general anesthesia for either MSCT or CMR imaging is an important drawback, especially for patients with advanced or decompensated cardiovascular disease. Although these imaging modalities may provide a more accurate assessment of left atrial volume, the risks associated with anesthesia may outweigh the benefit.
If left atrial volume assessed by 2‐D echocardiography were to be superior to LA : Ao in predicting clinical status or assessing the severity of MR, it could improve ability to accurately gauge long‐term prognosis at presentation. The use of left atrial volume derived from 2‐D echocardiographic dimensions to assess left atrial size in dogs with MMVD has not been investigated extensively. A recent study suggested 0.92 mL/kg as the upper limit of normal for left atrial volume obtained by the biplane area‐length method indexed to body weight.14
The aims of this study were (1) to propose a cut‐off for normal left atrial volume by 2‐D echocardiographic assessment using the biplane area‐length method in normal dogs and (2) to compare the level of agreement in identification of LAE between LA : Ao and left atrial volume using the biplane area‐length method in dogs with different stages of MMVD. Our hypothesis was that left atrial volume would be superior to LA : Ao in identification of mild LAE.
Materials and Methods
Healthy dogs owned by faculty, staff, and students of Virginia Maryland Regional College of Veterinary Medicine (VMRCVM) and healthy dogs presented for prebreeding cardiac screening to both the VMRCVM and the Kansas State University Veterinary Teaching Hospital (KSUVTH) made up the control group. Each of these dogs was judged to have normal cardiovascular status based on physical examination, Doppler blood pressure measurement,1 and complete echocardiographic examination. Evaluations were performed with approval of the Virginia Tech Institutional Animal Care and Use Committee. Doppler blood pressure measurements were performed in accordance with published recommendations.15 Standard M‐mode, 2‐D, and Doppler blood flow measurements were performed with continuous ECG monitoring in right and left lateral recumbency using an ultrasound unit2 equipped with 1.5–10 MHz phased array transducers. Exclusion criteria included the presence of a left apical systolic heart murmur or midsystolic click on physical examination, more than trivial MR or mitral valve prolapse identified by echocardiography, or any evidence of cardiovascular or systemic disease expected to affect cardiovascular function.
The study group comprised client‐owned dogs presented to the VMRCVM Veterinary Teaching Hospital or the KSUVTH for evaluation of MMVD. All patients had complete echocardiographic evaluation and measurement of Doppler blood pressure. Thoracic radiographs were obtained if clinically indicated. The diagnosis of MMVD was based on the echocardiographic identification of mitral valve thickening or prolapse, typically in combination with the presence of MR.
In all dogs, left atrial volume was calculated using the biplane area‐length method16 from the left apical 2‐ and 4‐chamber views (Fig 1) at the end of ventricular systole. End‐systole was defined as the frame immediately preceding opening of the mitral valve. Briefly, the endocardial border of the left atrium was traced to obtain atrial areas in both the left apical 4‐chamber (A1) and 2‐chamber (A2) views. The left auricular appendage and the confluence of the pulmonary veins were excluded, and the boundary of the left atrium and left ventricle was delineated by a straight line drawn from hinge point to hinge point across the mitral valve annulus. The length of the left atrium (L) was defined as the perpendicular distance from the midpoint of the delineation between atrium and ventricle to the dorsal aspect of the left atrial wall on the left apical 4‐chamber view. Left atrial volume was calculated using the equation [0.85 × A1 × A2]/L and was indexed to both body weight and body surface area.16 All measurements were repeated on 3 consecutive cardiac cycles and averaged. Average values were used in the statistical analysis. The LA : Ao was measured as previously reported5 and expressed as the average of 3 consecutive measurements. One operator (SW) conducted measurements on all dogs. In addition, the images from a randomly selected subset of 6 control dogs were measured again by operator SW 1 week after the original measurements and also by a second operator (MB). These data then were used to assess intra‐operator repeatability (ie, within‐operator variability) and inter‐operator reproducibility (ie, between‐operator variability) of the area‐length method.
Figure 1.
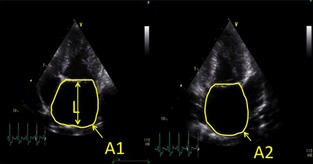
Measurement of left atrial volume using the biplane area‐length method [0.85 × A1 × A2]/L. A1, left atrial area from apical 4 chambers view; A2, left atrial area from 2 chamber view; L, length of left atrium.
All patients were classified based on the ACVIM staging system.17 Specifically, Stage B1 dogs were defined as asymptomatic patients with vertebral heart score (VHS) < 10.5, LA : Ao < 1.5 and normal left ventricular end‐diastolic dimensions based on previously published reference intervals,18 whereas Stage B2 dogs were defined as asymptomatic patients with either VHS > 10.5 or echocardiographic evidence of increased left ventricular end‐diastolic dimensions or LA : Ao > 1.5. Patients also were classified based on our own echocardiographically derived MR severity scoring system (outlined in Table 1) to assess overall disease severity using parameters other than left atrial size. The chosen parameters have been utilized previously for assessment of the severity of MR and included the area of the regurgitant jet assessed with color Doppler,19, 20 the peak velocity of the transmitral E wave,3, 21 left ventricular internal dimension at end‐diastole,22 the anatomy of the mitral valve leaflets,3, 23 and the density of the continuous wave Doppler MR signal.24 Individual scores assigned for each of the echocardiographic parameters were summed to obtain the mitral regurgitation severity score (MRSS). Although based on established echocardiographic parameters, this composite measure of disease severity has not been clinically validated in veterinary patients. The scores are presented only to provide additional descriptive data related to the severity of MMVD.
Table 1.
Mitral regurgitation severity scoring system outline.
| Echocardiographic Parameter | Normal | Mild | Moderate | Severe |
|---|---|---|---|---|
| Color Doppler regurgitant jet area | None, or trivial (0) | <20% of left atrial area (1) | 20–40% of left atrial area (2) | >40% of left atrial area (3) |
| Mitral inflow | E wave <1.2 m/s (1) | E wave <1.2 m/s (1) | E wave >1.2 m/s with E wave deceleration time >80 milliseconds (2) | E wave >1.2 m/s with E wave deceleration time <80 milliseconds (3) |
| Left ventricular internal dimension in diastole | Normal (1) | Normal (1) | Enlarged (<20% over upper reference limit for body weight) (2) | Enlarged (>20% over upper reference limit for body weight) (3) |
| Leaflet anatomy | Normal (1) | Normal (1) or mitral valve prolapse seen in 2 views (2) | Normal (1) or mitral valve prolapse seen in 2 views (2) | Flail leaflet (3) |
| Continuous wave Doppler mitral regurgitation jet density | None (0) | Incomplete/faint (1) | Dense (2) | Dense (2) |
| Score ranges | 3 | 4–7 | 8–11 | 12–14 |
Numbers in parenthesis represent the assigned scores for each echocardiographic parameter.
Statistical Analyses
A multiclass ROC analysis was conducted to estimate the multiclass area under the curve (AUC) and to classify the MRSS based on either LA : Ao, LA volume indexed to body weight (LA Vol/BW) or LA volume indexed to body surface area (LA Vol/BSA). An estimate of the multiclass AUC provides a measure of classification performance for each of the selected predictors to accurately separate the 4 classes of the MRSS (normal, mild, moderate, and severe). The estimated multiclass AUC for LA : Ao was 87.6%, for LA Vol/BW was 91.9% and for LA Vol/BSA was 87.0%. Although these AUC estimates show similar numerical magnitudes, no formal testing was conducted because statistical approaches to multiclass classification problems represents an active area of research for which the development and validation of statistical methodologies is ongoing. For this study, LA : Ao was considered the reference standard and further comparisons were conducted using LA Vol/BW, which showed the best classification performance of the 2 LA volumetric assessments.
Patients were classified as having either a normal or enlarged left atrium based on LA : Ao and LA Vol/BW. For LA : Ao, the accepted clinical threshold of 1.54, 25 was utilized as the upper limit and cut‐off between normal left atrial size and LAE. For LA Vol/BW, the empirical distribution of LA Vol/BW in the control group was evaluated to determine an upper normal threshold and cut‐off between normal left atrial size and LAE. Classification agreement for LAE between LA : Ao and LA Vol/BW was evaluated using a McNemar test of symmetry for 2 × 2 frequency tables.
A linear mixed model approach was used to quantify sources of random variability in the assessment of LA Vol/BW. The statistical model included random effects for patient, operator and residual; the latter 2 terms allowed for assessment of between‐operator reproducibility and within‐operator repeatability, respectively, following a standard gauge repeatability and reproducibility (GR&R) approach.26
Results
Twenty‐two dogs with normal cardiovascular status of 12 different breeds were enrolled in the control group. Ten dogs were of mixed breed origin, 2 were Beagles, and the remaining 10 breeds were represented by 1 dog each. The mean age of the control group was 4.6 years (range, 1.5–8.2 years) and mean weight was 17.4 kg (range, 4–37.1 kg).
Sixty client‐owned dogs with MMVD were enrolled in the study group. Twenty‐seven breeds were represented, with the most common breeds being Cavalier King Charles Spaniel (n = 9), Toy Poodle (n = 5), Pomeranian (n = 4), Shih Tzu (n = 3), Yorkshire Terrier (n = 3), and Labrador Retriever (n = 3). The mean age of this group was 10.5 years (range, 5.4–19.5 years) and mean weight was 13.7 kg (range, 3.5–51.5 kg). On average, animals in the study group were older than those in the control group (P < .0001), whereas there was no evidence for differences in body weight between the groups (P = .19). Within the study group there were 20 dogs classified as ACVIM stage B1, 25 classified as stage B2 and 15 classified as stage C. Of the 25 dogs classified as stage B2, 23 dogs were identified as having left heart enlargement based on echocardiographic parameters and 2 dogs were identified as having left heart enlargement based on radiographic parameters. The 2 dogs with radiographic enlargement included a Cavalier King Charles Spaniel with a VHS of 11.9 and a Shih Tzu with a VHS of 10.9. Seventeen of the study group patients had a mild MRSS, 20 patients had moderate scores and 13 had severe MRSS scores.
The empirical distribution of LA Vol/BW in the control group is summarized in the histogram presented in Figure 2. The mean LA Vol/BW of the control group was 0.88 mL/kg, and the median was 0.89 mL/kg. The 95th percentile value of LA Vol/BW was 1.07 mL/kg; this also was the second‐to‐last largest observed value. Thus, a cut‐off of 1.1 mL/kg (after rounding) was set as a reasonable upper working limit LA Vol/BW in normal dogs. Based on this cut‐off, LA Vol/BW identified LAE in 12 patients in the study group in which LA : Ao was considered normal. The diagnostic disagreement between LA : Ao and LA Vol/BW for identification of LAE was significant (P = .00012), as illustrated by the scatterplot in Figure 3. Of the 12 patients with discrepant diagnoses, 7 were ACVIM stage B1 and 5 were stage B2. Three of the stage B2 patients were classified as such based on increased echocardiographic left ventricular dimensions, whereas 2 of them had been identified as having cardiomegaly radiographically but had a normal LA : Ao and normal echocardiographic left ventricular dimensions. All 5 of the B2 patients and 3 of 7 of the B1 patients with discrepant diagnoses had a moderate MRSS, whereas the remaining 4 of 7 B1 patients had a mild MRSS. No diagnostic discrepancies between LA : Ao and LA Vol/BW were observed in dogs with a severe MRSS. Additionally, there were no cases that had LAE based on LA : Ao in which LA Vol/BW exceeded our cut‐off of 1.1 mL/kg.
Figure 2.
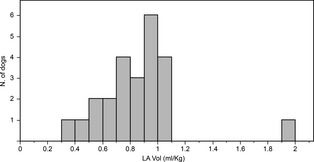
Histogram depicting the empirical distribution of left atrial volume indexed to body weight (LA Vol/BW) (mL/kg) in normal dogs.
Figure 3.
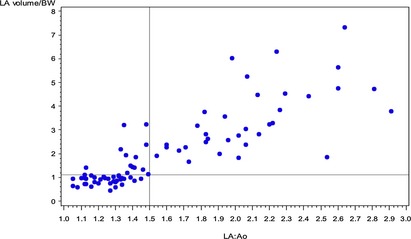
Scatterplot showing classification agreement between left atrial volume indexed to body weight (LA Vol/BW) and left atrial to aortic root ratio (LA : Ao). The threshold for identification of left atrial enlargement (LAE) was 1.5 for LA : Ao and 1.1 mL/kg for LA Vol/BW. Twelve dogs are identified in the upper left‐hand corner of the scatterplot. These dogs were identified as normal based on LA : Ao but exceeded the threshold for LAE based on LA Vol/BW.
For normal patients, most of the variation in the measurement of LA Vol/BW was attributed to variability among dogs. More specifically, the dog‐to‐dog variation accounted for approximately 90.8% of the total variability in measurement of LA Vol/BW, as indicated by the ratio of the corresponding variance component estimates (ie, between‐dog variance over total variance) and amounted to an estimated standard deviation of 0.45 mL/kg of LA Vol/BW among normal dogs. The remainder of the dispersion was explained by variability between operators (approximately 5.1%) and by measurement error of a single operator over repeated measures (approximately 4.1%).
After accounting for dog‐to‐dog variability, we assessed repeatability and reproducibility of LA Vol/BW measurements using corresponding variance component estimates.26 Intra‐operator repeatability refers to the proportion of the variability explained by repeated measurements taken by the same operator on a given dog and characterizes the variability within operators. It is also referred to as measurement error. After accounting for variability among dogs, repeatability for LA volume within operator was estimated as the ratio of the between‐operator variance and the sum of the between‐ and within‐operator variances. Estimated within‐operator repeatability in LA Vol/BW observations was approximately 55.6%, such that measurement error on LA Vol/BW among repeated measures taken by the same operator was characterized by an estimated standard deviation of approximately 0.10 mL/kg. This value characterizes measurement error and was obtained as the square root of the estimated within‐operator variance component.
In turn, reproducibility between operators in LA Vol/BW measurements was estimated at approximately 44.4% after accounting for dog‐to‐dog variation. Reproducibility quantifies the relative contribution of measurements by multiple operators to the overall variability in measurement of LA Vol/BW, after taking into consideration between‐dog variance. On average, the estimated standard deviation between operators was approximately 0.11 mL/kg of LA Vol/BW. This value was obtained as the square root of the estimated between‐operator variance component.
Dogs were the primary source of variability in the measurement of LA Vol/BW and explained >90% of the variability. After accounting for dog‐to‐dog variability, the point estimate for repeatability within operators was larger than that for reproducibility between operators, and both point estimates were very similar (55.6% versus 44.4%, respectively). These estimates support the consistency of measurement of LA Vol/BW by different operators, because the relative magnitude of the variability caused by multiple operators was not any larger than the proportion of the variability observed when a single operator was collecting repeated measurements. In addition, the magnitudes of the variance component estimates for between‐operator (ie, [0.11 mL/kg]2) and within‐operator variability (ie, [0.10 mL/kg]2) were small.
Discussion
This study identified a significant diagnostic disagreement between LA Vol/BW as determined by the biplane area‐length method and LA : Ao in identification of LAE in the dog. Specifically, LA Vol/BW was superior to LA : Ao in identification of mild LAE. The ability of volumetric methods to account for enlargement of the left atrium in more than just 1 dimension may explain why LA Vol/BW identified LAE in a subset of dogs that was not identified by LA : Ao. A unidimensional measurement of left atrial size must reflect a consistent relationship with the other dimensions of the left atrial chamber in order to accurately reflect true left atrial size.27 In humans, physical constraints imposed by the sternum and spine limit the amount of LAE that can occur in the anteroposterior dimension, thus this single dimension may underestimate true atrial size.28 Similarly, limitations specific to canine anatomy or simple variability from individual to individual may contribute to asymmetric enlargement of the left atrium in the dog. This is further supported by our finding that most of the variability observed in LA Vol/BW measurements was explained by dog‐specific factors (ie, dog‐to‐dog variance).
In both human and veterinary cardiology, multiple echocardiographic methods have been used to quantify left atrial size including M‐mode linear dimensions, 2‐D linear dimensions, left atrial areas and left atrial volumes. As of 2005, the American Society of Echocardiography established left atrial volume measured by either the biplane area‐length method or the biplane Simpson's method of disks as the standard for left atrial size assessment.16 The shift toward biplane volumetric assessment is based on the superiority of these measurements as prognostic markers and predictors of future cardiovascular risk in people,29, 30, 31 as well as their correlation with various imaging modalities.9, 10, 11, 13 Recent work in people suggests that, relative to the biplane Simpson's method of discs, the biplane area‐length method may provide superior estimates of left atrial volume.8
The MRSS system developed for this study was based on previously published recommendations for echocardiographic evaluation of the severity of native valvular regurgitation in humans,24 with modifications to simplify the scoring system in such a way that the selected parameters could easily be evaluated in every patient. Additionally, parameters related to left atrial size were eliminated to allow the MRSS to serve as an independent reflection of disease severity when comparing severity score to LA : Ao or LA Vol/BW obtained from individual patients. Eight of the 12 patients in which diagnostic disagreement was identified had a moderate MRSS. This suggests that in many of these discrepant cases there was echocardiographic evidence unrelated to left atrial size that was consistent with advanced MMVD. This observation lends support to the conclusion that LAE identified by the LA Vol/BW method in these discrepant cases actually was present. There was no diagnostic disagreement in those dogs with a severe MRSS, suggesting that in cases of advanced MMVD, LA Vol/BW and LA : Ao both reliably detect severe LAE.
Additional study is needed to determine whether the prognostic value of LA Vol/BW is superior to that of LA : Ao. The traditional LA : Ao measurement is simpler and less time consuming to calculate than is LA Vol/BW, thus an advantage in patient monitoring, prognostic relevance, or both should be sought before exclusive use of LA Vol/BW can be recommended. A prospective longitudinal study investigating the use of LA Vol/BW in dogs affected with MMVD is warranted.
Limitations of this study include a significantly older study group relative to the control group. Given the acquired and highly prevalent nature of MMVD in dogs, an age‐matched control group could not be obtained. Some studies in humans have detected an age‐related increase in left atrial size,32, 33 whereas others have not.11, 34 Increasing left atrial size may not reflect normal aging, but rather subclinical pathology.35 Recent research in dogs found no evidence for correlation between left atrial volume and age, but the median age of the dogs in that study was relatively low at 3.5 years.14
Furthermore, in this study, we did not consider breed‐related differences in left atrial volume between control and study populations. Significant differences in left atrial volume have been documented in dogs with normal cardiovascular status of different breeds.14 Notably, none of the previously reported breed‐specific left atrial volumes in normal dogs were above our established cut‐off of 1.1 mL/kg with the exception of the Petit Basset Griffon Vendeen, which had an upper 95% percentile volume of 1.12 mL/kg.14 Despite the many breeds represented in this study, we believe our suggested cut‐off of 1.1 mL/kg for LA Vol/BW provided a reasonable delineation between normal and abnormal dogs. More work is needed to further characterize this proposed threshold.
Finally, we did not include assessment of left atrial size by gold standard diagnostic imaging such as MSCT or CMR, nor did we compare 2‐D echocardiography and RT3‐D echocardiography. Comparison to MSCT or CMR would have better elucidated the accuracy of both LA : Ao and LA Vol/BW in their assessment of estimated left atrial size, and evaluation of RT3‐D echocardiography would have permitted assessment of agreement between 2‐D LA volume estimates obtained using a biplane method and RT3‐D‐derived LA volume. Although relationships between linear 2‐D measures of left atrial size and RT3‐D LA volumes have been described, correlations generally were weak.36 Agreement between estimates of left atrial volume from RT3‐D and 2‐D echocardiography has not been investigated. Ideally, further research should be conducted to provide such comparisons; however, the more clinically relevant questions are related to the ease and accessibility of obtaining the measurements as well as their prognostic value, not necessarily to their ability to obtain volumes identical to those obtained by RT3‐D echocardiography, MSCT or CMR.
In conclusion, we propose a cut‐off value of 1.1 mL/kg for LA Vol/BW measured in normal dogs using the biplane area‐length method. Significant diagnostic disagreement in identification of LAE exists between LA Vol/BW and the traditionally used LA : Ao. LA Vol/BW may be superior to LA : Ao in identification of mild LAE.
Acknowledgment
Grant support: The study was not supported by outside funding.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
The work was performed at the Veterinary Teaching Hospital at the Virginia‐Maryland Regional College of Veterinary Medicine and the Veterinary Teaching Hospital at the Kansas State University College of Veterinary Medicine.
The study was presented in abstract form at the 2013 ACVIM Forum, Seattle, WA.
Footnotes
811‐B; Parks Medical Electronics, Inc, Aloha, OR
Vivid 7; GE‐Medical, Milwaukee, WI
References
- 1. Borgarelli M, Haggstrom J. Canine degenerative myxomatous mitral valve disease: Natural history, clinical presentation and therapy. Vet Clin North Am Small Anim Pract 2010;40:651–663. [DOI] [PubMed] [Google Scholar]
- 2. Detweiler DK, Patterson DF. The prevalence and types of cardiovascular disease in dogs. Ann N Y Acad Sci 1965;127:481–516. [DOI] [PubMed] [Google Scholar]
- 3. Borgarelli M, Savarino P, Crosara S, et al. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med 2008;22:120–128. [DOI] [PubMed] [Google Scholar]
- 4. Haggstrom J, Boswood A, O'Grady M, et al. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: The QUEST study. J Vet Intern Med 2008;22:1124–1135. [DOI] [PubMed] [Google Scholar]
- 5. Hansson K, Haggstrom J, Kvart C, et al. Left atrial to aortic root indices using two‐dimensional and M‐mode echocardiography in cavalier King Charles spaniels with and without left atrial enlargement. Vet Radiol Ultrasound 2002;43:568–575. [DOI] [PubMed] [Google Scholar]
- 6. Rishniw M, Erb HN. Evaluation of four 2‐dimensional echocardiographic methods of assessing left atrial size in dogs. J Vet Intern Med 2000;14:429–435. [DOI] [PubMed] [Google Scholar]
- 7. Tsang MY, Barnes ME, Tsang TS. Left atrial volume: Clinical value revisited. Curr Cardiol Rep 2012;14:374–380. [DOI] [PubMed] [Google Scholar]
- 8. Al‐Mohaissen MA, Kazmi MH, Chan KL, et al. Validation of two‐dimensional methods for left atrial volume measurement: A comparison of echocardiography with cardiac computed tomography. Echocardiography 2013;30:1135–1142. [DOI] [PubMed] [Google Scholar]
- 9. Kircher B, Abbott JA, Pau S, et al. Left atrial volume determination by biplane two‐dimensional echocardiography: Validation by cine computed tomography. Am Heart J 1991;121:864–871. [DOI] [PubMed] [Google Scholar]
- 10. Vandenberg BF, Weiss RM, Kinzey J, et al. Comparison of left atrial volume by two‐dimensional echocardiography and cine‐computed tomography. Am J Cardiol 1995;75:754–757. [DOI] [PubMed] [Google Scholar]
- 11. Messika‐Zeitoun D, Bellamy M, Avierinos JF, et al. Left atrial remodelling in mitral regurgitation—Methodologic appro‐ach, physiological determinants, and outcome implications: A prospective quantitative Doppler‐echocardiographic and electron beam‐computed tomographic study. Eur Heart J 2007;28:1773–1781. [DOI] [PubMed] [Google Scholar]
- 12. Tidholm A, Westling AB, Hoglund K, et al. Comparisons of 3‐, 2‐dimensional, and M‐mode echocardiographical methods for estimation of left chamber volumes in dogs with and without acquired heart disease. J Vet Intern Med 2010;24:1414–1420. [DOI] [PubMed] [Google Scholar]
- 13. Rodevan O, Bjornerheim R, Ljosland M, et al. Left atrial volumes assessed by three‐ and two‐dimensional echocardiography compared to MRI estimates. Int J Card Imaging 1999;15:397–410. [DOI] [PubMed] [Google Scholar]
- 14. Hollmer M, Willesen JL, Tolver A, et al. Left atrial volume and phasic function in clinically healthy dogs of 12 different breeds. Vet J 2013;197:639–645. [DOI] [PubMed] [Google Scholar]
- 15. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007;21:542–558. [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 17. Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med 2009;23:1142–1150. [DOI] [PubMed] [Google Scholar]
- 18. Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Vet Intern Med 2004;18:311–321. [DOI] [PubMed] [Google Scholar]
- 19. Ljungvall I, Hoglund K, Carnabuci C, et al. Assessment of global and regional left ventricular volume and shape by real‐time 3‐dimensional echocardiography in dogs with myxomatous mitral valve disease. J Vet Intern Med 2011;25:1036–1043. [DOI] [PubMed] [Google Scholar]
- 20. Muzzi RA, de Araujo RB, Muzzi LA, et al. Regurgitant jet area by Doppler color flow mapping: Quantitative assessment of mitral regurgitation severity in dogs. J Vet Cardiol 2003;5:33–38. [DOI] [PubMed] [Google Scholar]
- 21. Borgarelli M, Crosara S, Lamb K, et al. Survival characteristics and prognostic variables of dogs with preclinical chronic degenerative mitral valve disease attributable to myxomatous degeneration. J Vet Intern Med 2012;26:69–75. [DOI] [PubMed] [Google Scholar]
- 22. Hezzell MJ, Boswood A, Chang YM, et al. The combined prognostic potential of serum high‐sensitivity cardiac troponin I and N‐terminal pro‐B‐type natriuretic peptide concentrations in dogs with degenerative mitral valve disease. J Vet Intern Med 2012;26:302–311. [DOI] [PubMed] [Google Scholar]
- 23. Terzo E, Di Marcello M, McAllister H, et al. Echocardiographic assessment of 537 dogs with mitral valve prolapse and leaflet involvement. Vet Radiol Ultrasound 2009;50:416–422. [DOI] [PubMed] [Google Scholar]
- 24. Zoghbi WA, Enriquez‐Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777–802. [DOI] [PubMed] [Google Scholar]
- 25. Ljungvall I, Hoglund K, Lilliehook I, et al. Serum serotonin concentration is associated with severity of myxomatous mitral valve disease in dogs. J Vet Intern Med 2013;27:1105–1112. [DOI] [PubMed] [Google Scholar]
- 26. Burdick RK, Borror CM, Montgomery DC. A review of methods for measurement systems capability analysis. J Qual Technol 2003;35:342–354. [Google Scholar]
- 27. Lester SJ, Ryan EW, Schiller NB, et al. Best method in clinical practice and in research studies to determine left atrial size. Am J Cardiol 1999;84:829–832. [DOI] [PubMed] [Google Scholar]
- 28. Schabelman S, Schiller NB, Silverman NH, et al. Left atrial volume estimation by two‐dimensional echocardiography. Cathet Cardiovasc Diagn 1981;7:165–178. [DOI] [PubMed] [Google Scholar]
- 29. Tsang TS, Abhayaratna WP, Barnes ME, et al. Prediction of cardiovascular outcomes with left atrial size: Is volume superior to area or diameter? J Am Coll Cardiol 2006;47:1018–1023. [DOI] [PubMed] [Google Scholar]
- 30. Le Tourneau T, Messika‐Zeitoun D, Russo A, et al. Impact of left atrial volume on clinical outcome in organic mitral regurgitation. J Am Coll Cardiol 2010;56:570–578. [DOI] [PubMed] [Google Scholar]
- 31. Tamura H, Watanabe T, Nishiyama S, et al. Increased left atrial volume index predicts a poor prognosis in patients with heart failure. J Card Fail 2011;17:210–216. [DOI] [PubMed] [Google Scholar]
- 32. Spencer KT, Mor‐Avi V, Gorcsan J 3rd, et al. Effects of aging on left atrial reservoir, conduit, and booster pump function: A multi‐institution acoustic quantification study. Heart 2001;85:272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nikitin NP, Witte KK, Thackray SD, et al. Effect of age and sex on left atrial morphology and function. Eur J Echocardiogr 2003;4:36–42. [DOI] [PubMed] [Google Scholar]
- 34. Thomas L, Levett K, Boyd A, et al. Compensatory changes in atrial volumes with normal aging: Is atrial enlargement inevitable? J Am Coll Cardiol 2002;40:1630–1635. [DOI] [PubMed] [Google Scholar]
- 35. Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part II: The aging heart in health: Links to heart disease. Circulation 2003;107:346–354. [DOI] [PubMed] [Google Scholar]
- 36. Tidholm A, Bodegard‐Westling A, Hoglund K, et al. Comparisons of 2‐ and 3‐dimensional echocardiographic methods for estimation of left atrial size in dogs with and without myxomatous mitral valve disease. J Vet Intern Med 2011;25:1320–1327. [DOI] [PubMed] [Google Scholar]


