Abbreviations
- b5
cytochrome b 5
- b5R
cytochrome b 5 reductase
- CYB5A
cytochrome b 5 gene
- CYB5R3
cytochrome b 5 reductase gene
- IV
intravenous
- MB
methylene blue
- NADH
nicotinamide adenine dinucleotide (reduced)
- NADPH
nicotinamide adenine dinucleotide phosphate (reduced)
The reduction of methemoglobin back to hemoglobin is catalyzed primarily by methemoglobin reductase, now recognized as the 2 enzyme system cytochrome b 5 (b5) and cytochrome b 5 reductase (b5R) (Fig 1).1, 2, 3 A negligible amount of methemoglobin is reduced by another enzyme, reduced nicotinamide adenine dinucleotide phosphate (NADPH) methemoglobin reductase.1 Treatment with methylene blue (MB) decreases methemoglobin concentrations dramatically by increasing the activity of the NADPH methemoglobin reductase pathway roughly 5‐ to 10‐fold.4 This report describes the diagnosis of a dog with congenital methemoglobinemia with normal b5R activity, 2 potentially deleterious variants in the cytochrome b 5 gene (CYB5A), and the preoperative treatment of this dog with MB.
Figure 1.
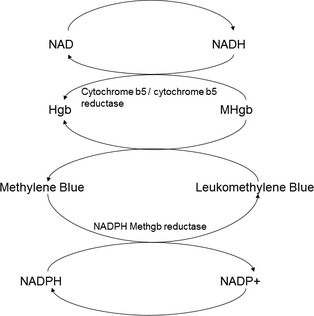
Reduction of methemoglobin.1 The reduction of methemoglobin as it occurs via the major cytochrome b 5 (b5) / cytochrome b 5 reductase (b5R) system and the minor NADPH methemoglobin reductase pathway. In the major pathway, b5R utilizes electrons from NADH to reduce ferricytochrome b 5 to ferrocytochrome b 5 and the resultant ferrocytochrome b 5 binds to ferriheme subunits in methemoglobin and transfers electrons to them. This electron transfer reduces methemoglobin back to hemoglobin (which can again bind oxygen) and regenerates ferricytochrome b 5.
A 4‐year‐old male castrated Pit Bull mix breed dog was referred for evaluation of markedly cyanotic mucous membranes and poor oxygen saturation, noted at the time of anesthetic induction for planned orthopedic correction for a right cranial cruciate ligament rupture. The surgery was aborted, but the dog reportedly experienced a prolonged anesthetic recovery. Results of a preanesthetic CBC and serum chemistry panel, as well as an electrocardiogram reportedly detected no abnormalities. Thoracic radiographs revealed a mildly enlarged right heart and mild interstitial pulmonary infiltrates.
There was no history of coughing, sneezing, collapse, syncope, exercise intolerance, or respiratory distress at home, and the dog had no known access to toxins including acetaminophen. He was fed a commercial adult canine food diet. The dog was adopted approximately 2–3 years before presentation and had suffered only chronic skin allergies until the cranial cruciate ligament rupture occurred. The owner reported that the dog's tongue and gums had always had a bluish discoloration. The patient was receiving carprofen,1 monthly milbemycin oxime—lufenuron,2 and spinosad.3 A course of antibiotic treatment with cefpodoxime4 had recently been discontinued.
Relevant physical examination findings included cyanotic mucous membranes including the tongue, gums, and penis (Fig 2). There was no noted improvement in the color of the mucous membranes with administration of supplemental oxygen. Thoracic auscultation was normal with no evidence of a heart murmur or arrhythmia and clear lungs sounds. The dog was panting but eupneic. A lameness of the right pelvic limb was noted.
Figure 2.
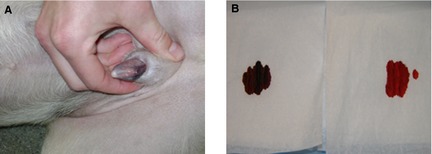
Clinical signs of cyanosis in a dog with congenital methemoglobinemia. Cyanosis of the penis at the time of presentation (A) as well as a “spot test” of venous blood from the patient (B; left) and a normal healthy control dog (B; right).
A CBC, serum chemistry panel, urinalysis, and coagulation profile with D‐dimer concentration were submitted for analysis.5 Abnormalities included erythrocytosis (HCT = 58%, reference interval 37–55%), increased total hemoglobin concentration (21.6 g/dL, reference interval 12–18 g/dL), mild hypokalemia (3.7 mEq/L, reference interval 4.0–5.6 mEq/L), hypercholesterolemia (412 mg/dL, reference interval 112–328 mg/dL), elevated aspartate aminotransferase (115 U/L, reference interval 5–55 U/L), and increased creatine kinase (890 U/L, reference interval 10–200 U/L). Coagulation parameters and D‐dimer concentration were within normal limits. Arterial and venous blood gas analyses6 were performed (Table 1), with a marked methemoglobinemia of approximately 38% (reference interval 0–1%) noted. An echocardiogram revealed normal cardiac structure and function, with no intracardiac or extracardiac shunts noted on intravenous (IV) agitated saline injection. The dog was discharged from the hospital with a diagnosis of methemoglobinemia, likely congenital.
Table 1.
Blood gas analysis on initial presentation and 2 weeks recheck in a chronically cyanotic dog showing hypoxemia and methemoglobinemia.
| Arterial Day 1 | Venous Day 1 | Venous Day 13 | Reference Interval | |
|---|---|---|---|---|
| pH | 7.46 | 7.44 | 7.43 | 7.36–7.47 (venous) |
| 7.4–7.5 (arterial) | ||||
| pCO2 | 28.6 | 29.2 | 35.0 | 27–40 mmHg (venous) |
| 30–50 mmHg (arterial) | ||||
| pO2 | 67.7 | 32 | 27.3 | 30–60 mmHg (venous) |
| 91–118 mmHg (arterial) | ||||
| HCO3 | 20.8 | 20.3 | 23.7 | 17–24 mmol/L |
| HCT | 58 | 59 | 61 | 40–50% |
| MetHb | 38.9 | 38 | 39.1 | 0–1% |
At recheck 2 weeks later, the dog remained clinically normal except for persistent cyanosis and right pelvic limb lameness. There was no change in oral medications or diet from the initial visit, and the owner had carefully examined the home environment to ensure there was no exposure to any toxins. A repeat venous blood gas analysis revealed persistently marked methemoglobinemia (Table 1), supporting a diagnosis of congenital methemoglobinemia. Blood samples from the patient and a healthy control dog were collected in potassium EDTA tubes for determination of nicotinamide adenine dinucleotide (NADH) ferricyanide reductase activity,7 which is used to detect b5R deficiency.5 The dog's blood methemoglobin level was quantified spectrophotometrically in a hemolysate by measuring the decrease in absorbance at 630 nm after the addition of cyanide which converts methemoglobin to cyanmethemoglobin.6 Methemoglobinemia was again documented to be markedly increased (53%), but the NADH‐ferricyanide reductase activity was similar to a control dog and within the reference interval for healthy dogs (Table 2).
Table 2.
Methemoglobin content and ferricyanide reductase activities in the dog, normal control dog assayed at the same time, and the canine reference intervals for these assays.
| Methemoglobin (%) | NADH‐Ferricyanide Reductase Activity (IU/g Hgb) | PCV (%) | |
|---|---|---|---|
| Control | 1.2 | 8.1 | 43 |
| Patient | 52.9 | 8.4 | 64 |
| Reference intervals | 0–1.2 | 8.1–15.5 | 38–56 |
Reference intervals represent minimum and maximum values from 39 healthy dogs for the methemoglobin assay, 30 healthy dogs for the ferricyanide reductase assay, and 58 healthy dogs (excluding greyhounds) for the centrifuged PCV.
Hgb, hemoglobin.
Surgical stabilization of the right stifle was performed 3 weeks after initial presentation. A preoperative venous blood gas was performed (Table 3), as well as gross “spot test” comparison of the blood sample with that from a control dog. The dog's blood sample was much darker in color than the control (Fig 2). Approximately 5 minutes after premedication, a sample for blood gas analysis (Table 3) was obtained from the left femoral artery. A single dose of MB8 1 mg/kg was administered IV over 20 minutes. A second venous blood gas sample was obtained 1 hour post‐MB administration (Table 3); the sample was grossly more red, and similar in color to the control than before treatment. In addition, the dog's mucous membranes were noticeably more pink than before treatment with MB (Fig 3).
Table 3.
Serial blood gas analyses to assess response to preoperative administration of methylene blue (MB) (1 mg/kg IV) to the described dog.
| 8:00 | 9:30a | 11:15 | 12:17 | 12:45 | 13:51 | 14:08 | 20:54 | 2:10 | Reference Interval | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Venous | Arterial | Venous | Arterial | Arterial | Arterial | Arterial | Venous | Venous | |
| pH | 7.44 | 7.38 | 7.4 | 7.29 | 7.27 | 7.34 | 7.35 | 7.41 | 7.36 | 7.36–7.47 (venous) |
| 7.4–7.5 (arterial) | ||||||||||
| pCO2 | 31.7 | 33.6 | 28.8 | 47.9 | 48 | 38.2 | 43.2 | 33.4 | 42.4 | 27–40 mmHg (venous) |
| 30–50 mmHg (arterial) | ||||||||||
| pO2 | 24.1 | 89.1 | 81.3 | 398.5 | 396.9 | 91.3 | 85.5 | 37.8 | 27.6 | 30–60 mmHg (venous) |
| 91–118 mmHg (arterial) | ||||||||||
| HCO3 | 22.1 | 20.4 | 18.1 | 23.3 | 22.3 | 20.8 | 24.2 | 21.3 | 24.0 | 17–24 mmol/L |
| HCT | 61 | 55 | 58 | 40 | 37 | 37 | 40 | 52 | 54 | 40–50% |
| MetHb | 37.2 | 39.7 | 5.9 | 3.9 | 3.4 | 3.1 | 3.4 | 4.9 | 6.8 | 0–1% |
| FiO2 (%) | 21 | 21 | 21 | 100 | 100 | 21 | 21 | 21 | 21 |
Sample obtained immediately before administration of MB.
Figure 3.
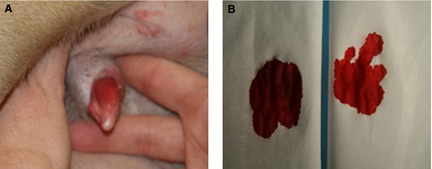
Resolution of cyanosis in a dog with congenital methemoglobinemia after treatment with methylene blue. Clinical resolution of cyanosis in this patient after treatment with MB (A) as well as a posttreatment “spot test” of venous blood from the patient (B; left) and a normal healthy control dog (B; right).
A routine tibial tuberosity advancement with meniscectomy of the right stifle was performed without complication. Transection of a persistent penile frenulum was also performed uneventfully. Serial arterial blood gas6 monitoring was performed every 15 minutes while the dog was anesthetized. After extubation, venous blood gas monitoring was performed 6 and 12 hours later (Table 3). Postoperative recovery was uneventful.
At the time of suture removal 10 days later, the dog was reportedly doing well at home and the incision site had healed well. The mucous membranes were again cyanotic, and the owner reported that the bluish discoloration returned approximately 3 days after the surgery. A repeat venous blood gas analysis confirmed return of the marked methemoglobinemia at 38%.
Further investigation of the methemoglobinemia was conducted at the University of Wisconsin‐Madison. First a RBC cytochrome c reduction assay was performed, which reflects primarily b5R activity, with some modulation by b5 (unpublished data, Trepanier 2006). The patient's cytochrome c reduction (95.47 nmol/μL RBC/min) was within the reference interval (48.89–253.33 nmol/μL RBC/min), established using RBC lysates from 15 control dogs. In light of these results, a defect in b5 was suspected, but there was no assay available that was specific for b5 activity. Therefore, we resequenced the coding exons of CYB5A, the b5 gene,9 and compared the sequence to 34 noncyanotic control dogs.7 In addition, CYB5R3, the b5R gene, was resequenced for completeness. Novel coding variants were analyzed for potential functional effects using 4 in silico modeling programs10, 11, 12, 13; novel promoter variants were analyzed for effects using the MAPPER search engine8 and novel intronic variants were analyzed for alteration of splice sites.14 For both genes, we included sequencing of the alternative exons that code for the soluble form of the enzymes that are found in erythrocytes. The primers used were described in a previous study.7 As no reference sequences existed for canine soluble mRNA CYB5A and CYB5R3 isoforms in GenBank,15 comparative genomic techniques16 were used to predict the location of these alternative exons. In the case of CYB5R3, a known bovine‐expressed sequence tag containing the DNA sequence corresponding to the alternative exon 1S showed 92% sequence similarity with a location on chromosome 10 of the canine genome (canFam3.1 assembly, Build 1 Version 1). It was predicted that exon 1S was located at chr 10:22822896‐22822602. For the alternative exon 4 of CYB5A, we aligned the entire canine gene (known to be located on chromosome 1 via the sequenced microsomal variant NM_001193298)7 with a number of mammalian genomic sequences. Based on these results and known mammalian expressed sequence tags, the alternative exon 4 was predicted to be located at chr 1:5103750‐5103772. This region corresponds exactly to part of a CYB5A transcript recently sequenced from canine blood (CUFF.77.1 or CUFF.202.1) accessible via the Broad Improved Canine Annotation v1 Track Data Hub.9 The PCR primer sequences (5′ to 3′) used to amplify these exons from canine genomic DNA (in both patient and controls) were as follows: CYB5R3 exon 1S: F:ACCCAGCCCTACTGCTCAC, R: CACAGGCTCCACATGGTCT; CYB5A exon 4: F: TCGCTAAGATTTAACATTGCTTGA, R: CGATGAGGCAAAGCTACACA.
Gene resequencing and variant analysis17 revealed 2 novel variants each in CYB5A and CYB5R3 in this dog (Table 4). Consistent with the normal b5R activity assay, neither polymorphism in CYB5R3 was predicted to be deleterious. Specifically, the promoter insertion polymorphism at −316_−315 altered predicted binding sites for several transcription factors, but none that have been shown to bind experimentally to the CYB5R3 promoter region in any mammalian species. The intronic CYB5R3 variant at I1S+97 was not predicted to affect potential splice sites or the binding of regulatory proteins.
Table 4.
List of polymorphisms found in the cyanotic dog for (a) CYB5A and (b) CYB5R3—The loci and genotypes of novel variants are in bold. All other variants were also found in 34 noncyanotic control dogs, as sequenced in Funk‐Keenan et al7 and minor allele frequencies (MAF) are listed for each.
| Count | Type | Locus | Genotype | Notes | MAF in 34 Control Dogs7 |
|---|---|---|---|---|---|
| (a) CYB5A (6 exons sequenced) | |||||
| 1 | SNV | −392 | TT | Promoter | 0.21 |
| 2 | SNV | −358 | CC | Promoter | 0.51 |
| 3 | DIV | −324_−287 | +/− | Promoter, 37 bp deletion | 0.00 |
| 4 | SNV | −104 | GG | Promoter | 0.45 |
| 5 | SNV | −76 | GG | Promoter | 0.30 |
| 6 | SNV | +68 G>A | AA | Exon 1, coding Ser23Asn | 0.00 |
| 7 | SNV | I1−63 | TT | Intron 1 | 0.28 |
| (b) CYB5R3 (10 exons sequenced) | |||||
| 1 | SNV | −442 | CG | Exon 1M promoter | 0.31 |
| 2 | SNV | −350 | CC | Exon 1M promoter | 0.20 |
| 3 | DIV | −316_−315 | +25/+25 a |
Exon 1M promoter 25 bp insertion |
0.00 |
| 4 | DIV | I1M+196_205 | −10/−10b | Intron 1M | 0.49 |
| 5 | SNV | I1S+97 | GT | Intron 1S | 0.00 |
| 6 | SNV | I2−35 | AA | Intron 2 | 0.08 |
| 7 | SNV | I3+6 | TT | Intron 3 | 0.08 |
| 8 | SNV | I3+50 | AA | Intron 3 | 0.36 |
| 9 | SNV | I3+102 | TT | Intron 3 | 0.36 |
| 10 | SNV | +303 | CC |
Exon 4, CDS Asp101 (syn) |
0.38 |
| 11 | SNV | *833 | AA | Exon 9, 3′UTR | 0.43 |
SNV, single nucleotide variant; DIV, deletion‐insertion variant; UTR, untranslated region.
Insertion: CAGCCCCCGGAGTGCAGGCCGGGCG.
Deletion: CGCATCCCCC.
In CYB5A, however, a nonsynonymous variant was identified at locus 68 G>A in exon 1 of CYB5A, with a predicted amino acid change of Ser23Asn. This Ser residue is conserved in at least 17 mammalian species, as well as 8 other vertebrates including Xenopus sp. and is in a position that influences protein conformational mobility.10 The Ser23Asn variant was not present in any of 34 noncyanotic dogs sequenced in a previous study,7 and was predicted by 2 of 4 in silico modeling programs10 , 11 to be deleterious to the b5 protein. The second novel variant in CYB5A, a heterozygous 37 bp promoter deletion at −324_−287, was also of interest. This promoter region is well conserved between dogs, humans, and rodents,15 and this 38 bp deletion (chr1:5070633‐5070670) was predicted to remove all 3 Sp3 transcription factor binding sites in the predicted 700 bp promoter sequence. Binding of Sp3 normally promotes CYB5A gene expression, and mutation of Sp3 binding sites decreases CYB5A expression in vitro.11 It is therefore likely that one or both of these CYB5A variants contributed to decreased b5 protein expression and impaired methemoglobin reduction in this dog.
Discussion
Methemoglobinemia is a condition resulting from the oxidation of ferrous iron (Fe2+) to ferric iron (Fe3+) within hemoglobin molecules.12 Methemoglobin impairs the normal transport of oxygen to tissues leading to significant tissue hypoxia and at levels >20%, cyanosis, exercise intolerance, and erythrocytosis.1, 13 Ferriheme groups cannot bind oxygen. As a result of the allosteric interactions among heme groups within the erythrocyte, the formation of ferriheme in one or more of the heme groups of the tetramer leads to decreased release of oxygen from the heme groups bound with oxygen. When assayed in vitro, the oxyhemoglobin dissociation curve is shifted to the left in methemoglobinemic blood.14
There are both congenital and acquired causes of methemoglobinemia reported in veterinary medicine.6, 15, 16, 17, 18 Acquired causes of methemoglobinemia are usually the result of exposure to a toxic oxidizing agent, including ingestion of acetaminophen, nitrates, nitrites, topical benzocaines and naphthalene, or inhalation of smoke from various combustible substances.18 Before this case, the only documented cause for congenital methemoglobinemia in dogs was associated with deficiency of b5R.5, 6, 16, 19, 20 Because of the technical difficulties associated with preparing methemoglobin free of the b5R enzyme and slow reaction rates using methemoglobin, b5R is typically assayed using ferricyanide rather than methemoglobin as the substrate.5, 21 In the case of this dog, NADH‐ferricyanide reductase activity was normal, indicating the b5R activity was likely normal, and suggested a deficiency or defect in the b5 protein. Because there are no specific hematologic enzymatic assays that isolate b5 function, the next step was to resequence the CYB5A and CYB5R3 genes. Given the dog's clinical response to MB, a defect in the minor pathway comprising NADPH methemoglobin reductase could be eliminated in this dog.
Gene resequencing in this dog identified 2 variants each in CYB5A and CYB5R3, as compared to 34 noncyanotic dogs. Based on sequence evolutionary conservation, the coding CYB5A single nucleotide variant that leads to Ser23Asn is predicted to be damaging to the b5 protein function. While this variant is a candidate for the cause of the dog's methemoglobinemia, we also found a promoter deletion in CYB5A in this dog that removes 3 key transcription factor binding sites. Either of these variants could therefore lead to decreased b5 protein expression in vivo. However, liver tissue was not available for this dog for expression analyses. While immunoblotting of erythrocytes would have been helpful, in our experience it has been difficult to detect either b5R or b5 by immunoblotting of RBCs because of interference by the abundance of hemoglobin proteins. Therefore, the relative contributions of these 2 CYB5A variants will need to be determined by functional characterization in vitro.
CYB5A variants are rare in human patients and have been associated with more catastrophic phenotypes, such as abnormal neurologic development and hermaphrodism.22 However, CYB5R3 variants can occur in humans with only cyanosis as the phenotype (Type I methemoglobinemia). This has been hypothesized to result from decreased protein expression over the lifetime of an anucleate red blood cell, which might be compensated for in a nucleated somatic cell that could continually synthesize new b5 protein. Such a scenario for CYB5A could explain the finding of cyanosis with no other abnormalities in this dog.
Other causes of congenital methemoglobinemia described in humans, including hemoglobin M disorders and b5 deficiency,1, 2, 3, 21 have not yet been documented in dogs. An inherited RBC flavin adenine dinucleotide (FAD) deficiency has been described in horses with persistent methemoglobinemia.23 Affected horses have low b5R activity because FAD is a cofactor for b5R.18 The dog of this report has congenital methemoglobinemia not associated with b5R deficiency but rather dysfunction of the b5 protein.
Methylene blue is considered the treatment of choice for patients with clinical signs due to methemoglobinemia after toxin exposure. In the erythrocyte, MB is reduced to leukomethylene blue via an NADPH methemoglobin reductase enzyme.1 The NADPH methemoglobin reductase enzyme is a generalized reductase that also has an affinity for dyes including MB. Leukomethylene blue has a high affinity for methemoglobin and nonenzymatically reduces methemoglobin back to hemoglobin. Under normal conditions, this reduction pathway plays a negligible role in reducing methemoglobin, accounting for only roughly 5% of reduced methemoglobin in the body in humans. However, in the presence of MB, this reduction pathway can increase 5‐ to 10‐fold, making MB an ideal treatment for methemoglobinemia even when the b5/b5R pathway is not functional.
Patients with congenital methemoglobinemia often have a mild erythrocytosis, an expected response in the setting of impaired oxygen carrying capacity and delivery. In this dog's case, the peripheral packed cell volume (PCV) was approximately 60% with approximately 38% of the hemoglobin as oxidized methemoglobin. Consequently, the dog had an estimated functional PCV of approximately 37%, considered adequate to sustain normal bodily function and the reason for this dog's lack of clinical signs of hypoxia despite markedly cyanotic mucous membranes. Patients that are acutely and severely affected by methemoglobinemia secondary to toxin exposure often do not have adequate time to compensate for a marked decrease in oxygen carrying capabilities, and may require treatment with MB to remain stable.
The dog in this case was given MB as a preoperative treatment before orthopedic surgery. Based on the above discussion, it could be argued that this treatment was not necessary for the dog to remain stable under anesthesia. The decision to pursue this treatment was made because the dog had a previously reported prolonged recovery from anesthesia. The dog's response to MB was also helpful in supporting the diagnosis of congenital methemoglobinemia.
This is a case of congenital methemoglobinemia in a dog that is possibly attributed to a genetic variant in the CYB5A gene. The dog's normal NADH‐ferricyanide reductase activity and clinical response to MB ruled out involvement of b5R and NAPDH methemoglobin reductase respectively, leaving a defect in b5 as the likely cause of methemoglobinemia. Further functional characterization of both novel CYB5A variants is warranted.
Acknowledgments
The authors thank Melanie Pate and Eva Bachar for their contributions to this case.
Grant support: This work was not supported by a grant or any other funding.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
The work contributing to this case report was performed at Advanced Critical Care, Emergency & Specialty Services, and the veterinary schools of the University of Florida, University of Wisconsin and University of Pennsylvania.
Footnotes
Rimadyl; Zoetis, New York, NY
Sentinel; Novartis, East Hanover, NJ
Comfortis; Elanco, Greenfield, IN
Simplicef; Pfizer, Irvine, CA
IDEXX Laboratories, North Grafton, MA
Stat Profile‐Critical Care Xpress; Nova Biomedical, Waltham, MA
University of Florida College of Veterinary Medicine, Gainesville, FL
Injectable American Regent Inc, Shirley, NY
BigDye Direct Cycle Sequencing; Applied Biosystems, Foster City, CA
Align GVGD, International Agency for Research on Cancer, Lyon, France
Panther classification system; Thomas lab ‐ USC, Los Angeles, CA; http://www.pantherdb.org
PolyPhen‐2 server; genetics.bwh.harvard.edu/pph2
SIFT web server, J. Craig Venter Institute, Rockville, MD; sift.jvci.org
Human Splicing Finder (HSF, MAxEnt, and ESE Finder); www.umd.be/HSF/
UCSC Genome Bioinformatics, http://www.genome.ucsc.edu/
Staden Package software
References
- 1. Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: Etiology, pharmacology, and clinical management. Ann Emerg Med 1999;34:646–656. [DOI] [PubMed] [Google Scholar]
- 2. Hegesh E, Hegesh J, Kaftory A. Congenital methemoglobinemia with a deficiency of cytochrome b5. N Engl J Med 1986;314:757–761. [DOI] [PubMed] [Google Scholar]
- 3. Kitao T, Sugita Y, Yoneyama Y, et al. Methemoglobin reductase (cytochrome b5 reductase) deficiency in congenital methemoglobinemia. Blood 1974;44:879–884. [PubMed] [Google Scholar]
- 4. Wendel WB. The control of methemoglobinemia with methylene blue. J Clin Invest 1939;18:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harvey JW, King RR, Berry CR, et al. Methaemoglobin reductase deficiency in dogs. Comp Haematol Int 1991;1:55–59. [Google Scholar]
- 6. Harvey JW, Ling GV, Kaneko JJ. Methemoglobin reductase deficiency in a dog. J Am Vet Med Assoc 1974;164:1030–1033. [PubMed] [Google Scholar]
- 7. Funk‐Keenan J, Sacco J, Wong YY, et al. Evaluation of polymorphisms in the sulfonamide detoxification genes CYB5A and CYB5R3 in dogs with sulfonamide hypersensitivity. J Vet Intern Med 2012;26:1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marinescu VD, Kohane IS, Riva A. MAPPER: A search engine for the computational identification of putative transcription factor binding sites in multiple genomes. BMC Bioinformatics 2005;6:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoeppner MP, Lundquist A, Pirum M, et al. An improved canine genome and a comprehensive catalogue of coding genes and non‐coding transcripts. PLoS One 2014;9:e91172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang L, Cowley AB, Terzyan S, et al. Comparison of cytochrome b 5 from insects and vertebrates. Proteins 2007;67:293–304. [DOI] [PubMed] [Google Scholar]
- 11. Huang N, Dardis A, Miller WL. Regulation of cytochrome b5 gene transcription by Sp3, GATA‐6, and steroidogenic factor 1 in human adrenal NCI‐H295A cells. Mol Endocrinol 2005;19:2020–2034. [DOI] [PubMed] [Google Scholar]
- 12. Umbreit J. Methemoglobin—It's not just blue: A concise review. Am J Hematol 2007;82:134–144. [DOI] [PubMed] [Google Scholar]
- 13. Rockwood GA, Armstrong KR, Baskin SI. Species comparison of methemoglobin reductase. Exp Biol Med (Maywood) 2003;228:79–83. [DOI] [PubMed] [Google Scholar]
- 14. Darling RC, Roughton FJW. The effect of methemoglobin on the equilibrium between oxygen and hemoglobin. Am J Physiol 1942;137:56–68. [Google Scholar]
- 15. Fine DM, Eyster GE, Anderson LK, et al. Cyanosis and congenital methemoglobinemia in a puppy. J Am Anim Hosp Assoc 1999;35:33–35. [DOI] [PubMed] [Google Scholar]
- 16. Atkins CE, Kaneko JJ, Congdon LL. Methemoglobin reductase deficiency and methemoglobinemia in a dog. J Am Anim Hosp Assoc 1981;17:829–832. [Google Scholar]
- 17. Harvey JW, Dahl M, High ME. Methemoglobin reductase deficiency in a cat. J Am Vet Med Assoc 1994;205:1290–1291. [PubMed] [Google Scholar]
- 18. Rahilly L, Mandell DC. Methemoglobinemia In: Silverstein DC, Hopper K, eds. Small Animals Critical Care Medicine. St. Louis, MO: Saunders; 2009:374–378. [Google Scholar]
- 19. Harvey JW. Pathogenesis, laboratory diagnosis and clinical implications of erythrocyte enzyme deficiencies in dogs, cats and horses. Vet Clin Pathol 2006;35:144–156. [DOI] [PubMed] [Google Scholar]
- 20. Letchworth GJ, Bentnick‐Smith J, Bolton GR, et al. Cyanosis and methemoglobinemia in two dogs due to NADH methemoglobin reductase deficiency. J Am Anim Hosp Assoc 1977;13:75–79. [Google Scholar]
- 21. Percy MJ, McFerran NV, Lappin TR. Disorders of oxidized haemoglobin. Blood Rev 2005;19:61–68. [DOI] [PubMed] [Google Scholar]
- 22. Giordano SJ, Kaftory A, Steggles AW. A splicing mutation in the cytochrome b5 gene from a patient with congenital methemoglobinemia and pseudohermaphrodism. Human Genet 1994;93:568–570. [DOI] [PubMed] [Google Scholar]
- 23. Harvey JW, Stoackham MA, Scott PJ, et al. Methemoglobinemia and eccentrocytosis in equine erythrocyte flavin adenine dinucleotide deficiency. Vet Pathol 2003;40:632–342. [DOI] [PubMed] [Google Scholar]


