Abstract
Background
Endothelial dysfunction (ED) has been suggested to be associated with myxomatous mitral valve disease (MMVD) in dogs. Tetrahydrobiopterin (BH4) is an important cofactor for production of the endothelium‐derived vasodilator nitric oxide (NO). Under conditions of oxidative stress, BH4 is oxidized to the biologically inactive form dihydrobiopterin (BH2). Thus, plasma concentrations of BH2 and BH4 may reflect ED and oxidative stress.
Objective
To determine plasma concentrations of BH2 and BH4 in dogs with different degrees of MMVD.
Animals
Eighty‐four privately owned dogs grouped according to ACVIM guidelines (37 healthy control dogs including 13 Beagles and 24 Cavalier King Charles Spaniels [CKCSs], 33 CKCSs with MMVD of differing severity including 18 CKCSs [group B1] and 15 CKCSs [group B2], and 14 dogs of different breeds with clinical signs of congestive heart failure [CHF] because of MMVD [group C]).
Methods
Dogs underwent clinical examination including echocardiography. Plasma concentrations of BH2 and BH4 were measured using high‐performance liquid chromatography with fluorescence detection.
Results
Higher plasma BH4 and BH2 concentrations were found with dogs in CHF compared with all other groups (control, B1 and B2; P ≤ .001). Females had higher concentrations of BH4 and BH4/BH2 (P ≤ .0003). BH4/BH2 was found to decrease with age (P < .0001). Cardiovascular risk factors in humans such as passive smoking (P ≤ .01) and increased body weight (P ≤ .009) were associated with lower BH4 concentrations.
Conclusions and Clinical Importance
Age, sex, body weight, passive smoking, and cardiac status are associated with plasma biopterin concentration in dogs. Additional studies should clarify the clinical implications of the findings.
Keywords: Endothelial dysfunction, Mitral regurgitation, Oxidative stress, Tetrahydrobiopterin
Abbreviations
- ACE‐I
angiotensin‐converting enzyme inhibitor
- ACVIM
American College of Veterinary Internal Medicine
- BCS
body condition score
- BH2
dihydrobiopterin
- BH4
tetrahydrobiopterin
- BW
body weight
- CBC
complete blood count
- CHF
congestive heart failure
- CKCS
Cavalier King Charles Spaniel
- CV
coefficient of variation
- DAP
diastolic arterial pressure
- DTE
1,4‐Dithioerythritol
- ED
endothelial dysfunction
- eNOS
endothelial nitric oxide synthase
- FMD
flow‐mediated vasodilatation
- FS
fractional shortening
- IVSDN
interventricular septal thickness in diastole normalized for body weight
- IVSSN
interventricular septal thickness in systole normalized for body weight
- LA/Ao
left atrial‐to‐aortic root ratio
- LVIDDN
left ventricular end‐diastolic diameter normalized for body weight
- LVIDSN
left ventricular end‐systolic diameter normalized for body weight
- LV
left ventricular
- LVPWDN
left ventricular free wall thickness in diastole normalized for body weight
- LVPWSN
left ventricular free wall thickness in systole normalized for body weight
- MAP
mean arterial pressure
- MMVD
myxomatous mitral valve disease
- MR
mitral regurgitation
- NO
nitric oxide
- NS
nonsignificant
- SAP
systolic arterial pressure
Myxomatous mitral valve disease (MMVD) is the most common cardiovascular disease in dogs.1, 2 The Cavalier King Charles Spaniel (CKCS) is a predisposed breed in which MMVD causes greater cardiac‐specific morbidity and mortality compared with other breeds.3, 4 MMVD is a slowly progressive disease, but duration of the preclinical period often is unpredictable.5 The uncertain pathogenesis of MMVD and medical treatment restricted to management of clinical signs5, 6 warrant additional research to identify new diagnostic, therapeutic, and prognostic targets.
Impaired endothelial function and oxidative stress have long been associated with cardiovascular disease including heart failure and valvular disease in human patients.7, 8, 9, 10 Endothelial dysfunction (ED) also has been associated with cardiovascular risk factors in humans including hypertension, hypercholesterolemia, aging, male sex, and cigarette smoking11, 12, 13, 14 and has been suggested to have prognostic potential for human cardiovascular disease.8, 10, 15
Veterinary studies have reported altered antioxidant status and biomarkers for oxidative stress in dogs with both spontaneous and experimentally induced cardiovascular disease including MMVD.16, 17, 18 Other studies of ED examining both circulating biomarkers6, 19, 20, 21, 22 and functional measurements6, 23, 24 suggest that ED may play a role in the pathogenesis of MMVD in dogs. Thus, oxidative stress and ED may serve as future targets with regard to improved diagnosis, treatment, or prognosis for dogs with MMVD.6, 18, 19, 24
Nitric oxide (NO) generated by the vascular endothelium is a key regulator of vascular homeostasis and normal endothelial function that inhibit disease processes such as inflammation, thrombosis, and oxidative stress.25 Tetrahydrobiopterin (BH4) is an important cofactor for endothelial NO synthase (eNOS) and thereby for production of NO.26, 27 Under conditions of oxidative stress, BH4 in the vascular tissue is oxidized to dihydrobiopterin (BH2).28 BH2 lacks the ability to donate electrons to eNOS and may even decrease BH4 activity by competitive binding to eNOS.29 Insufficiency of BH4 causes eNOS to uncouple and produce superoxide rather than NO, a situation that further exacerbates oxidative stress and ED.29, 30, 31
Thus, the aim of the present study was to determine plasma concentrations of BH2 and BH4 and the BH4/BH2 ratio in dogs with different degrees of MMVD and to investigate if concentrations correlated with disease severity and selected cardiovascular risk factors known to occur in humans.
Materials and Methods
We recruited healthy privately owned dogs ≥4 years with varying severity of MMVD. Written informed consent was obtained from all owners and the study was approved by the Danish Animal Experiments Inspectorate, license no. 2012‐15‐2934‐00700. All dogs lived in private homes and were fed commercial diets, homemade diets, or a combination of these. Dogs receiving medical treatment apart from cardiac treatment and dogs with a history or signs of noncardiac organ‐related disease were excluded. All dogs were subjected to the following protocol: interview with the owner, collection of blood samples, clinical examination, and echocardiography. Passive smoking was defined as dogs living in a home where the owner smoked indoors. Body condition score (BCS) was graded 1–9.32 Left apical systolic murmur intensity was graded 1–6.33
Blood pressure was measured using high‐definition oscillometry equipment on the proximal part of the tail. Measurements were repeated 5 times as previously described.34 Thoracic radiographs (laterolateral and dorsoventral) were performed on dogs in congestive heart failure (CHF) (except in 4 dogs because of logistic reasons) to rule out concomitant pulmonary disease. All dogs were part of a previous study that evaluated variation in mitral regurgitation (MR).35
Blood Sampling
Blood samples were drawn from the jugular vein using a vacutainer system connected to a 21‐G butterfly catheter. Dogs were fasted 6–18 hours before blood sampling. Analysis included a complete blood count (CBC) and serum biochemistry profile. For biopterin analysis, blood was collected in K3 EDTA tubes (4 mL) with 100 μL freshly made 1,4‐Dithioerythritol (DTE) solution (40 mg DTE in 1,000 μL Milli‐Q [18.2 MΩ] water) added to minimize oxidation during analysis.36 Samples were mixed gently before centrifugation (3,000 × g, 3 minutes, at 4°C) and plasma was frozen immediately at −80°C until analysis. Plasma BH4 and BH2 concentrations were measured by high‐performance liquid chromatography with fluorescence detection employing iodine oxidation as described by Fukushima and Nixon.37
After individual analysis, plasma from the 5 highest and 5 lowest BH2‐ and BH4‐containing samples (regardless of group) was pooled and analyzed 5 times per day for 5 consecutive days to determine within‐day and between‐day variation. Within‐day and between‐day coefficients of variation of BH4 and BH2 measurements were below 7% (Table 1).
Table 1.
Within‐day and between‐day variation in plasma BH4, plasma BH2, and total biopterin results.
| BH4 | BH2 | Total Biopterin | |
|---|---|---|---|
| Within‐day CV% | |||
| High | 2.62 | 3.18 | 1.49 |
| Low | 6.11 | 1.77 | 4.32 |
| Between‐day CV% | |||
| High | 1.92 | 2.94 | 1.08 |
| Low | 6.38 | 4.87 | 3.75 |
High, pool of the 5 samples highest in BH2 and BH4, respectively; Low, pool of the 5 samples lowest in BH2 and BH4.
BH4, tetrahydrobiopterin; BH2, dihydrobiopterin; CV, coefficient of variation.
Echocardiography
Standardized transthoracic echocardiography was performed from multiple parasternal and apical windows38 using a 3S and 5S ultrasound transducer and digitally stored.1 Echocardiography was performed and analyzed2 by 1 operator (LHO). The observer was blinded to the identity and clinical data of the dog during assessment. The following echocardiographic variables were assessed: Severity of MR classified as mild, moderate, or severe based on regurgitant jet area relative to left atrial area,19 left atrial‐to‐aortic root ratio (LA/Ao),39 and left ventricular (LV) dimensions normalized to body weight (BW).40 LV dimensions were measured from M‐mode short‐axis view and used to calculate fractional shortening (FS).
Classification of Disease Severity
Dogs were staged based on American College of Veterinary Internal Medicine (ACVIM) consensus statement classification guidelines as: control group (healthy dogs with no auscultatory heart murmur including 13 Beagles and 24 CKCSs), group B1 (CKCSs with auscultatory heart murmur and LA/Ao ≤ 1.5), group B2 (CKCSs with auscultatory heart murmur and LA/Ao > 1.5), and group C (dogs in CHF including 9 CKCSs and 1 of each of the following breeds: Crossbreed, Springer Spaniel, Dachshund, Bull Terrier, Yorkshire Terrier).5, 39 Dogs with CHF were defined as having clinical signs of CHF (eg, cough, dyspnea, nocturnal restlessness, exercise intolerance), echocardiographic changes compatible with CHF and response to diuretic treatment. Cardiac medication was given to all dogs in group C (Table 2).
Table 2.
Canine characteristics and conventional echocardiographic variables.
| ACVIM Group | Control | B1 | B2 | C |
|---|---|---|---|---|
| Total number | 37 | 18 | 15 | 14 |
| Cardiac treatment | 0 | 0 | 0 | 14a |
| Sex (female/male) | 21/16 | 13/5 | 8/7 | 2/12 |
| Age (years) | 5.9C (4.9–7.6) | 7.2C (6.3–8,5) | 8.1C (5.3–9,0) | 11.0Control,B1,B2 (9.8–13.0) |
| BW (kg) | 9.3 (8.5–12.3) | 9.1 (8.0–10.0) | 9.1 (8.0–10.4) | 11.5 (10.1–13.6) |
| BCS 3/4/5/6/7 | 0/7/19/11/0 | 0/3/7/6/1 | 0/3/6/4/2 | 1/1/3/4/3 |
| Cholesterol (mmol/L) | 5.8C (4.5–6.8) | 6.9 (5.4–8.2) | 6.3 (4.7–6.8) | 8.0Control (6.4–8.9) |
| Smoking (n/y) | 31/6 | 13/5 | 11/4 | 11/3 |
| SAP (mmHg) | 149.4.5 (140.5–162.5) | 154.5 (148.8–162.5) | 147.3 (137.5–154.9) | 150.5 (143.0–162.3) |
| DAP (mmHg) | 79.9 (75.0–87.3) | 77.0 (74.8–86.5) | 77.5 (75.3–81.6) | 84.8 (78.8–91.2) |
| MAP (mmHg) | 104.6 (99.5–111.0) | 106.5 (100.0–109.0) | 101.5 (98.4–106.6) | 108.0 (101.5–115.5) |
| Storage time (days) | 319.0 (275.0–333.0) | 326.5 (298.0–371.0) | 319.0 (297.5.0–337.0) | 309.0 (287.0–349.0) |
| MR (no/mi/mo or se) | 26/11/0 | 2/10/6 | 0/3/12 | 0/0/14 |
| LA/Ao | 1.4B2,C (1.3–1.4) | 1.4B2,C (1.4–1.5) | 1.7Control,B1,C (1.6–1.8) | 2.2Control,B1,B2 (2.0–2.4) |
| LVIDDN | 1.5B2,C (1.4–1.6) | 1.6C (1.4–1.7) | 1.8Control,C (1.6–1.8) | 2.1Control,B1,B2 (1.9–2.2) |
| LVIDSN | 1.1 (1.0–1.1) | 1.0 (1.0–1.1) | 1.1 (1.0–1.2) | 1.2 (1.1–1.3) |
| FS (%) | 25.8B2,C (22.5–32.0) | 27.8 (23.2–35.4) | 36.1Control (27.7–39.4) | 42.0Control (33.5–45.7) |
| LVPWDN | 0.5 (0.5–0.5) | 0.5 (0.4–0.5) | 0.5 (0.4–0.5) | 0.5 (0.4–0.5) |
| LVPWSN | 0.6 (0.5–0.6) | 0.6 (0.5–0.6) | 0.6 (0.6–0.7) | 0.6 (0.6–0.6) |
| IVSDN | 0.4 (0.4–0.5) | 0.4 (0.4–0.5) | 0.4 (0.4–0.5) | 0.4 (0.4–0.4) |
| IVSSN | 0.5 (0.4–0.6) | 0.5 (0.5–0.6) | 0.5 (0.4–0.6) | 0.6 (0.5–0.7) |
Within each row, superscripts Control,B1,B2,C represent the group from which there is statistically significant difference. Values reported are median and interquartiles.
ACVIM, American College of Veterinary Internal Medicine; BW, body weight; BCS, body condition score; SAP, systolic arterial pressure; DAP, diastolic arterial pressure; MAP, mean arterial pressure; MR, mitral regurgitation using jet area method where no = no, mi = mild, mo = moderate, se = severe (no: <20%, mi: 20–50%, mo or se: >50%); LA/Ao, ratio of left atrium to aortic root; LVIDDN, left ventricular end diastolic diameter normalized for BW; LVIDSN, left ventricular end systolic diameter normalized for BW; FS, fractional shortening; IVSDN, interventricular septal thickness in diastole normalized for BW; IVSSN, interventricular septal thickness in systole normalized for BW; LVPWDN, left ventricular free wall thickness in diastole normalized for BW; LVPWSN, left ventricular free wall thickness in systole normalized for BW.
Diuretics (Diu) (n = 1), Diu+Pimobendan (Pimo) (n = 3), Diu+Angiotensin converting enzyme inhibitor (ACE‐i) (n = 1), Diu+ACE‐i+Pimo (n = 6), Diu+ACE‐i+Digoxin (Dig) (n = 1), Diu+ACE‐i+Pimo+Dig (n = 1), Diu+ACE‐i+Pimo+Dig+Hydralazin+Potassium gluconate supplementation (n = 1).
Statistical Analysis
Data were analyzed using statistical software3 and for statistical analysis, a significance level of P < .05 was used.
For descriptive statistics, continuous variables were tested for Gaussian distribution within groups using a Shapiro‐Wilk test combined with visual evaluation of histograms and normal quantile plots. Differences among groups were investigated using a nonparametric Kruskal‐Wallis test as many groups did not follow a normal distribution. When significant associations were detected, pair‐wise comparisons using Wilcoxon rank sum test were performed with Bonferroni adjustment. Group differences for categorical data were investigated using Fisher's exact test.
Using a multiple linear regression model, differences in concentrations of BH2, BH4, and BH4/BH2 were analyzed. Explanatory variables used were ACVIM group (or LA/Ao ratio or degree of MR), age, sex, passive smoking (yes/no), BW, BCS, systolic arterial pressure (SAP), serum cholesterol concentrations, and plasma biopterin storage time. For the purpose of statistical analysis, BCS groups 3 and 4 were merged because only 1 dog was recorded as BCS 3.
Response variable residuals were tested for homogeneity of variation based on visual inspection of residual and QQ plots and Shapiro‐Wilk test. When transformation of data was considered necessary, a boxcox analysis was used to find the optimal transformation. BH4 results were transformed to BH4−0.5 and BH2 to BH2−1. Subsequently, the multiple linear regression models were reduced through backward selection based on P values. For class variables that remained significant, differences between groups were investigated by performing posthoc testing using Tukey‐Kramer adjustment when appropriate for multiple testing.
Results
Three dogs were excluded because of abnormalities in serum biochemistry (n = 1), heart disease other than MMVD diagnosed by echocardiography (n = 1), and medical treatment for CHF without echocardiographic signs compatible with CHF (n = 1). Twelve dogs (8 CKCSs and 4 Beagles) received diet supplements including fish oil, glucosamine, algae, or some combination of these. The final study population consisted of 84 dogs allocated in ACVIM groups as follows: control group (n = 37), group B1 (n = 18), group B2 (n = 15), and group C (n = 14). Baseline characteristics and conventional echocardiographic results of the final study population are given in Table 2. Sex differed among groups (P = .01), but passive smoking did not (P = .7). No differences in baseline characteristics and conventional echocardiographic values were found between CKCSs and other dog breeds in group C.
Factors with influence on plasma concentrations of BH4, BH2, and BH4/BH2 are summarized in Table 3. Plasma concentrations of BH4 and BH2 were associated with MMVD severity (Figs 1, 2). Higher concentrations of BH4 and BH2 were found in ACVIM group C compared with the other groups. Additionally, both BH4 and BH2 correlated positively with LA/Ao ratio and degree of MR. Lower BH4 plasma concentrations were found in male dogs and in dogs from homes with indoor smoking. In the LA/Ao model, dogs with BCS 6 had lower plasma BH4 than dogs with BCS 5 (P = .04). In the ACVIM disease group model and the MR model, BCS was no longer significant in posthoc analysis after Tukey‐Kramer adjustment. Finally, BH4 plasma concentrations decreased with increasing BW, but increased with increasing serum cholesterol concentration. BH2 was positively correlated with age (but only in the statistical models including LA/Ao or degree of MR as disease variable) and serum cholesterol concentration. The BH4/BH2 ratio was not associated with disease severity, but the ratio decreased with increasing age and females had a higher BH4/BH2 ratio than males (Fig 3). No influence of SAP or sample storage time was found. Identical statistical ACVIM disease group models were performed with the control group divided into Beagles and CKCSs and the same explanatory variables reached significance.
Table 3.
Overall P values of biopterin results in the multivariate statistical models including ACVIM disease group, LA/Ao, and MR jet area as disease severity variables in 84 dogs with or without MMVD.
| Explanatory Factors | BH4−0.5 | BH2−1 | BH4/BH2 Ratio |
|---|---|---|---|
| Models with ACVIM group as disease variable | |||
| Group | <.0001 | <.0001 | NS |
| Age (years) | NS | NS | <.0001 |
| Sex | .0001 | NS | <.0001 |
| Smoking | .01 | NS | NS |
| BW (kg) | .0004 | NS | NS |
| BCS | .03 | NS | NS |
| Cholesterol (mmol/L) | .005 | .04 | NS |
| SAP (mmHg) | NS | NS | NS |
| Storage time (days) | NS | NS | NS |
| Models with LA/Ao as disease variable | |||
| LA/Ao | <.0001 | .005 | NS |
| Age (years) | NS | .005 | <.0001 |
| Sex | .0003 | NS | <.0001 |
| Smoking | .01 | NS | NS |
| BW (kg) | .001 | NS | NS |
| BCS | .03 | NS | NS |
| Cholesterol (mmol/L) | <.0001 | .007 | NS |
| SAP (mmHg) | NS | NS | NS |
| Storage time (days) | NS | NS | NS |
| Models with degree of MR as disease variable | |||
| Degree of MR | <.0001 | .01 | NS |
| Age (years) | NS | .005 | <.0001 |
| Sex | .0003 | NS | <.0001 |
| Smoking | .01 | NS | NS |
| BW (kg) | .009 | NS | NS |
| BCS | .049 | NS | NS |
| Cholesterol (mmol/L) | .0005 | .03 | NS |
| SAP (mmHg) | NS | NS | NS |
| Storage time (days) | NS | NS | NS |
Final models with ACVIM groups as disease variable have an adjusted R2 of 0.57 (BH4), 0.42 (BH2), and 0.37 (BH4/BH2). Final models with LA/Ao as disease variable have an adjusted R2 of 0.48 (BH4), 0.38 (BH2), and 0.37 (BH4/BH2). Final models with MR as disease variable have an adjusted R2 of 0.50 (BH4), 0.37 (BH2), and 0.37 (BH4/BH2).
ACVIM, American College of Veterinary Internal Medicine; BW, body weight; BCS, body condition score; SAP, systolic arterial pressure; LA/Ao, left atrial‐to‐aortic root ratio; MR, mitral regurgitation; NS, Nonsignificant.
Figure 1.
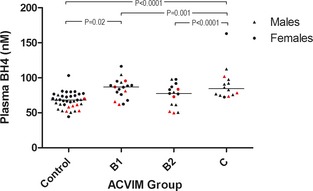
Plots of plasma tetrahydrobiopterin (BH4) concentrations and three different measurements of myxomatous mitral valve disease severity. Red symbols indicate dogs from homes with smoking. ▲, males; ●, females. Medians are indicated with horizontal lines. Horizontal bars indicate statistically significant comparisons and their P values. Plasma BH4 concentrations were significantly higher in group C compared with all other groups and in group B1 compared with the control group. Males and dogs from homes with smoking had significantly lower plasma BH4 concentrations. ACVIM, American College of Veterinary Internal Medicine.
Figure 2.

Plots of plasma dihydrobiopterin (BH2) concentrations and three different measurements of myxomatous mitral valve disease severity. Medians are indicated with horizontal lines. Horizontal bars indicate statistically significant comparisons and their P values. Plasma BH2 concentrations were significantly higher in group C compared with all other groups. ACVIM, American College of Veterinary Internal Medicine.
Figure 3.
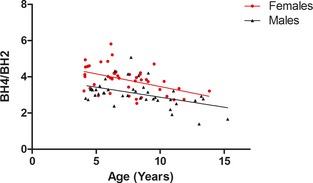
Plot of plasma BH4/BH2 ratio as a function of age with fitted linear regression lines for males and females. ▲, males; ●, females. BH4/BH2 ratio was negatively associated with age. Males had significantly lower BH4/BH2 ratio.
Discussion
In the present study, we found significantly increased concentrations of BH4 and BH2 in dogs in CHF and a positive association with mitral valve disease severity, suggesting development of altered endothelial function and an association with oxidative stress as disease advances. Furthermore, several cardiovascular risk factors in humans including age, sex, smoking, BW, BCS, and serum cholesterol concentration influenced biopterin status in dogs.
The presence of increased plasma BH2 concentration in relation to CHF suggests that oxidative stress and ED are present in dogs in the late stages of MMVD. This finding agrees with findings of a study in humans in which a negative relationship between flow‐mediated vasodilatation (FMD) and plasma BH2 concentration was found.41 Increased oxidative stress has been shown to be involved in the pathogenesis and progression of ED42 because eNOS‐mediated NO production in the endothelium decreases as BH4 is oxidized to BH2 in exchange for increased superoxide production.28, 29
However, the observed increasing plasma concentrations of BH4 with increasing disease severity and in CHF dogs were unexpected. Although higher plasma BH4 concentrations were found in these dogs, the ability to produce NO may not be increased equally because BH2 has been shown to competitively bind to eNOS.29 BH4 concentrations most likely reflect the complex balance among compensatory de novo synthesis, oxidative loss, salvage pathways, and transport between extra‐ and intracellular compartments.25 Thus, a possible explanation for increased BH4 concentrations may be an adaptive increase in synthesis with disease progression perhaps in response to receptor downregulation, decreased receptor sensitivity, or decreased bioavailability. Another possibility is that increased plasma BH4 concentrations result from increased recycling from BH2 as disease develops.25 It could also be speculated that a leakage of BH4 from the endothelium occurs as disease progresses and that increased plasma BH4 concentrations thus reflect decreased intracellular concentrations of BH4. This hypothesis is supported by a study in humans that found decreased endothelial function in patients with high plasma BH4 concentrations and an inverse relationship between plasma and vascular BH4.43
Increasing age was shown to be associated with increased BH2 and decreased BH4/BH2 ratio. This is an expected finding because both MMVD and ED in dogs are conditions that progress with age.4, 24 Similarly, age and BH4/BH2 ratio have been suggested to be negatively associated in a study in humans.41 In older individuals, an age‐associated accumulated oxidative damage may have influenced the results.44
Females had significantly higher plasma BH4 concentrations and BH4/BH2 ratio than males, similar to findings in humans, suggesting that female sex has a protective effect on oxidative stress and ED.41 This finding supports other studies in dogs that have observed more severe MMVD in male dogs than in females of the same age4, 45, 46 and a study in humans that found decreased FMD in male patients.12
In this study, BW was negatively associated with BH4 consistent with the risk profile in humans of obesity as a risk factor in cardiovascular disease.14, 41 Accordingly, BCS, which better describes obesity or underweight body condition, also was associated with plasma BH4 concentration because 1 statistical model found significantly lower BH4 concentrations in dogs with BCS 6 compared with BCS 5. Dogs included in this study were estimated as having BCSs between 3 and 7 (with only 1 dog categorized as BCS 3), meaning that no severely obese or cachexic dogs were included.
Active smoking and passive smoking represent cardiovascular risk factors in humans and have been shown to be associated with plasma biopterin status41 and ED in human patients. 11, 12, 14, 47, 48 We found that dogs from homes with indoor smoking had significantly lower plasma BH4 concentrations, suggesting that a higher degree of BH4 oxidation occurs with passive smoking exposure in dogs. The importance of passive smoking as a risk factor for disease progression in dogs is unknown. To the authors' knowledge, circulating concentrations of BH4 have not been evaluated in passive smoking in humans.
Hypercholesterolemia represents another cardiovascular risk factor in humans, but there are discrepancies in research concerning the association with ED.12, 13, 14 In this study, serum cholesterol concentrations correlated positively with plasma BH4 and BH2 concentrations in dogs, but none of the dogs had severe hypercholesterolemia. Furthermore, the lipoprotein composition in dogs differs from that of humans.49 In dogs, the high‐density lipoproteins are predominant and the main carriers of cholesterol, whereas in humans, low‐density lipoproteins constitute the major lipoprotein.50, 51 This species difference may be of importance and it is uncertain whether or not hypercholesterolemia is a cardiovascular risk factor in dogs.
Limitations of the study include the use of privately owned dogs because potential selection bias cannot be avoided. Although the CKCSs were selected at random in a database, this database may not be representative of the CKCSs population because it is mainly based on dogs used for breeding. Different dietary regimens and dietary supplements may have influenced results because commercial dog foods are enriched with different amounts of antioxidants. Although all dogs had been fasted 6–18 hours before examination, the exact duration of fasting could have influenced results. Moreover, it cannot be ruled out that cardiac treatment in group C, which for a number of dogs included angiotensin‐converting enzyme inhibitors, pimobendan, or both, could have influenced ED and oxidative stress and thereby BH2 and BH4 concentrations because these substances have been suggested to alter NO availability.52, 53 However, for ethical reasons, we did not attempt to stop or alter treatment. Finally, minor deviations from reference in CBC and serum biochemistry results among dogs in group C may be of importance because plasma BH2 concentration has been shown to be increased and BH4/BH2 ratio decreased with increasing severity of renal failure in humans.54 Hence, we cannot discount the possibility that renal status might have influenced the results.
Conclusion
Myxomatous mitral valve disease severity and certain cardiovascular risk factors of humans including age, sex, BW, BCS, and passive smoking are associated with biopterin status in dogs, indicating the presence of ED and oxidative stress. Additional studies are warranted to investigate the clinical implications of these findings.
Acknowledgments
The authors thank Christina Tirsdal Kjempff, Joan Elisabeth Frandsen, and Susanne Kronborg at the Department of Veterinary Disease Biology and Dennis Jensen at the Department of Veterinary Clinical and Animal Sciences, University of Copenhagen, Denmark, for excellent technical assistance. The study was supported financially by fellowship grants from the LifePharm Centre for In Vivo Pharmacology to MJR and AM and grants from the Danish National Research Council (Project no. 271‐08‐0998) and Simon Fougher Hartmann's family Foundation.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
This study was performed at the Departments of Veterinary Clinical and Animal Sciences and Veterinary Disease Biology, University of Copenhagen, Denmark, and Din Veterinär Animal Hospital, Helsingborg, Sweden.
Preliminary results were presented as an oral presentation at the 2013 European College of Veterinary Internal Medicine—Companion Animals (ECVIM‐CA) Forum, Liverpool, 12–14th September 2013.
Footnotes
Vivid i echocardiograph; GE‐medical, Milwaukee, WI
EchoPAC PC. Version 112; GE Vingmed Ultrasound AS, Horten, Norway
R studio, version 0.97.336, © 2009‐2012 RStudio, Inc., Boston, MA
References
- 1. Detweiler DK, Patterson DF. The prevalence and types of cardiovascular disease in dogs. Ann N Y Acad Sci 1965;127:481–516. [DOI] [PubMed] [Google Scholar]
- 2. Buchanan JW. Chronic valvular disease (endocardiosis) in dogs. Adv Vet Sci Comp Med 1977;21:75–106. [PubMed] [Google Scholar]
- 3. Egenvall A, Bonnett BN, Haggstrom J. Heart disease as a cause of death in insured swedish dogs younger than 10 years of age. J Vet Intern Med 2006;20:894–903. [DOI] [PubMed] [Google Scholar]
- 4. Haggstrom J, Hansson K, Kvart C, Swenson L. Chronic valvular disease in the Cavalier King Charles Spaniel in Sweden. Vet Rec 1992;131:549–553. [PubMed] [Google Scholar]
- 5. Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med 2009;23:1142–1150. [DOI] [PubMed] [Google Scholar]
- 6. Cunningham SM, Rush JE, Freeman LM. Systemic inflammation and endothelial dysfunction in dogs with congestive heart failure. J Vet Intern Med 2012;26:547–557. [DOI] [PubMed] [Google Scholar]
- 7. Nakamura M, Yoshida H, Arakawa N, et al. Endothelium‐dependent vasodilatation is not selectively impaired in patients with chronic heart failure secondary to valvular heart disease and congenital heart disease. Eur Heart J 1996;17:1875–1881. [DOI] [PubMed] [Google Scholar]
- 8. Fischer D, Rossa S, Landmesser U, et al. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur Heart J 2005;26:65–69. [DOI] [PubMed] [Google Scholar]
- 9. Sugamura K, Keaney JF Jr. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med 2011;51:978–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heitzer T, Schlinzig T, Krohn K, et al. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 2001;104:2673–2678. [DOI] [PubMed] [Google Scholar]
- 11. Ruef J, Marz W, Winkelmann BR. Markers for endothelial dysfunction, but not markers for oxidative stress correlate with classical risk factors and the severity of coronary artery disease. (A subgroup analysis from the Ludwigshafen Risk and Cardiovascular Health Study). Scand Cardiovasc J 2006;40:274–279. [DOI] [PubMed] [Google Scholar]
- 12. Celermajer DS, Sorensen KE, Bull C, et al. Endothelium‐dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol 1994;24:1468–1474. [DOI] [PubMed] [Google Scholar]
- 13. Egashira K, Inou T, Hirooka Y, et al. Impaired coronary blood flow response to acetylcholine in patients with coronary risk factors and proximal atherosclerotic lesions. J Clin Invest 1993;91:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benjamin EJ, Larson MG, Keyes MJ, et al. Clinical correlates and heritability of flow‐mediated dilation in the community: The Framingham Heart Study. Circulation 2004;109:613–619. [DOI] [PubMed] [Google Scholar]
- 15. Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow‐mediated vasodilatation of brachial artery: A meta‐analysis. Int J Cardiovasc Imaging 2010;26:631–640. [DOI] [PubMed] [Google Scholar]
- 16. Prasad K, Gupta J, Kalra J, et al. Oxidative stress as a mechanism of cardiac failure in chronic volume overload in canine model. J Mol Cell Cardiol 1996;28:375–385. [DOI] [PubMed] [Google Scholar]
- 17. Nishijima Y, Sridhar A, Bonilla I, et al. Tetrahydrobiopterin depletion and NOS2 uncoupling contribute to heart failure‐induced alterations in atrial electrophysiology. Cardiovasc Res 2011;91:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Freeman LM, Rush JE, Milbury PE, Blumberg JB. Antioxidant status and biomarkers of oxidative stress in dogs with congestive heart failure. J Vet Intern Med 2005;19:537–541. [DOI] [PubMed] [Google Scholar]
- 19. Pedersen HD, Schutt T, Sondergaard R, et al. Decreased plasma concentration of nitric oxide metabolites in dogs with untreated mitral regurgitation. J Vet Intern Med 2003;17:178–184. [DOI] [PubMed] [Google Scholar]
- 20. de Laforcade AM, Freeman LM, Rush JE. Serum nitrate and nitrite in dogs with spontaneous cardiac disease. J Vet Intern Med 2003;17:315–318. [DOI] [PubMed] [Google Scholar]
- 21. Pedersen LG, Tarnow I, Olsen LH, et al. Body size, but neither age nor asymptomatic mitral regurgitation, influences plasma concentrations of dimethylarginines in dogs. Res Vet Sci 2006;80:336–342. [DOI] [PubMed] [Google Scholar]
- 22. Moesgaard SG, Falk T, Teerlink T, et al. Brain‐natriuretic peptide and cyclic guanosine monophosphate as biomarkers of myxomatous mitral valve disease in dogs. Vet J 2011;189:349–352. [DOI] [PubMed] [Google Scholar]
- 23. Jones ID, Fuentes VL, Boswood A, et al. Ultrasonographic measurement of flow‐mediated vasodilation in dogs with chronic valvular disease. J Vet Cardiol 2012;14:203–210. [DOI] [PubMed] [Google Scholar]
- 24. Moesgaard SG, Klostergaard C, Zois NE, et al. Flow‐mediated vasodilation measurements in Cavalier King Charles Spaniels with increasing severity of myxomatous mitral valve disease. J Vet Intern Med 2012;26:61–68. [DOI] [PubMed] [Google Scholar]
- 25. Schmidt TS, Alp NJ. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci (Lond) 2007;113:47–63. [DOI] [PubMed] [Google Scholar]
- 26. Tayeh MA, Marletta MA. Macrophage oxidation of L‐arginine to nitric oxide, nitrite, and nitrate. Tetrahydrobiopterin is required as a cofactor. J Biol Chem 1989;264:19654–19658. [PubMed] [Google Scholar]
- 27. Mortensen A, Lykkesfeldt J. Does vitamin C enhance nitric oxide bioavailability in a tetrahydrobiopterin‐dependent manner? In vitro, in vivo and clinical studies. Nitric Oxide 2014;36:51–57. [DOI] [PubMed] [Google Scholar]
- 28. Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: Implications for vascular endothelial function. Biochem Biophys Res Commun 1999;263:681–684. [DOI] [PubMed] [Google Scholar]
- 29. Vasquez‐Vivar J, Martasek P, Whitsett J, et al. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: An EPR spin trapping study. Biochem J 2002;362:733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 2003;111:1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stroes E, Hijmering M, van Zandvoort M, et al. Origin of superoxide production by endothelial nitric oxide synthase. FEBS Lett 1998;438:161–164. [DOI] [PubMed] [Google Scholar]
- 32. Ettinger SJ. The physical examination of the dog and cat In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine: Diseases of the Dog and the Cat, 7th ed St. Louis, MO: Saunders Elsevier; 2010:1–9. [Google Scholar]
- 33. Gompf RE. The clinical approach to heart disease: History and physical examination In: Fox PR, ed. Canine and Feline Cardiology. New York, NY: Churchill Livingstone; 1988:29–42. [Google Scholar]
- 34. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007;21:542–558. [DOI] [PubMed] [Google Scholar]
- 35. Reimann MJ, Moller JE, Haggstrom J, et al. R‐R interval variations influence the degree of mitral regurgitation in dogs with myxomatous mitral valve disease. Vet J 2014;199:348–354. [DOI] [PubMed] [Google Scholar]
- 36. Mortensen A, Lykkesfeldt J. Kinetics of acid‐induced degradation of tetra‐ and dihydrobiopterin in relation to their relevance as biomarkers of endothelial function. Biomarkers 2013;18:55–62. [DOI] [PubMed] [Google Scholar]
- 37. Fukushima T, Nixon JC. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal Biochem 1980;102:176–188. [DOI] [PubMed] [Google Scholar]
- 38. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 39. Haggstrom J, Hansson K, Karlberg BE, et al. Plasma concentration of atrial natriuretic peptide in relation to severity of mitral regurgitation in Cavalier King Charles Spaniels. Am J Vet Res 1994;55:698–703. [PubMed] [Google Scholar]
- 40. Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Vet Intern Med 2004;18:311–321. [DOI] [PubMed] [Google Scholar]
- 41. Takeda M, Yamashita T, Shinohara M, et al. Plasma tetrahydrobiopterin/dihydrobiopterin ratio: A possible marker of endothelial dysfunction. Circ J 2009;73:955–962. [DOI] [PubMed] [Google Scholar]
- 42. Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ Res 2000;87:840–844. [DOI] [PubMed] [Google Scholar]
- 43. Antoniades C, Shirodaria C, Crabtree M, et al. Altered plasma versus vascular biopterins in human atherosclerosis reveal relationships between endothelial nitric oxide synthase coupling, endothelial function, and inflammation. Circulation 2007;116:2851–2859. [DOI] [PubMed] [Google Scholar]
- 44. Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A 1993;90:7915–7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Swenson L, Haggstrom J, Kvart C, Juneja R. Relationship between parental cardiac status in Cavalier King Charles Spaniels and prevalence and severity of chronic valvular disease in offspring. J Am Vet Med Assoc 1996;208:2009–2012. [PubMed] [Google Scholar]
- 46. Olsen LH, Fredholm M, Pedersen HD. Epidemiology and inheritance of mitral valve prolapse in Dachshunds. J Vet Intern Med 1999;13:448–456. [DOI] [PubMed] [Google Scholar]
- 47. Shinozaki K, Hirayama A, Nishio Y, et al. Coronary endothelial dysfunction in the insulin‐resistant state is linked to abnormal pteridine metabolism and vascular oxidative stress. J Am Coll Cardiol 2001;38:1821–1828. [DOI] [PubMed] [Google Scholar]
- 48. Celermajer DS, Adams MR, Clarkson P, et al. Passive smoking and impaired endothelium‐dependent arterial dilatation in healthy young adults. N Engl J Med 1996;334:150–154. [DOI] [PubMed] [Google Scholar]
- 49. Maldonado EN, Romero JR, Ochoa B, Aveldano MI. Lipid and fatty acid composition of canine lipoproteins. Comp Biochem Physiol B Biochem Mol Biol 2001;128:719–729. [DOI] [PubMed] [Google Scholar]
- 50. Lehmann R, Bhargava AS, Gunzel P. Serum lipoprotein pattern in rats, dogs and monkeys, including method comparison and influence of menstrual cycle in monkeys. Eur J Clin Chem Clin Biochem 1993;31:633–637. [DOI] [PubMed] [Google Scholar]
- 51. Mahley RW, Weisgraber KH. Canine lipoproteins and atherosclerosis. I. Isolation and characterization of plasma lipoproteins from control dogs. Circ Res 1974;35:713–721. [DOI] [PubMed] [Google Scholar]
- 52. Mombouli JV, Illiano S, Nagao T, et al. Potentiation of endothelium‐dependent relaxations to bradykinin by angiotensin I converting enzyme inhibitors in canine coronary artery involves both endothelium‐derived relaxing and hyperpolarizing factors. Circ Res 1992;71:137–144. [DOI] [PubMed] [Google Scholar]
- 53. Iwasaki A, Matsumori A, Yamada T, et al. Pimobendan inhibits the production of proinflammatory cytokines and gene expression of inducible nitric oxide synthase in a murine model of viral myocarditis. J Am Coll Cardiol 1999;33:1400–1407. [DOI] [PubMed] [Google Scholar]
- 54. Yokoyama K, Tajima M, Yoshida H, et al. Plasma pteridine concentrations in patients with chronic renal failure. Nephrol Dial Transplant 2002;17:1032–1036. [DOI] [PubMed] [Google Scholar]


