Abstract
Background
The duration of antacid‐induced hypergastrinemia after cessation of administration of omeprazole and famotidine apparently has not been determined in dogs.
Hypothesis
That serum gastrin will return to basal concentrations by 7 days after cessation of famotidine or omeprazole administration.
Animals
Nine healthy, adult, male, research colony dogs.
Methods
Randomized, cross‐over design. Serum gastrin was determined daily for 7 days to establish baseline concentrations. Famotidine (1.0 mg/kg q24h) or omeprazole (1.0 mg/kg q24h) was administered PO for 7 days followed by a 14‐day washout. Serum concentrations of gastrin were determined daily during 7 days of administration and daily for 7 days after cessation of administration. Each drug was evaluated in 8 of the 9 dogs.
Results
Omeprazole caused a significant increase in serum gastrin concentration (37.2 ± 7.3 to 71.3 ± 19.0 ng/L; P = .006). Famotidine induced a transient increase in serum gastrin (37.2 ± 7.3 to 65.5 ± 38.5 ng/L; P = .02) that peaked at administration day 3 and declined thereafter. By day 7 after cessation of both drugs, there was no difference in serum gastrin concentrations compared to those before administration (famotidine P = .99; omeprazole P = .99). During or after administration, gastrin concentrations above 3 times the upper reference range were rare (12 of 224 samples).
Conclusions and Clinical Importance
A 7‐day withdrawal from short‐term administration of famotidine or omeprazole is sufficient for serum gastrin to return to baseline concentrations. Withholding famotidine or omeprazole for longer before investigating pathologic causes of hypergastrinemia is unnecessary.
Keywords: Antacid, Gastrinoma, Hypergastrinemia, Ulcer, Vomiting
Abbreviations
- AUC
area under the curve
- Cmax
maximum concentration
- H2‐RA
histamine type 2‐receptor antagonist
- MSU
Michigan State University
- PPI
proton‐pump inhibitor
Medications that suppress gastric acid secretion, such as famotidine, a histamine type 2‐receptor antagonist (H2‐RA), or omeprazole, a proton‐pump inhibitor (PPI), are often used in dogs for empirical treatment of vomiting, gastroduodenitis, esophagitis, and prevention of gastrointestinal ulcers.1, 2, 3, 4 When the condition is not self‐limiting or when a definitive diagnosis is not obtained by performing routine diagnostics, uncommon causes of vomiting, regurgitation, or gastrointestinal ulceration, such as a gastrinoma, may be considered. Gastrinomas are non‐β islet cell neuroendocrine tumors that produce abnormal amounts of gastrin.5 The primary action of gastrin is to stimulate gastric acid secretion by the parietal cells of the stomach.6 The diagnosis of a gastrinoma is made by documenting hypergastrinemia, gastrointestinal ulceration, and by detection of a neuroendocrine tumor.4, 7 Because the tumor itself can be difficult to detect, a tentative diagnosis of gastrinoma is sometimes made on the basis of compatible clinical and laboratory findings, and the finding of hypergastrinemia. Dogs with confirmed gastrinomas typically have serum gastrin concentrations greater than 3 times (often 10–200 times) the upper end of the reference range.4, 7
Histamine type 2‐receptor antagonists and PPIs suppress gastric acid secretion. It is the acidic gastric environment that normally would inhibit excessive gastrin secretion.8, 9 Because of the lack of negative feedback caused by pharmacologic suppression of gastric acid secretion, gastrin increases, resulting in hypergastrinemia. Hypergastrinemia could be the cause of the clinical signs, rather than merely the result of the empirical antacid administration. This raises the question of when the finding of hypergastrinemia should be considered abnormal rather than a physiologic response to the drug. To answer the question for an individual animal, administration of the drug must be discontinued.9 In so doing, its beneficial effects will be lost, perhaps to the detriment of the patient. Appropriate withdrawal times after treatment with H2‐RAs or PPIs to assure that serum gastrin has returned to basal concentrations apparently have not been determined for dogs. The current veterinary recommendation1 to abstain from antacid treatment for 14 days before measuring fasting serum gastrin concentrations was extrapolated from human medicine.10 The purpose of this study was to determine if a 7‐day withdrawal time from famotidine and from omeprazole is sufficient for serum gastrin to return to basal concentrations in normal dogs.
Materials and Methods
A randomized, cross‐over study was performed at the Michigan State University (MSU) College of Veterinary Medicine. All procedures were approved by the MSU Institutional Animal Care and Use Committee. The study population consisted of 9 research colony dogs. Breeds included 6 Beagles, 2 Cardigan Welsh Corgis, and 1 Beagle‐Briard cross. The dogs ranged in age from 1.5 to 6.5 years (median, 4.5 years) and all were intact males. Weights ranged from 11.3 to 23.6 kg (median, 14.0 kg). All dogs were determined to be healthy on the basis of normal results of physical examination, CBC, serum biochemical profile and urinalysis.
The dogs were acclimated to once daily am feedings for their entire lives. Throughout the study, all samples were collected between 8:00 and 9:00 am (after withholding food for 24 hours), before administration of medication and before the am feeding. Blood was collected into serum tubes (no serum separator gel) from the jugular or cephalic veins. Samples were allowed to clot for 30 minutes at room temperature and centrifuged at 1,350 × g for 15 minutes with an LW Scientific Ultra 8F centrifuge. The serum was harvested and stored at −80°C until analysis by the MSU Diagnostic Center for Population and Animal Health Endocrinology Laboratory, using a [125I] radioimmunoassay2 for gastrin. The assay had previously been validated for use with canine serum by measuring circulating concentrations of gastrin with assay procedures performed per manufacturer's protocol. Synthetic human gastrin17‐I standards were used to make the displacement curve, with the highest standard of 1,000 ng/L. The manufacturer‐reported percent cross‐reactivity with related compounds was gastrin 17‐I (100%), gastrin 17‐II (77%), gastrin 34‐I (42%), gastrin 5‐17 (54%), cholecystokinin‐PZ (<0.1%), cholecystokinin‐8 (10.9%). The manufacturer‐reported sensitivity of detection was 3 ng/L. For intra‐assay repeatability (12 replicates), the % coefficients of variation were 7.3%, 6.8%, and 4.9% for canine samples with respective gastrin concentrations of 48, 53, and 93 ng/L. For interassay repeatability (10 assays), the % coefficients of variation were 11.4%, 7.8%, and 11.8% for canine sample pools with respective gastrin concentrations of 59, 92, and 322 ng/L. A canine sample pool with a high concentration of gastrin (322 ng/L) was diluted with 0 standard at rates of 1:2, 1:4, 1:8, 3:8, 5:8, 1:20, and 19:20. The respective recovery rates (% observed/expected) at these dilutions were 108%, 107%, 117%, 96%, 105%, 118%, and 99%.
Blood was collected daily for 7 days to establish baseline serum gastrin concentrations. The dogs were randomly assigned to receive either famotidine3 (1 mg/kg, PO, q24h) or omeprazole4 (1 mg/kg, PO, q24h) for 7 days, followed by a 14‐day washout period, followed by the opposite drug for 7 days. For more accurate dosing the omeprazole was dissolved in an 8.4% sodium bicarbonate solution to a concentration of 20 mg of omeprazole/84 mg of sodium bicarbonate per mL as previously described for pediatric patients.11, 12, 13 The omeprazole dose ranged from 0.9 to 1.1 mg/kg for individual dogs, with a group mean dose of 1.0 mg/kg. The 20 mg famotidine tablets were quartered, and dosed to the nearest 5 mg. The famotidine dose ranged from 0.9 to 1.1 mg/kg for individual dogs, with a group mean dose of 1 mg/kg. The drugs were administered PO without the aid of food treats. After sample collection and drug administration, dogs were returned to their kennels and fed as usual.5 The time between drug administration and feeding was approximately 15–30 minutes. Serum gastrin concentrations were determined daily during administration of each drug to confirm that each drug induced hypergastrinemia. Daily sample collection continued for 7 days after cessation of each medication to evaluate the withdrawal time period.
Because the gastrin assays were run in batches, the study was under way before it was discovered that from the onset one of the dogs was persistently and significantly hypergastrinemic (average baseline [7 days] gastrin concentration 329 ng/L, range 80–453 ng/L; reference range 10–40 ng/L). This dog was dropped from the study and was replaced by 2 additional dogs. As with the other dogs, blood was collected daily for 7 days to establish baseline serum gastrin concentrations. Thus, baseline data were from 9 normal dogs. However, one of the additional 2 dogs was randomly assigned to receive only famotidine and the other received only omeprazole. Doing so was justifiable because the duration of hypergastrinemic effects of each of the 2 drugs was separately evaluated in 8 dogs over time. The 2 drugs’ effects were not compared to each other. Replacing 1 dog that would have received both drugs with 2 dogs that would each receive only 1 drug shortened the study time for these 2 dogs by 28 days and enabled the study to be completed on time. Thus, 8 normal dogs received famotidine and 8 normal dogs received omeprazole, and generated the treatment and withdrawal data.
Statistics
Gastrin concentrations from the 7 days before drug administration were averaged for all dogs to generate a baseline value denoted as Day 0. Daily serum gastrin concentrations over the 7‐day administration protocol were compared to this baseline Day 0 value using a one‐tailed, linear contrast. In this analysis, data from all 7 days of during drug administration were considered an equally weighted block of data that was compared to the baseline Day 0 value using a linear contrast matrix of the form [−7,1,1,1,1,1,1,1]. In addition, serum gastrin concentrations on withdrawal day 7, the single day, were compared to baseline Day 0 using a two‐tailed linear contrast. Results are reported as the mean and standard deviation. For all tests, the nominal type‐1 error rate of 5% (P ≤ .05) was considered the threshold for statistical significance. Statistics were performed using a data analysis software system.6
Results
The reference range for dogs for serum concentrations of gastrin was 10–40 ng/L. The mean (±SD) baseline serum gastrin concentration (Day 0) of the dogs (n = 9) was 37.2 ± 7.3 ng/L, with a range of 18–116 ng/L. Three of the 9 dogs had serum concentrations of gastrin within the reference range on all 7 days before drug administration. On 14 of 63 occasions during the 7 days before drug administration, gastrin concentrations were above the reference range: twice for Dog 1 (101 and 116 ng/L), once for Dog 4 (42 ng/L), 3 times for Dog 5 (41, 46, and 87 ng/L), twice for Dog 6 (41 and 47 ng/L), 3 times for Dog 7 (50, 55, and 77 ng/L), and 3 times for Dog 8 (45, 55, and 59 ng/L).
Serum gastrin concentration increased during 7 days of famotidine administration (P = .02; Fig 1) though the effect peaked by day 3, declining back toward baseline values during the remainder of administration. By day 7 of administration, concentrations were similar to baseline. The mean (±SD) serum gastrin concentration of the dogs (n = 8) during administration of famotidine was 43.6 ± 8.2 ng/L, with a range of 19–152 ng/L. All but 1 dog (Dog 8) had gastrin concentrations within the reference range on 1 or more days during administration of famotidine. During famotidine administration Dog 8's gastrin concentrations ranged from 41 to 61 ng/L. On 26 of 56 occasions during 7 days of administration, gastrin concentrations above 40 ng/L (41–152 ng/L) occurred at least once for each dog. Serum concentrations of gastrin above 120 ng/L (3 times the upper limit of the reference range of 40 ng/L) occurred once (Dog 1, 152 ng/L). The mean serum gastrin concentration of the dogs (n = 8) during withdrawal from famotidine was 39.9 ± 2.7 ng/L. When comparing the withdrawal data to day 7 of famotidine administration, there was no significant difference (P = .29) in serum gastrin concentrations. This was because of the transient nature of the rise in gastrin levels during drug administration. During the 7 days after famotidine administration (withdrawal), serum gastrin concentrations ranged from 15 to 126 ng/L. On 20 of 56 occasions, gastrin concentrations above 40 ng/L (42–126 ng/L) occurred at least once for each dog. Serum gastrin concentration above 120 ng/L occurred once (Dog 1, 126 ng/L). Specifically comparing serum gastrin concentrations on famotidine withdrawal day 7 (the single day) with baseline Day 0, there was no significant difference (P = .99; Fig 1).
Figure 1.
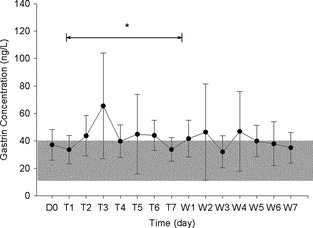
Mean (±SD) serum gastrin concentrations from 9 dogs at baseline (D0), and from 8 dogs during famotidine administration (Days T1–T7) and withdrawal (Days W1–W7). The line indicates the days of drug administration data considered as a block. *Indicates significant difference between baseline and during drug administration, and between withdrawal and during drug administration. Shaded area represents reference range of 10–40 ng/L.
Administration of omeprazole was associated with a statistically significant increase in serum gastrin concentrations (P = .006; Fig 2). The mean serum gastrin concentration of the dogs (n = 8) during omeprazole administration was 71.3 ± 19.0 ng/L, with a range of 16–280 ng/L. All but one dog (Dog 3) had gastrin concentrations within the reference range on at least 1 day during omeprazole administration. On 34 of 56 occasions during 7 days of drug administration, serum gastrin concentrations above 40 ng/L occurred at least once for each dog. On all 7 days during omeprazole administration, Dog 3 had serum gastrin concentrations above 40 ng/L (46–163 ng/L). On 9 of the 34 occasions during omeprazole administration when gastrin concentrations exceeded the reference range, they did so by more than 3 times the upper limit. In other words, they were greater than 120 ng/L: twice for Dog 1 (144 and 280 ng/L), 3 times for Dog 3 (124, 129, and 163 ng/L), once for Dog 4 (121 ng/L), and three times for Dog 8 (131, 167, and 203 ng/L). When comparing withdrawal data to day 7 of omeprazole administration, there was a significant reduction (P = .004) in serum gastrin concentrations. The mean serum gastrin concentration of the dogs (n = 8) during withdrawal from omeprazole was 41.8 ± 8.8 ng/L. During the 7 days after omeprazole administration (withdrawal), serum gastrin concentrations ranged from 15 to 127 ng/L. On 22 of 56 occasions during withdrawal, serum gastrin concentrations above 40 ng/L (41–127 ng/L) occurred in 6 of the 8 dogs. Serum gastrin concentration above 120 ng/L occurred once (Dog 1, 127 ng/L). Two dogs (Dog 4 and Dog 6) had gastrin concentrations within the reference range (10–40 ng/L) on all 7 days after omeprazole administration. Cessation (withdrawal) of omeprazole administration was associated with a reduction in serum gastrin levels that was not statistically different from Day 0 baseline values (P = .25). Specifically comparing serum gastrin concentrations on withdrawal Day 7 (the single day) with baseline Day 0, there was no significant difference in (P = .99; Fig 2).
Figure 2.
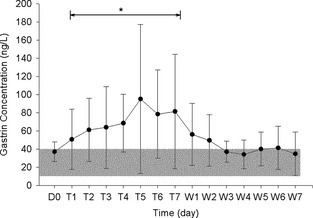
Mean (±SD) serum gastrin concentrations from 9 dogs at baseline (D0), and from 8 dogs during omeprazole administration (Days T1–T7) and withdrawal (Days W1–W7). The line indicates the days of drug administration data considered as a block. *Indicates significant differences between baseline and during drug administration, and between withdrawal and during drug administration. Shaded area represents reference range of 10–40 ng/L.
Discussion
This study shows that in healthy dogs serum gastrin concentrations returned to baseline values within 7 days after short‐term administration of either famotidine or omeprazole was discontinued. Specifically, serum gastrin increased significantly during administration of omeprazole, decreased after administration, and had returned to basal concentrations by day 7 of withdrawal. Serum gastrin concentrations increased significantly during administration of famotidine, began to decline by day 4 during administration, thereafter were similar to baseline values, and had returned to baseline concentrations by day 7 of withdrawal. These findings indicate that the recommended 14‐day withdrawal from short‐term treatment with either famotidine or omeprazole for accurate determination of endogenous fasting serum gastrin concentrations is unnecessarily long for healthy dogs. This has clinical importance for dogs with hypergastrinemia because of gastrinoma, which because of their rarity, are often empirically administered antacids while more common causes of gastroenteritis or ulcers are being excluded. It has been recommended that gastric acid suppressing drugs, which also cause hypergastrinemia, be discontinued to pursue the diagnosis of a gastrinoma.7, 10 Discontinuation of antacid treatment from a dog with an as‐yet‐undiagnosed gastrinoma can result in recurrence of clinical signs and patient discomfort, and increase the possibility of gastric ulceration and perforation.4, 14 Therefore, a short withdrawal time is highly desirable.
Because the anticipation of a meal (cephalic phase) as well as the presence of food in the stomach (gastric phase) stimulate gastrin secretion, substantial inter‐ and intraindividual variation in serum gastrin concentrations is not surprising. Although the effects of the gastric phase can be managed by withholding food, the cephalic phase cannot. This is true in research as well as clinical settings. Fourteen of the 63 baseline samples from these healthy dogs were above the upper end of the reference range of 40 ng/L (47–116 ng/L). The findings of this study might suggest therefore that gastrin concentrations of this magnitude are not necessarily abnormal. They occur sporadically in healthy dogs.
The presence or absence of food in the stomach not only affects gastrin release but also affects absorption or bioavailability of some drugs. The purpose of this study was not to evaluate the magnitude of famotidine or omeprazole effects but rather to determine if their hypergastrinemic effects had abated by 7 days after drug administration. Had not the drugs first induced a significant increase in gastrin during administration, there would be no need for a withdrawal period before evaluating endogenous gastrin concentrations. To minimize uncertainty regarding the presence of food, food was withheld for 24 hours and then a dose‐to‐feeding interval was maintained at 15–30 minutes for both drugs throughout the study. Doing so was probably unnecessarily strict for famotidine because the presence or absence of food in the stomach does not alter famotidine's effectiveness.15 Famotidine has been shown to achieve high serum concentrations when mixed with food,16 to significantly increase serum gastrin concentrations in dogs when administered either 30 minutes before feeding15 or 60 minutes after feeding,15, 17 to significantly increase gastric pH when administered 60 minutes before or 60 minutes after feeding,8 and to decrease the severity and prevalence of exercise‐induced gastritis when administered with meals.16, 17 In a study of famotidine in 11 privately owned dogs maintained at home during the study, serum gastrin concentrations increased significantly.18 There was no standardization of the dose‐feed interval, the frequency of feeding, the type of food fed, or the duration of time food was withheld (which was defined simply as at least 12 hours) before sample correction.18
Although in people, the absorption of omeprazole is delayed by the presence of food in the stomach and the maximum concentration (C max) is decreased when administered with food, there is little change in bioavailability19 or the area under the curve.19, 20 Omeprazole effectively reduces the severity of exercise‐induced gastritis in dogs when administered without regard to the timing of recent or future meals.21 Omeprazole raises gastric pH15 and decreases both the prevalence and severity of exercise‐induced gastritis17 when dogs are feed 30 minutes or within an hour of its administration. Given the diversity of breeds, diets, husbandry conditions, and decades of clinical use in veterinary medicine,1, 2, 3, 4, 8, 14, 15, 16, 17, 18, 21, 22, 23 the results of the present study are likely to applicable to the clinical setting.
Some authors have observed that dogs with confirmed gastrinomas typically have serum gastrin concentrations greater than 3 times the upper end of the reference range.7 It is interesting to note that although in these healthy dogs in which there was a statistically significant increase in serum gastrin concentrations during famotidine and omeprazole administration, on only 12 of 224 occasions both during and after administration were concentrations greater than 3 times the upper limit (40 ng/L) of the reference range, or above 120 ng/L. Dog 1 was the only dog with gastrin concentrations greater than 120 ng/L during (152 ng/L) or after (126 ng/L) treatment with famotidine. During omeprazole administration there were 9 occasions involving 4 dogs (Dogs 1, 3, 4, 8) where serum gastrin concentrations were 121–280 ng/L. One dog (Dog 1), on 1 occasion during the 7 days after omeprazole administration, had a serum gastrin concentration of 127 ng/L. Yet each of these 4 dogs also had gastrin concentrations within the reference range on at least 1 occasion both during and after administration of both famotidine and omeprazole.
The results of the study show that in healthy dogs after food has been withheld for 24 hours gastrin concentrations sporadically (14 of 63 evaluations) exceed the upper limits (40 ng/L) of the reference range, sometimes by nearly 3‐fold (range 41–116 ng/L). They also show that both during and after administration of famotidine and omeprazole gastrin concentrations often (123 of 224 samples) remain in the reference range, and if not, rarely (12 of 224 samples) exceed a 3‐fold increase (range 121–280 ng/L). Extremely high serum concentrations of gastrin, such as are common in dogs with confirmed gastrinomas, did not occur. Serum gastrin concentrations similar to those found in the present study have been reported for dogs before and during oral administration of famotidine, 0.5 mg/kg q12h, for14 days.18 A potential limitation of the study is that drug administration was short term, lasting for only 7 days, whereas in a clinical setting dogs may have received antacids for weeks or months before a diagnosis of gastrinoma is pursued. Long‐term treatment with omeprazole has been documented to delay the diagnosis of gastrinoma in people because it so effectively relieves clinical signs.24 On the other hand, it has also been shown that famotidine administration for 14 days or longer causes only a transient rise in serum concentrations of gastrin in dogs and in people.18, 25 After an initial, transient increase, serum gastrin concentrations in healthy dogs return to normal by day 14 of famotidine administration.18 This is consistent with the findings of the present study. It has been shown in dogs that omeprazole administered PO at 1.5–2.6 mg/kg q24h (which is twice the standard dose23 that was used in the present study) was more effective at maintaining intragastric pH ≥ 3 for a longer duration than the standard dose, and that famotidine given PO at 1.0–1.3 mg/kg q12h, twice the standard dose, was no more effective than placebo.15 Given the direct relationship between increased gastric pH and serum gastrin concentrations,6, 26 their finding that famotidine did not raise gastric pH more than did placebo is consistent with our finding that famotidine induced a transient increase in serum gastrin concentrations, which then declined toward baseline values by treatment‐day 4, despite continuing administration. After administration of a similar total daily omeprazole dose of 1.1 mg/kg, twice daily for 15 days, by 3 days after cessation gastrin concentrations were reportedly no different from baseline values.22 Although postadministration gastrin data were not provided in that abstract, the statement is consistent with the findings of the present study.
In conclusion, this study shows that the often‐recommended 14‐day withdrawal from short‐term use of famotidine or omeprazole before measuring serum gastrin concentrations is unnecessarily long in normal dogs. Furthermore, because during treatment and for 7 days thereafter, serum gastrin concentrations were often (123 of 224 samples) within the reference range and rarely (12 of 224 samples) as high as those reported to be typical of gastrinoma, the results suggest that current or recent treatment with famotidine or omeprazole may not confound the clinical interpretation of serum gastrin concentrations as often or as greatly as has been thought.
Acknowledgments
The authors thank Dr Petersen‐Jones for allowing access to the research dogs and Sharon Steck, Michele Fritz, Janice Querbin, Kristi Aybar and Kristin Koehl, Susan Beyerlein and Dr Patricia Schenk for their technical support. The study was supported by internal funding.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
The work was performed at Michigan State University College of Veterinary Medicine.
Footnotes
MSU Diagnostic Center for Population and Animal Health Endocrinology Laboratory, Lansing, MI
Gastrin [125I] Radioimmunoassay Kit; MP Biomedicals, Orangeburg, NY
Pepcid AC (20 mg tablets); Wackhardt Limited, Mumbai, India
Prilosec–OTC (20 mg tablet); Proctor & Gamble, Cincinnati, OH
Advanced Fitness; Hill's Pet Nutrition, Topeka, KS
StatSoft, Inc. (2010). STATISTICA (data analysis software system), version 9.1. http://www.statsoft.com.
References
- 1. Papich MG. Antiulcer therapy. Vet Clin North Am Small Anim Pract 1993;23:497–512. [DOI] [PubMed] [Google Scholar]
- 2. Neiger R. Gastric Ulceration. Kirk's Current Veterinary Therapy XIV. St. Louis, MO: Saunders; 2009:497–501. [Google Scholar]
- 3. Stanton ME, Bright RM. Gastroduodenal ulceration in dogs. Retrospective study of 43 cases and literature review. J Vet Intern Med 1989;3:238–244. [DOI] [PubMed] [Google Scholar]
- 4. Hughes SM. Canine gastrinoma: A case study and literature review of therapeutic options. N Z Vet J 2006;54:242–247. [DOI] [PubMed] [Google Scholar]
- 5. Zollinger RM, Ellison EH. Primary peptic ulceration of the jejunum associated with islet cell tumors of the pancreas. Ann Surg 1955;142:709–728. [PMC free article] [PubMed] [Google Scholar]
- 6. Guyton and Hall . Secretory functions of the alimentary tract In: Guyton and Hall eds. Textbook of Medical Physiology, 11th ed Philadelphia, PA: Elsevier Inc.; 2006:795–801; 820–821. [Google Scholar]
- 7. Ward CR. Gastrointestinal endocrine disease In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 7th ed St. Louis, MO: WB Saunders; 2010:1857–1865. [Google Scholar]
- 8. Bersenas AME, Mathews KA, Allen DG, Conlon PD. Effects of ranitidine, famotidine, pantoprazole, and omeprazole on intragastric pH in dogs. Am J Vet Res 2005;66:425–431. [DOI] [PubMed] [Google Scholar]
- 9. Pounder R, Smith J. Drug‐induced changes of plasma gastrin concentration. Gastroenterol Clin North Am 1990;19:141–153. [PubMed] [Google Scholar]
- 10. Dhillo WS, Jayasena CN, Lewis CJ, et al. Plasma gastrin measurement cannot be used to diagnose a gastrinoma in patient on either proton pump inhibitors or histamine type‐2 receptor antagonists. Ann Clin Biochem 2006;43:153–155. [DOI] [PubMed] [Google Scholar]
- 11. Quercia RA, Fan C, Liu X, et al. Stability of omeprazole in an extemporaneously prepared oral liquid. Am J Health Syst Pharm 1997;54:1833–1836. [DOI] [PubMed] [Google Scholar]
- 12. DiGiacinto JL, Griener JC, TenEick AP. Stability of suspension formulations of lansoprazole and omeprazole stored in amber‐colored plastic oral syringes. Ann Pharmacother 2000;34:600–605. [DOI] [PubMed] [Google Scholar]
- 13. Nationwide Children's Hospital Pharmacy, Compounding Formulas. http://www.nationwidechildrens.org/outpatient-pharmacy-services. Accessed April 14, 2014.
- 14. Brooks D, Watson GL. Omeprazole in a dog with gastrinoma. J Vet Intern Med 1991;11:379–381. [DOI] [PubMed] [Google Scholar]
- 15. Tolbert K, Bissett S, King A, et al. Efficacy of oral famotidine and 2 omeprazole formulations for the control of intragastric pH in dogs. J Vet Intern Med 2011;25:47–54. [DOI] [PubMed] [Google Scholar]
- 16. Williamson KK, Willard MD, McKenzie EC, et al. Efficacy of famotidine for the prevention of exercise‐induced gastritis in racing Alaskan sled dogs. J Vet Intern Med 2007;21:924–927. [DOI] [PubMed] [Google Scholar]
- 17. Williamson KK, Willard MD, Payton ME, et al. Efficacy of omeprazole versus high‐dose famotidine for prevention of exercise‐induced gastritis in racing Alaskan sled dogs. J Vet Intern Med 2010;24:285–288. [DOI] [PubMed] [Google Scholar]
- 18. Mordecai A, Sellon RK, Mealey KL. Normal dogs treated with famotidine for 14 days have only transient increases in serum gastrin concentrations. J Vet Intern Med 2011;25:1248–1252. [DOI] [PubMed] [Google Scholar]
- 19. Pilbrant A, Cederberg C. Development of an oral formulation of omeprazole. Scand J Gastroenterol 1985;108:113–120. [DOI] [PubMed] [Google Scholar]
- 20. Drug Facts and Comparisons. Philadelphia, PA: Wolters Kluwer; http://www.factsandcomparisons.com. Accessed May 14, 2014. [Google Scholar]
- 21. Davis MS, Willard MD, Nelson SL, et al. Efficacy of omeprazole for the prevention of exercise‐induced gastritis in racing Alaskan sled dogs. J Vet Intern Med 2003;17:163–166. [DOI] [PubMed] [Google Scholar]
- 22. Dossin O, Jones K, Ridgway M, et al. Effect of omeprazole on serum gastrin concentrations and calcium metabolism in healthy dogs In: Proceedings of the ACVIM Forum; 2011 June 15–18; Denver, CO. Malden, MA: Wiley; 2011. Abstract GI‐5. [Google Scholar]
- 23. Plumbs DC. Veterinary Drug Handbook, 7th ed Ames, IA: Wiley‐Blackwell Publishing; 2011:pp 408 and 751. [Google Scholar]
- 24. Wong H, Yau T, Chan P, et al. PPI‐delayed diagnosis of gastrinoma: Oncologic victim of pharmacologic success. Pathol Oncol Res 2009;16:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Komazawa Y, Adachi K, Mihara T, et al. Tolerance to famotidine and ranitidine treatment after 14 days of administration in healthy subjects without Helicobacter pylori infection. J Gastroenterol Hepatol 2003;18:678–682. [DOI] [PubMed] [Google Scholar]
- 26. Simoens C, Woussen‐Colle MC, De Graef J. Effect of cimetidine, ranitidine and omeprazole on postprandial gastrin and somatostatin release in conscious dogs. Regul Pept 1988;22:285–293. [DOI] [PubMed] [Google Scholar]


