Abstract
Background
Canine cutaneous T‐cell lymphoma (CTCL) is an uncommon disease for which efficacious therapies are lacking. The novel anticancer nucleotide prodrug VDC‐1101 (formerly known as GS‐9219) has shown efficacy in dogs with multicentric lymphoma. One of the observed adverse effects with this drug was a skin change characterized by hair loss, erythema, and pruritus, implying delivery of VDC‐1101 to the skin.
Hypothesis/Objectives
The primary study objective was to identify the objective response rate (ORR) to VDC‐1101 in canine CTCL; secondary objectives included characterization of progression‐free survival (PFS) and adverse events (AEs).
Animals
Twelve dogs with chemotherapy‐naïve or relapsed, histologically and immunohistochemically confirmed CTCL.
Methods
Dogs received VDC‐1101 as a 30‐minute IV infusion once every 21 days. Prednisone (1 mg/kg PO q48h) was administered concurrently.
Results
In 11 evaluable patients, responses included 1 complete response (CR), 4 partial responses (PR), 2 stable disease (SD), and 4 progressive disease for an ORR of 45% and biologic response rate (CR/PR/SD) of 64%. The median PFS was 37.5 days (26 to >399 days), which includes 1 durable and ongoing CR (>1 year). Gastrointestinal and hematologic AEs were mild; no dogs developed grade 3 or 4 AEs. Three dogs developed dermatopathies and 1 of these dogs was removed from the study as a result of this AE.
Conclusions and Clinical Importance
VDC‐1101 has activity against canine CTCL and could provide another treatment option in a disease process with a poor prognosis.
Keywords: Cancer, Chemotherapy, Dog, Mycosis fungoides
Abbreviations
- AE
adverse event
- CETL
cutaneous epitheliotropic T cell lymphoma
- CR
complete response
- CTCL
cutaneous T cell lymphoma
- DLT
dose limiting toxicity
- ECOG
Eastern Cooperative Oncology Group
- NHL
non‐Hodgkin's lymphoma
- ORR
objective response rate
- PD
progressive disease
- PFS
progression‐free survival
- PMEG
acyclic nucleotide phosphonate 9‐(2‐phosphonylmethoxyethyl) guanine
- PMEGpp
PMEG disphosphate
- PO
per os
- PR
partial response
- RECIST
Response Evaluation Criteria in Solid Tumors
- SD
stable disease
Canine lymphoma is one of the most common cancers encountered in veterinary oncology, representing 20% of all malignancies in some studies, whereas cutaneous T‐cell lymphoma (CTCL), including both epitheliotropic and nonepitheliotropic forms, represents a less common presentation of the disease,1 with an estimated prevalence of 1% of skin tumors in dogs.2 Cutaneous epitheliotropic T‐cell (CETL) lymphoma represents the majority of cases with estimated reported prevalence of 0.02–0.7%.3, 4, 5 Although most canine multicentric lymphoma patients initially respond to currently available chemotherapy protocols, response rates are considerably lower in patients with CTCL, and generally responses are brief in duration.
Cutaneous T‐cell lymphoma is caused by the infiltration of malignant T cells into the skin. CTCL is a heterogenous group of disorders with a variety of clinical presentations, and consists of nonepitheliotropic and epitheliotropic forms. Clinical presentation is extremely variable. Dogs usually are presented with a history of chronic dermatitis, and mucocutaneous junctions or the oral mucosa sometimes is affected. Involvement of other organs including lymph nodes, viscera, and peripheral blood can be observed in the late stages of disease.1, 6 The Scott classification has been used to describe the clinical presentation of CETL and includes exfoliative erythroderma, plaques or nodules, ulcerative disease of the oral mucosa, and a mucocutaneous form,4, 7 but a more recent publication found frequent overlap of presentations with concurrent presence of all lesion types.5 In this study, the majority of lesions were disseminated over the trunk (83.3%) or localized to the head (63%). The footpads were involved in 26.6% of cases. Pruritus was observed in 40% of cases and lymph node enlargement in 20%.
Several therapies have been evaluated for the treatment of CTCL in dogs, including radiation therapy,8 differentiating agents (eg, retinoids, linoleic acid),9, 10 and cytotoxic chemotherapeutic agents such as lomustine and dacarbazine.11, 12, 13 Although objective response rates (CR/PR) of 45–80% have been reported with these therapies, complete responses (CR) are rare and responses generally are brief in duration. Nearly all dogs with CTCL eventually succumb to progressive disease. Novel therapies for this disease clearly are necessary to improve outcomes.
VDC‐1101,1 previously referred to as GS‐9219, is a double prodrug of the acyclic nucleotide phosphonate 9‐(2‐phosphonylmethoxyethyl) guanine (PMEG), which was designed to preferentially deliver and accumulate PMEG and its active phosphorylated metabolite, PMEG disphosphate (PMEGpp), in lymphoid cells, while avoiding systemic exposure of PMEG.14 Delivery of PMEG/PMEGpp results in cytotoxicity because of inhibition of nuclear DNA polymerases α, δ, and ε.15 PMEG's clinical utility is limited by poor cellular permeability and gastrointestinal and renal toxicity.16, 17, 18 VDC‐1101, however, is hydrolyzed intracellularly to 9‐(2‐phosphonylmethoxyethyl)‐N6‐cyclopropyl‐2,6‐diaminopurine (cPrPMEDAP), deaminated to PMEG and then rapidly converted to PMEGpp.1 In normal laboratory dogs, VDC‐1101 selectively depletes replicating lymphoid tissues at doses that spare other tissues, and demonstrates substantial antineoplastic activity in dogs with naturally occurring non‐Hodgkin lymphoma (NHL).14, 19, 20 One of the observed adverse effects with this drug was a skin change characterized by hair loss, erythema, and pruritus, implying deposition of VDC‐1101 in the skin.
The purpose of this study was to identify the objective response rate (ORR) to VDC‐1101 in canine CTCL; secondary endpoints included the characterization of progression‐free survival (PFS) and adverse events (AEs).
Materials and Methods
Enrollment in this clinical trial was according to a Simon Minimax design, with a rule‐out response probability of 5% and a minimum useful response probability of 25%. Twelve dogs were found to be an adequate number to detect these changes in a pilot efficacy study. The goal of this prospective trial was to complete initial accrual within 9 months with an additional 3 months of follow‐up.
Owners of dogs with CTCL presenting as patients to the Colorado State University Veterinary Medical Center (CSU‐VMC), School of Veterinary Medicine, University of Wisconsin‐Madison (UW‐SVM), University of Georgia Veterinary Teaching Hospital (UGA‐VTH), and Tufts University Foster Hospital (TU‐FH) for Small Animals were offered study entry for treatment with VDC‐1101 under compliance with the Animal Care and Use Committees of all institutions. Signed informed consent was obtained from all owners. Before entry, dogs were evaluated by physical examination, complete blood count (CBC), serum biochemistry profile, urinalysis, and thoracic radiography. Dogs were included if they had a histologic and immunohistochemical diagnosis of CTCL, adequate organ function, and a modified Eastern Comparative Oncology Group (ECOG) constitutional performance score of 0 or 1 on Day 1 (Table 1). Any clinical presentation or histologic form of CTCL was included as long as at least 1 lesion occurred on haired skin. All histopathologic sections were confirmed to be CTCL by positive immunohistochemical staining for CD3. Because histopathologic sections were submitted from several institutions, information regarding specific histologic subtypes was not available.
Table 1.
Modified ECOG performance status. Dogs must have had a performance score of 0 or 1 to be eligible.
| 0 | Normal activity |
| 1 |
Restricted activity Decreased activity from predisease status |
| 2 |
Compromised Ambulatory only for vital activities |
| 3 |
Disabled, needs to be force‐fed Is unable to confine urination and defecation to acceptable areas |
| 4 | Dead |
Concurrent antineoplastic treatment was not allowed, with the exception that all dogs received prednisone (1 mg/kg PO q48h). Previous cytotoxic chemotherapy and radiation treatment was allowed with 3‐week and 6‐week washout periods, respectively. Dogs were excluded if they were receiving homeopathic or alternative therapies. There was a 1‐week washout period for dogs that had received retinoids. Dogs of the West Highland White Terrier Breed were excluded because of their genetic predisposition for pulmonary fibrosis, an uncommon but documented AE of VDC‐1011.21, 22
VDC‐1101 as the succinate salt was administered by a 30‐minute IV infusion in 0.9% NaCl for injection (2 mL/kg) at a dosage of 1.0 mg/kg every 21 days until progressive disease (PD) or dose‐limiting toxicity, defined as any uncomplicated grade 4 neutropenia or thrombocytopenia or any grade 3 or 4 nonhematologic toxicity (excluding dermatopathy). This dosage and schedule represents those that were previously defined as optimal in pet dogs with non‐Hodgkin lymphoma, based on efficacy and tolerability.19 Patients that experienced a CR were treated for 2 cycles beyond confirmation of CR or 5 total cycles, whichever was longer.
At each visit, owner history, physical examination, CBC, serum biochemistry, and urinalysis were performed. Response to treatment was assessed by modified Response Evaluation Criteria in Solid Tumors (RECIST) criteria for patients with measurable lesions. Briefly, up to 5 target lesions were identified per patient. Caliper measurements of each target lesion were performed by 1 evaluator. At each response assessment, the sum of the longest diameters was recorded and compared to baseline. Photographs of lesions at each visit were encouraged. A CR was defined as resolution of all measurable disease. A PR was defined as a ≥30% decrease in the sum of diameters, whereas PD was defined as a ≥20% increase in the sum of diameters, or the development of any new lesions. A patient was classified as stable disease (SD) when not meeting the criteria for CR, PR, or PD. The ORR was defined as the percentage of dogs experiencing a CR or PR at any time point. Biologic response rate included dogs with SD that persisted for a minimum of 1 treatment cycle. PFS was defined as the interval from VDC‐1101 treatment initiation to PD. We were unable to assess PFS from the onset of lesions because often this information was not available from RDVM records and would have to have been approximated from owner recollection. Dogs were censored from analysis if they were in CR at the time of last treatment or lost to follow‐up. The PFS was calculated using the Kaplan‐Meier product limit method, which accounts for dogs that were in remission at the time of last follow‐up or lost to follow‐up and are statistically referred to as censored. All statistical analyses were performed by using a commercial software package.2
Adverse events were graded according to the Veterinary Cooperative Oncology Group Common Terminology Criteria for Adverse Events v1.0,23 based on client history, physical examination, CBC, biochemistry profile, and urinalysis. Assessment of AEs was performed pretreatment and at all visits thereafter. If dogs experienced ≥ grade 3 AEs, dose reduction or delay was instituted unless the abnormality was judged not attributable to VDC‐1101. Because of difficulty in discriminating cutaneous toxicity from PD of CTCL, a biopsy was performed if a dermatopathy was observed. Because of the previous observation of pulmonary fibrosis as a late complication in a small percentage of VDC‐1101 treated dogs,17 all dogs underwent baseline thoracic radiography and repeat thoracic radiography every other month for duration of the trial. Whenever possible, postmortem examinations were performed at the time of death.
Results
Treatment and Outcome
Twelve dogs with CTCL were enrolled prospectively in a clinical trial of VDC‐1101 with prednisone. Patient and disease characteristics are presented in Table 2. Ten dogs had epitheliotropic T‐cell lymphoma, whereas 2 dogs were diagnosed with nonepitheliotropic lymphoma. Clinical presentation of dogs varied, and the specific clinical presentation of each patient was not consistently provided in the medical records for each individual case. Investigators were asked to report upon cutaneous target lesions (up to 5 per dog) and these were the lesions that were followed up for response assessment. Therefore, information regarding total number of lesions or specific characterization of lesions was not available. The 5 pretreated dogs received a variety of cytotoxic chemotherapy protocols (all received CCNU with or without additional protocols), and all had PD at study enrollment. Four of these 5 dogs had also received treatment with corticosteroids. An additional 4 dogs had previously received corticosteroids. If dogs were receiving prednisone at the time of trial entry, their dosage was adjusted to 1 mg/kg q48h. Of the 11 dogs eligible for response assessment, 1 experienced a CR (Fig 1) and 4 experienced PR, for an overall response rate of 45% and biologic response rate of 64% (including 2 dogs with SD). The median PFS of all dogs was 37.5 days. The dog experiencing a CR had multifocal lesions that were erythematous and scaling, and localized to the lateral thorax bilaterally. Histologically, this dog was diagnosed with nonepitheliotropic cutaneous lymphoma. The other dog in the study with nonepitheliotropic lymphoma experienced rapid progressive disease 9 days after treatment. The 4 dogs that experienced PR had diverse clinical presentations. All had diffuse skin lesions and 1 dog had concurrent mucocutaneous involvement. Two of these dogs were naïve to treatment and 2 had received previous treatment.
Table 2.
Patient characteristics: Pretreated dogs include dogs that received previous cytotoxic chemotherapy.
| Median age, years (range) | 9 (4–14) |
| Median weight, kg (range) | 36 (10.1–45.7) |
| Sex | |
| Male | 4 |
| Female | 8 |
| Breed | |
| Golden retriever | 4 |
| Labrador retriever | 2 |
| Mix | 1 |
| Other (1 each) | 5 |
| Pretreatment | |
| Yes | 5 |
| No | 7 |
Figure 1.
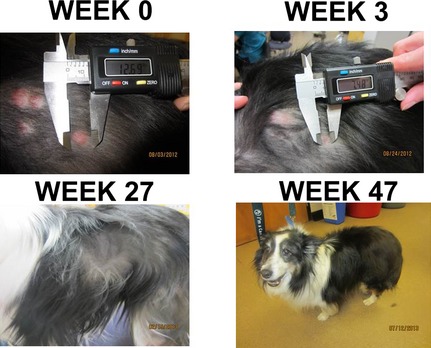
Photographs of the complete responder before treatment, and 3 weeks, 6 months, and 11 months after treatment. Thoracic radiographs continue to be normal.
The response rate in previously treated dogs was 50% (2/4) and in chemotherapy‐naïve dogs was 43% (3/7). One dog died 2 days after treatment. On initial presentation, this dog had severe regenerative anemia (hematocrit, 20%) and an inflammatory leukogram. Clinically, at the time of enrollment, the dog had a poor appetite and was reported to be lethargic but met the inclusion requirement based on performance score. This dog did not meet the inclusion criteria based on its hematocrit, but was approved for enrollment by the study investigator because the dog had exhausted all other therapeutic options. Postmortem examination identified widespread lymphoma with secondary severe bacterial pyoderma. In addition to the skin, lymphoma was found in the lymph nodes, heart, kidneys, and bone marrow. The acute cause of death was suspected to be cardiac failure secondary to lymphoma infiltration. Although this dog was not evaluable for response assessment, it was included in PFS outcome analysis.
Two dogs were censored in the PFS analysis: 1 dog achieved CR that has persisted for >399 days without relapse at the time of data analysis, and the second dog had a dose‐limiting dermatopathy followed by loss to follow‐up. The overall median PFS was 37.5 days (2 to >399 days). The median PFS of naïve dogs was 43 days (9 to >399 days) compared to 21 days (2–84 days) in pretreated dogs (P = .25; Fig 2). The dog that achieved long‐term CR had not previously received chemotherapy or corticosteroids.
Figure 2.
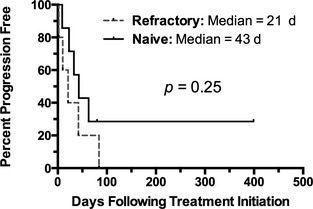
Kaplan‐Meier curve depicting progression‐free survival of naïve (n = 7) versus refractory dogs (n = 5). There was no significant difference in outcome associated with pretreatment.
Safety and Adverse Events
Adverse events were transient and self‐limiting in most dogs and are described in Table 3. No grade 3 or 4 events were observed. However, 3 dogs (25%) developed dermatopathy. Biopsies were performed in 2 dogs and supported that the changes were consistent with drug‐induced toxicity as opposed to PD. More specifically, histopathology in these 2 dogs disclosed mixed dermatitis and hyperkeratosis, without evidence of neoplasia. One dog was removed from the trial prematurely after the lesions did not resolve after a 21‐day dose delay. This patient continued to have SD throughout the dose delay and then was lost to follow‐up. Another dog that developed dermatopathic AE received a 20% dose reduction and the dermatopathy improved. This dog developed new target lesions 3 weeks later and was withdrawn. A biopsy was not performed in this dog, but the reported lesions were consistent with those reported previously with drug‐associated toxicity19 (ie, erythema and alopecia of the pinnae) and clinically were distinct from the lymphoma lesions.
Table 3.
Summary of adverse events in 12 dogs with CTCL receiving VDC‐1101.
| Adverse Event | Grade | n |
|---|---|---|
| Anorexia | 1 | 3 |
| 2 | 1 | |
| Vomiting | 1 | 1 |
| 2 | 1 | |
| Nausea | 1 | 1 |
| Diarrhea | 1 | 1 |
| Lethargy | 1 | 2 |
| Anemia | 1 | 2 |
| Neutropenia | 1 | 1 |
| Thrombocytopenia | 1 | 1 |
| Dermatopathy | 1 | 1 |
| 2 | 2 | |
| Othera | 1 | 6 |
| 2 | 1 |
This includes events of unknown etiology (proteinuria, low albumin, hyperphosphatemia, elevated blood urea nitrogen, elevated creatinine kinase, and hypokalemia). There were 4 events related to elevated liver enzyme activities that were deemed secondary to prednisone administration and are not included in the Table.
Postmortem examinations were performed in 3 dogs. All 3 necropsies showed evidence of lymphoma with no drug‐associated pathologic findings noted. There was no evidence of pulmonary fibrosis in these 3 dogs. No dogs developed clinical or radiographic evidence of pulmonary fibrosis while on study.
Discussion
Despite the relatively high response rates observed with classical treatment of canine CTCL, response durations generally are not durable leading to the need for additional therapies. VDC‐1101, a novel prodrug of the guanine nucleotide analog PMEG, has antitumor activity and acceptable tolerability in dogs with NHL.14, 19, 20, 21 One of the observed toxicities was a dermatopathy, implying delivery of drug to the skin. Based on this possible therapeutic target, we investigated the activity of VDC‐1101 in dogs with naturally occurring CTCL.
VDC‐1101 showed activity against canine CTCL with an ORR of 45% and median PFS in all patients of 37.5 days. The activity observed in both untreated and chemotherapy‐refractory patients indicates potential utility for VDC‐1101 in both induction and rescue chemotherapy settings. A statistical difference in PFS was not identified between naïve and refractory patients. Some of the patients in the naïve category had received corticosteroids previously, and it is unknown how this could have affected response to treatment. In addition, VDC‐1101 resulted in SD in an additional 2 dogs yielding a biologic response rate of 64%. In patients with advanced CTCL, the ability to achieve SD is a benefit to patients because it helps prolong an acceptable quality of life.
Objective response assessment of CTCL lesions can be challenging. The authors attempted to standardize assessment as much as possible. In dogs with multiple lesions, up to 5 target lesions were identified and these lesions were followed, measured, and recorded at each visit. Photographs also were obtained based on individual clinician preference to help in response assessment. In some cases of CTCL, lesions can progress in severity but not in size. Therefore, the subjective assessment of response by the primary clinician also was provided. While this subjectivity may affect the response rate, every attempt was made to standardize response assessment.
Dogs were treated that had variable clinical presentations, severity of lesions, duration of clinical signs, and previous treatments. The authors acknowledge that specific variants of CTCL are known to exist and that these may carry different prognoses. Furthermore, dogs presenting with advanced clinical presentations in general have a poorer prognosis. The aim of this study was to demonstrate responses with VDC‐1101 in a wide variety of CTCL cases. Based on the lack of statistical power with this initial investigation into the activity of VDC‐1101in CTCL, no conclusions regarding responses in different clinical or histologic forms could be made. Currently, there is no variation in treatment recommendations for these clinical and histologic variants and more information is needed to assess how these specific clinical forms of disease respond to differences in treatment.
A limitation of the study was the small number of cases reported. One aim of this initial prospective phase II clinical trial was to identify a >25% response rate. It was determined that 12 dogs would be adequate to assess this change and the authors identified a 45% response rate. Although it would be beneficial to evaluate a larger number of patients, this initial information provides preliminary evidence that VDC‐1101 has efficacy in some dogs with CTCL and is a potential treatment for this disease. Additional studies would be necessary to compare VDC‐1101 to conventional chemotherapy treatments.
Another potential limitation of the study was the concurrent use of prednisone in conjunction with VDC‐1101. Prednisone was prescribed concurrently at an anti‐inflammatory dosage in effort to delay or prevent a dose‐limiting cutaneous or pulmonary toxicity. We acknowledge that corticosteroids can induce short‐term clinical improvement in dogs with lymphoid neoplasia. However, we feel that any relevant antineoplastic effects are unlikely at the dosing schedule used in this study. Many patients (n = 9) had been exposed to high doses of corticosteroids before enrollment in the study (alone or in combination with other chemotherapy) with documented PD. Therefore, it seems unlikely that any responses in this study were secondary to the relatively low dosage of prednisone used. Furthermore, responses to prednisone, if observed, would be expected to be brief in duration. Corticosteroid use did make it difficult to attribute any possible antipruritic effects to VDC‐1101.
VDC‐1101 was well tolerated. Acute AEs were mild and manageable with no grade 3 or 4 toxicities observed. These events were consistent with those described in normal dogs and in dogs with NHL treated with GS‐9219, with the exception that there were no cases in this study that developed pulmonary fibrosis.14, 19 The lack of pulmonary fibrosis observation could be secondary to concurrent administration of prednisone in this study, the small numbers of dogs necropsied, or the relatively short overall survival times observed with CTCL patients. The dog that experienced CR continued to have normal thoracic auscultation and unremarkable thoracic radiographs >1 year after treatment.
Two dogs that progressed while on study (ie, development of new lesions) were continued on VDC‐1101 based on compassionate use because the owners reported improved quality of life while the dogs were receiving the drug, as a result of stabilization or improvement in originally identified lesions. One of these dogs received an additional 3 treatment cycles before decreases in quality of life were observed, and the other dog received an additional dose before treatment was changed to lomustine based on the emergence of new lesions. It is therefore possible that VDC‐1101 can improve quality of life in patients even in the face of mild disease progression.
In conclusion, VDC‐1101 in combination with prednisone exhibits activity against naïve and refractory CTCL in dogs with an acceptable AE profile. The observation of a long‐term CR in 1 dog is encouraging. Pulmonary fibrosis was not observed, but because of the limited number of dogs and short survival times, continued monitoring of thoracic radiographs is recommended.
Acknowledgments
The authors acknowledge The American Kennel Club and VetDC, Inc. We also thank Dr. Jennifer Pendergraft for her assistance with manuscript revisions.
Grant support: This study was supported by grant # 01569 from the American Kennel Club Canine Health Foundation. VDC‐1101 was provided by VetDC, Inc.
Conflict of Interest Declaration: Dr Doug Thamm is a consultant and shareholder in VetDC, Inc.
Cases were enrolled at the Flint Animal Cancer Center, Colorado State University Veterinary Medical Center (CSU‐VMC), School of Veterinary Medicine, University of Wisconsin‐Madison (UW‐SVM), University of Georgia Veterinary Teaching Hospital (UGA‐VTH), and Tufts University Foster Hospital (TU‐FH) for Small Animals.
This study was presented in abstract form at the Veterinary Cancer Society meeting (poster), October 2013, Minneapolis, MN.
Footnotes
VetDC, Fort Collins, CO
Prism v6.0; GraphPad Software, LaJolla, CA
References
- 1. Vail DM, Pinkerton ME, Young DM. Hematopoietic tumors In: Withrow SJ, Vail DM, Page RL, eds. Small Animal Clinical Oncology, 5th ed St. Louis, MO: Saunders Elsevier; 2013:608–627. [Google Scholar]
- 2. Goldschmidt MH, Shofer FS. Skin Tumors of the Dog and Cat. Oxford: Pergamon Press; 1992:22–264. [Google Scholar]
- 3. Santora D, Marsalla R, Hernandez J. Investigation on the association between atopic dermatitis and the development of mycosis fungoides in dogs: A retrospective case‐control study. Vet Dermatol 2007;18:101–106. [DOI] [PubMed] [Google Scholar]
- 4. Fontaine J, Bovens C, Bettenay S, Meuller RS. Canine cutaneous epitheliotropic T‐cell lymphoma: A review. Vet Compar Oncol 2009;7:1–14. [DOI] [PubMed] [Google Scholar]
- 5. Fontaine J, Heimann M, Day MJ. Canine cutaneous epitheliotropic T‐cell lymphoma: A review of 30 cases. Vet Dermatol 2010;21:267–275. [DOI] [PubMed] [Google Scholar]
- 6. Morrison WB. Cancer in the Dog and Cat‐Medical and Surgical Management, 2nd ed Jackson, WY: Teton NewMedia; 2001:641–670. [Google Scholar]
- 7. Scott DW, Miller WH, Griffin CE. Muller and Kirk's Small Animal Dermatology, 6th ed Philadelphia, PA: Saunders; 2001:1330–1340. [Google Scholar]
- 8. DeBoer DJ, Turrel M, Moore PF. Mycosis fungoides in a dog: Demonstration of T‐cell specificity and response to radiotherapy. J Am An Hosp Assoc 1990;26:566–572. [Google Scholar]
- 9. Iwamoto KS, Bennett LR, Norman A, et al. Linoleate produces remission in canine mycosis fungoides. Cancer Lett 1992;64:17–22. [DOI] [PubMed] [Google Scholar]
- 10. White SD, Rosychuk RA, Scott KV, et al. Use of isotretinoin and etretinate for the treatment of benign cutaneous neoplasia and cutaneous lymphoma in dogs. J Am Vet Med Assoc 1993;202:387–391. [PubMed] [Google Scholar]
- 11. Risbon RE, de Lorimier LP, Skorupski K, et al. Response of canine cutaneous epitheliotropic lymphoma to lomustine (CCNU): A retrospective study of 46 cases (1999–2004). J Vet Intern Med 2006;20:1389–1397. [DOI] [PubMed] [Google Scholar]
- 12. Williams LE, Rassnick KM, Power HT, et al. CCNU in the treatment of canine epitheliotropic lymphoma. J Vet Intern Med 2006;20:136–143. [DOI] [PubMed] [Google Scholar]
- 13. Lemarie SL, Eddlestone SM. Treatment of cutaneous T‐cell lymphoma with dacarbazine in a dog. Vet Dermatol 1997;8:41–46. [DOI] [PubMed] [Google Scholar]
- 14. Reiser H, Wang J, Chong L, et al. GS‐9219—A novel acyclic nucleotide analogue with potent antineoplastic activity in dogs with spontaneous non‐Hodgkin's lymphoma. Clin Cancer Res 2008;14:2824–2832. [DOI] [PubMed] [Google Scholar]
- 15. Kramata P, Downey KM, Paborsky LR. Incorportation and excision of 9‐(2‐phosphonylmethoxyethyl)guanine (PMEG) by DNA polymerase delta and epsilon in vitro. J Biol Chem 1998;273:21966–21971. [DOI] [PubMed] [Google Scholar]
- 16. Rose WC, Crosswell AR, Bronson JJ, Martin JC. In vivo antitumor activity of 9‐[(2‐phosphonylmethoxy)ethyl]‐guanine and related phosphonate neucleotide analogues. J Natl Cancer Inst 1990;82:510–512. [DOI] [PubMed] [Google Scholar]
- 17. Nasesens L, Hatse S, Segers C, et al. 9‐(2‐phosphonylmethoxyethyl)‐N6‐cyclopropyl‐2,6‐diaminopurine: A novel prodrug of 9‐(2‐phosphonylmethoxyethyl)guanine with improved antitumor efficacy and selectivity in choriocarcinoma‐bearing rats. Oncol Res 1999;11:195–203. [PubMed] [Google Scholar]
- 18. Valerianova M, Votruba I, Holy A, et al. Antitumour activity of N6‐substituted PMEDAP derivatives against T‐cell lymphoma. Anticancer Res 2001;21:2057–2064. [PubMed] [Google Scholar]
- 19. Vail DM, Thamm DH, Reiser H, et al. Assessment of GS‐9219 in a pet dog model of non‐Hodgkin's lymphoma. Clin Cancer Res 2009;15:3503–3510. [DOI] [PubMed] [Google Scholar]
- 20. Lawrence J, Vanderhoek M, Barbee D, et al. Use of 3'‐deoxy‐3'‐[18F]fluorothymidine PET/CT for evaluating response to cytotoxic chemotherapy in dogs with non‐Hodgkin's lymphoma. Vet Radiol Ultrasound 2009;50:660–668. [DOI] [PubMed] [Google Scholar]
- 21. Corcoran BM, Cobb M, Martin MW, et al. Chronic pulmonary disease in West Highland white terriers. Vet Rec 1999;29:611–616. [DOI] [PubMed] [Google Scholar]
- 22. Heikkilä HP, Lappalainen AK, Day MJ, et al. Clinical, bronchoscopic, histopathologic, diagnostic imaging, and arterial oxygenation findings in West Highland White Terriers with idiopathic pulmonary fibrosis. J Vet Intern Med 2011;25:433–439. [DOI] [PubMed] [Google Scholar]
- 23. Vail DM. Veterinary Co‐operative Oncology Group‐Common Terminology Criteria for Adverse Events (VCOG‐CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Vet Comp Oncol 2004;2:194–213. [DOI] [PubMed] [Google Scholar]


