Abstract
Background
Symmetric dimethylarginine (SDMA) has been shown to be an accurate and precise biomarker for calculating estimated glomerular filtration rate (GFR) in humans, as well as a more sensitive biomarker than serum creatinine concentration (sCr) for assessing renal dysfunction.
Objectives
The purpose of this retrospective study was to report on the utility of measuring serum SDMA concentrations in cats for detection of chronic kidney disease (CKD) before diagnosis by conventional measurement of sCr.
Animals
Chronic kidney disease cats (n = 21) included those persistently azotemic for ≥3 months (n = 15), nonazotemic cats with GFR >30% decreased from median GFR of normal cats (n = 4), and nonazotemic cats with calcium oxalate kidney stones (n = 2). Healthy geriatric cats (n = 21) were selected from the same colony.
Methods
Symmetric dimethylarginine concentrations (liquid chromatography‐mass spectroscopy) and sCr (enzymatic colorimetry) were determined retrospectively from historical data or banked serum samples in azotemic cats or at the time GFR (iohexol clearance) was measured in nonazotemic cats.
Results
Serum SDMA (r = −0.79) and sCr (r = −0.77) concentrations were significantly correlated to GFR (both P < .0001). Symmetric dimethylarginine became increased before sCr in 17/21 cats (mean, 17.0 months; range, 1.5–48 months). Serum SDMA had higher sensitivity (100%) compared with sCr (17%), but lower specificity (91% versus 100%) and positive predictive value (86% versus 100%).
Conclusion and Clinical Importance
Using serum SDMA as a biomarker for CKD allows earlier detection of CKD in cats compared with sCr, which may be desirable for initiating renoprotective interventions that slow progression of CKD.
Keywords: Endogenous, Feline, Pet foods, Predictor
Abbreviations
- ADMA
asymmetric dimethylarginine
- BUN
blood urea nitrogen
- CKD
chronic kidney disease
- DDAH
dimethylarginine dimethylaminohydrolase
- GFR
glomerular filtration rate
- IRIS
International Renal Interest Society
- MMA
monomethylarginine
- NOS
nitric oxide synthase
- NPV
negative predictive value
- PPV
positive predictive value
- sCr
serum creatinine concentration
- SDMA
symmetric dimethylarginine
- UPC
urine protein : creatinine
- USG
urine specific gravity
Chronic kidney disease (CKD) is a major cause of morbidity in cats, especially as they age.1, 2 Although irreversible, the rate of progression in cats is not predictable, and CKD in many cats can be successfully managed for months or even years.1, 2 Early recognition of CKD is desirable in order to know when to initiate renoprotective interventions that may slow its progression (eg, dietary modifications).2, 3 However, early decreases in glomerular filtration rate (GFR) are not recognized by commonly used diagnostic tests. CKD usually is diagnosed on the basis of later indicators including impaired urine concentrating ability and azotemia (increased serum creatinine [sCr] and blood urea nitrogen [BUN] concentrations). Cats with CKD are staged according to guidelines developed by the International Renal Interest Society (IRIS) and accepted by the American and European Societies of Veterinary Nephrology and Urology.4 The 4‐tier IRIS CKD staging system is based on sCr concentration, magnitude of proteinuria as measured by urine protein : creatinine (UPC) ratio, and blood pressure.5
Determination of GFR is a valuable tool for estimating renal function and staging kidney disease in dogs and cats.6 Renal clearance of inulin is the gold standard for measuring GFR.6 If a marker is eliminated solely by the kidneys via glomerular filtration, and is neither reabsorbed nor secreted by the tubules, then renal clearance is equal to plasma clearance and provides an accurate estimate of GFR.6 Measuring iohexol plasma clearance appears to be the simplest and most accurate method for determining GFR in clinical practice.6 Because measuring GFR is technically cumbersome and expensive, sCr concentration remains the standard surrogate for GFR.5 However, sCr has limitations as a marker of kidney function, most notably insensitivity because it remains in the normal reference interval (with a flat slope over much of the GFR range) until GFR is decreased approximately 75%.7 In addition, other nonrenal factors can influence sCr, including endogenous production by muscle such that muscle mass, breed, and sex may influence sCr concentration. Although serial evaluation of sCr in the same cat does increase its sensitivity for detecting progressive changes in GFR,5 baseline concentrations often are unavailable because they are not commonly monitored in clinical practice. Thus, there is a need for a better biomarker for earlier detection of CKD.
Symmetric dimethylarginine (SDMA) has been shown to be an accurate and precise biomarker for calculating estimated GFR in humans,8 as well as a more sensitive biomarker than sCr for assessing renal dysfunction.9 A meta‐analysis of 18 studies involving human patients showed that 1/SDMA concentrations correlated highly with inulin clearance (r = 0.85), and SDMA concentrations correlated highly with sCr concentrations (r = 0.75).10 In another study using human subjects, a close correlation of GFR (measured by an iodothalamate clearance technique) with sCr and SDMA was reported (r = −0.84 and −0.89, respectively).11 Plasma SDMA concentrations also have been shown to be increased in cats with CKD and to correlate with plasma Cr concentration (r = 0.74).12
In brief, SDMA is a byproduct of protein methylation. There are 3 main species of methylated arginine: monomethylarginine (MMA), asymmetric dimethylarginine (ADMA), and SDMA.13 Subsequent protein degradation of methylated proteins yields individual methylated arginine amino acids. Symmetric dimethylarginine is excreted primarily (≥90%) by renal clearance.14, 15 Because SDMA is eliminated by the kidneys, plasma concentrations are affected by changes in GFR.
The purpose of this retrospective study was to report on the utility of measuring serum SDMA concentrations to detect CKD in cats before diagnosis by conventional single‐point sCr measurements. Our goal was to measure serum SDMA concentrations in all CKD cats before the time they became azotemic, provided banked serum samples were available for measurement, in order to compare the sensitivity of sCr and serum SDMA as biomarkers for early detection of renal dysfunction.
Materials and Methods
Animals and Study Design
All study protocols were reviewed and approved by the Institutional Animal Care and Use Committee, Hill's Pet Nutrition, Inc., Topeka, KS. Each cat had an annual physical examination, CBC, serum biochemical analyses, urinalysis, and urine culture if indicated by the urinalysis results. In addition, after 2010, serum was frozen at −70°C and banked for retrospective analyses. Cats were housed individually or in groups and allowed exercise in indoor runs. Cats had access to natural light that varied with seasonal changes. All cats were provided with regular opportunities to exercise, with access to toys. All cats were owned by the commercial funders of this research or their affiliates, who gave permission for them to be included in this study.
All cats had been used historically for palatability studies. Many different types of commercial and non‐commercial foods of varying nutrient compositions were fed, including dry and canned cat foods. All foods met the requirements established by the Association of American Feed Control Officials (AAFCO) for complete and balanced pet foods for adult cats.
Cats with CKD (n = 21) came from the colony of over 400 domestic short hair cats. Cats in the colony ranged in age from 1 to 19 years (mean, 7 years) with approximately 25% of cats >10 years. The cats (n = 21) with CKD included cats that were persistently azotemic over an extended period, typically for ≥3 months (n = 15). CKD was diagnosed in nonazotemic cats (with stable sCr concentrations) based on GFR testing by iohexol clearance (n = 4). Nonazotemic cats with calcium oxalate kidney stones also were considered as having CKD (n = 2). Cats with CKD had no evidence of other confounding disease, notwithstanding history and physical examination findings (changes in urine volume or changes in kidney size or shape) that were consistent with CKD. Mean age of cats with CKD was 14.3 years (range, 8.0–18.5 years). There were 15 ovariohysterectomized females and 6 neutered males. Mean body weight was 4.0 kg (range, 2.6–5.7 kg).
Acute kidney disease may have been present in some cats with CKD and gone undiagnosed. For example, because an unremarkable urine sediment does not definitively rule out infection, especially in cats with CKD, cats with occult pyelonephritis may have gone undiagnosed. In cats with chronic urolithiasis, stones could have moved, leading to partial ureteral obstruction and acute kidney injury.
A similar number of healthy geriatric cats (n = 21) was selected from the same colony. Criteria for inclusion were age >10 years, requirement of 3 normal GFR tests, 3 normal sCr concentrations, and 3 urine specific gravity (USG) measurements >1.040 over a 6‐month period. In addition, these cats lacked historical or physical evidence of confounding disease at the time of inclusion, and had banked serum samples available for determination of SDMA concentrations. Mean age of healthy geriatric cats was 11.7 years (range, 10.2–13.1 years). There were 12 ovariohysterectomized females and 9 neutered males. Mean body weight was 4.5 kg (range, 3.1–6.3 kg).
Retrospective data was used to document sCr concentrations in cats with CKD. Serum SDMA concentrations were determined from serum stored in serum banks. sCr and SDMA concentrations also were measured from blood collected prospectively in CKD cats.
Analyses
Serum Biomarkers
Serum Cr and BUN concentrations were determined by enzymatic colorimetric methods.1 Reference ranges for sCr (0.7–2.1 mg/dL) and BUN (15.0–31.0 mg/dL) in other adult cats were previously established for this in‐house laboratory.
Urinalysis
Urine specific gravity was determined using a refractometer. Urine creatinine concentration was used as an internal reference and measured with the same assay as sCr. Urine protein concentrations were determined using urine supernatant. In brief, urine was pre‐incubated in an alkaline solution containing EDTA, which denatures protein and eliminates interference from magnesium ions. Benzethonium chloride then was added, producing turbidity, which is assessed by a turbidimetric method.1 Urine protein : creatinine ratio results are reported as mg/dL protein : mg/dL creatinine.
Glomerular Filtration Rate
Serum concentrations of iohexol were measured by a commercial laboratory2 using an inductively coupled plasma‐atomic emission spectroscopy (ICP‐AES) method.16 In brief, a single IV injection of iohexol, an iodinated radiographic contrast agent (300 mg I/kg), was administered and 3 serum samples were collected at 2, 3, and 4 hours after injection. GFR was estimated from calculations made using a one‐compartment model for serum iohexol clearance. Median (range) GFR for normal healthy cats in this study determined from 3 iohexol clearance tests per cat over a 6‐month period was 1.94 mL/min/kg (range, 1.34–3.79 mL/min/kg).
Symmetric Dimethylarginine
Symmetric dimethylarginine concentrations were determined using liquid chromatography‐mass spectroscopy (LC‐MS). In brief, 50 μL of serum from cats, or 50 μL of standard, were transferred to centrifuge tubes and diluted with 50 μL of internal standard solution3 in water. Next, 300 μL of high performance liquid chromatography (HPLC)‐grade acetonitrile4 was added to extract the SDMA. The samples were centrifuged and the supernatant was decanted and tested by LC‐MS for SDMA concentration.
The LC‐MS procedure was performed using an API 4000 coupled with Shimadzu Nexera HPLC system.5 Ten μL of serum extract was injected into the HPLC equipped with a C18 column6 (5 μm, 4.6 × 30 mm). The SDMA was eluted using 0.1% formic acid4 and 0.5 mM perfloroheptanoic acid7 in water (Mobile Phase A) and acetonitrile with 0.1% formic acid (Mobile Phase B) with a flow rate of 1 mL/min and column temperature of 25°C. Under these conditions, the retention times for SDMA and internal standard D‐7‐ADMA are both 1.72 minutes. The effluent of HPLC was directed to the electrospray ionization source of the mass spectrometer, which produced single charged adduct ions [M+H]+. The [M+H]+ ions of SDMA and D‐7‐ADMA were analyzed in the multiple reaction monitoring (MRM) mode of the mass spectrometer. Fragmentation was accomplished using collision energy of 20 V. The observed MRM transitions for SDMA and D‐7‐ADMA were 203.1 to 172.1 and 210.2 to 46.1 m/z, respectively.
A standard curve for SDMA quantitation was prepared using calibrator solutions. First, a solution of SDMA stock standard was prepared (1 mg/mL) in water. The stock standard was diluted 50‐fold in water to obtain working standard solutions (20 μg/mL). Next, 100 μL of working standard was spiked into 1,900 μL of charcoal‐stripped feline serum to obtain 100 μg/dL SDMA. Serial dilutions of SDMA were similarly prepared in charcoal‐stripped feline serum ranging from 100 to 1.56 μg/dL of SDMA. The calibrator samples were analyzed using the same protocol described for samples. The standard curve, prepared following the LC‐MS protocol for SDMA detection, was linear with a correlation coefficient >0.999. The intra‐assay (n = 5) and inter‐assay (n = 25) coefficients of variation for SDMA concentrations were 2.2 and 2.5%, respectively. The normal reference limit for SDMA in other healthy cats previously was determined to be <14 μg/dL.8
Statistical Analysis
Statistical analyses were performed using Statistical Analysis Software version 9.2.9 Data were assessed for normality by the Shapiro–Wilk test. To investigate the relationships between SDMA and GFR, sCr and GFR, and SDMA and sCr, correlation coefficients were measured between these response variables. To accomplish this, SDMA and sCr were plotted against GFR, and equations were derived from linear, exponential, logarithmic, or quadratic plots. Multiple equations were assessed to better approximate changes at the edges of the plots. These analyses were performed in Excel and verified using a regression model in SAS. Significance was accepted as P < .05. Using derived equations, the GFR that corresponded to the upper limit of the normal reference interval for SDMA (14 μg/dL) and sCr (2.1 mg/dL) concentrations, was calculated. The sensitivity, specificity, positive predictive value (PPV), and negative predictive values (NPV) also were calculated for SDMA and sCr concentrations to detect CKD.
All healthy geriatric cats had 3 evaluations for sCr, BUN, and SDMA concentrations, USG, and GFR over a 6‐month period. These measurements were used to calculate standard deviation and coefficient of variation for these variables. The data for GFR measurements were not normally distributed. To describe the variability of GFR in healthy geriatric cats, median, range, and percentiles were determined. The lower 2.5 percentile was used to establish the lower limit of normal. All cats with GFR below the lower 2.5 percentile were considered to have abnormal renal function whereas cats above this threshold were considered to have normal renal function.
Healthy geriatric cats then were compared with cats with CKD at the time SDMA concentrations were first increased (>14 μg/dL) and at the time sCr concentrations were first increased (>2.1 mg/dL). Comparisons included age; body weight; sCr, BUN, and SDMA concentrations; USG; UPC ratios; and, GFR measurements. In addition, the estimated time that serum SDMA concentrations were increased before sCr concentrations were increased was calculated. Data are reported as mean (range) unless otherwise indicated. A P < .05 was considered statistically significant.
Results
Concurrent serum SDMA and sCr concentrations were plotted for CKD cats and healthy geriatric cats (1 time point only) using a scatter plot (Fig 1; r = 0.72; P < .0001). In cats with CKD, repeated measures (2 sets of data) within the same animal were included (ie, at the time when SDMA first increased above normal and at the time when sCr was first increased above normal; Fig 1; Table 1). There were no situations in which serum SDMA concentration was within the normal reference interval and serum sCr concentration was increased above the normal reference interval (ie, no points in the upper left quadrant). All healthy geriatric cats had serum SDMA and sCr concentrations in the normal reference interval (lower left quadrant). As CKD cats became azotemic, serum SDMA and sCr data points moved into the upper right quadrant (see Fig 1).
Figure 1.
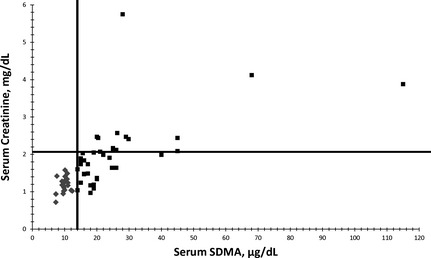
Relationship between serum symmetric dimethylarginine (SDMA; μg/dL) and serum creatinine (sCr; mg/dL) concentrations in 21 healthy geriatric cats (mean age, 11.7 years; range, 10.2–13.1 years; diamonds) and 21 cats with chronic kidney disease (CKD; mean age, 14.3 years; range, 8.0–18.5 years; squares). There is a positive linear relationship between serum SDMA and sCr concentrations (r = 0.72). No cats with sCr concentrations above the normal reference interval (>2.1 mg/dL) had normal serum SDMA concentrations (<14 μg/dL).
Table 1.
Demographic data for healthy cats and cats with CKD at time SDMA first elevated and at time when sCr first elevated
| Healthy Cats | Cats with CKD Data When SDMA First Detected as >14 μg/mL | Cats with CKD Data When sCr First Detected as >2.1 mg/dL | |||
|---|---|---|---|---|---|
| N | 21 | 21 | P Value | 21 | P Value |
| Age (years) | 11.7 (10.2–13.1) | 12.8 (4.9–17.2) | .08 | 14.3 (8.0–18.5) | <.001 |
| Body weight (kg) | 4.5 (3.1–6.3) | 4.1 (2.6–5.7) | >.10 | 4.0 (2.6–5.7) | >.10 |
| Creatinine concentration (mg/dL) | 1.2 (0.7–1.6) | 1.6 (1.0–2.5) | .001 | 2.6 (1.2–5.8)a | <.001 |
| Blood urea nitrogen concentration (mg/dL) | 19.7 (16.7–26.0) | 28.6 (19.8–41.6) | <.001 | 46.0 (28.7–88.9) | <.001 |
| Urine specific gravity | 1.059 (1.039–1.078) | 1.030 (1.009–1.056) | <.001 | 1.020 (1.009–1.047) | <.001 |
| Urine protein to creatinine ratio | 0.2 (0.1–0.3) | 0.2 (0.1–0.4) | >.10 | 0.3 (0.1–1.2) | >.10 |
| Glomerular filtration rate (mL/min/kg) | 1.94 (1.34–3.79)b | 1.17 (0.87–1.45) | <.001 | 0.98 (0.49–1.45) | <.001 |
| SDMA (μg/dL) | 9.9 (7.3–12.4) | 19.9 (14.0–45.0) | <.001 | 32.1 (15.0–115.0) | <.001 |
| Approximate time SDMA increased before creatinine (months) | 0.0 (0.0–0.0) | 17.0 (1.5–48.0)c | <.001 | ||
Data are presented as mean (range).
CKD, chronic kidney disease; SDMA, symmetric dimethylarginine; sCr, serum creatinine.
Six of 21 cats had sCr < 2.1 mg/dL. In 4 cats, the diagnosis of CKD was based on GFR being <1.36 mL/min/kg. In 2 cats with calcium oxalate kidney stones, sCr never increased above the normal reference interval, although serum SDMA concentrations were >14 μg/mL in both cats.
Median (range) GFR for normal healthy cats in this study determined from 3 iohexol clearance tests per cat over a 6‐month period.
Serum SDMA concentrations increased before sCr in 17/21 cats. In 4/21 cats, banked serum samples were not available to measure SDMA concentrations before the time cats developed azotemia.
The median (range) of GFR measurements in healthy geriatric cats was 1.94 mL/min/kg (1.34–3.79 mL/min/kg). The lower 2.5 percentile, which was used to establish the lower limit of normal, was determined to be 1.36 mL/min/kg. All cats with GFR < 1.36 mL/min/kg (which corresponds to a >30% decrease below the median GFR) were considered to have CKD whereas cats above this threshold were considered to have normal renal function.
Using serum from data banks to measure SDMA concentrations, serum SDMA concentrations were shown to increase above the normal reference interval before sCr concentrations increased above the normal reference interval in 17/21 cats by a mean of 17.0 months (range, 1.5–48.0 months). A representative example of the relationship between serum SDMA concentrations and sCr concentrations across time is shown for 1 cat in a time versus analytes concentration bar graph (Fig 2). In 4/21 cats, banked serum samples were not available to measure SDMA concentrations before the time cats developed azotemia. For the latter, the time that serum SDMA was increased before an increase in sCr was recorded as 0 months. In 4/21 cats with CKD, sCr concentration did not increase above the normal reference interval and the diagnosis of CKD was based on GFR being >30% below median GFR for healthy cats. In these 4 cats, the estimated time that serum SDMA concentration was increased before sCr increased was recorded as months until death in 3 cats (17, 28.5, and 48 months), and 41 months in 1 cat that was still alive. In 2/21 cats (both cats with calcium oxalate kidney stones), sCr never increased above the normal reference interval, although serum SDMA concentrations were increased above normal in both cats. One of the cats was euthanized because of anorexia of 2 weeks' duration with a sCr of 2.03 mg/dL; serum SDMA had been increased for 16 months (same duration as the nephrolithiasis diagnosis). The other cat was alive with a sCr of 1.74 mg/dL; serum SDMA has been increased for 9 months. Nephrolithiasis had been diagnosed 14 months before. Three azotemic cats also had calcium oxalate nephrolithiasis; nephrolithiasis had been diagnosed 13 and 17 months before death in 2 cats, but was not diagnosed before necropsy in the third cat.
Figure 2.
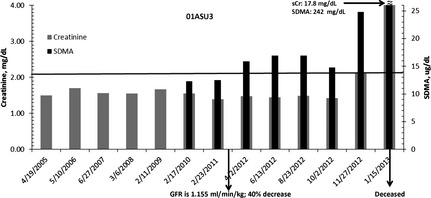
Representative cat (01ASU3; born September 2001; neutered male) with serum symmetric dimethylarginine (SDMA; black bars) and sCr (gray bars) concentrations indicated across time. Glomerular filtration rate was measured in March 2011 and found to be 40% below the mean of 1.94 mL/min/kg for the feline population of the reference laboratory. Serum SDMA was increased in April 2012 (16 μg/dL). The cat became azotemic in November 2012 (sCr, 2.12 mg/dL), approximately 8 months after serum SDMA was increased. The cat died in January 2013.
The best fit equation for the relationship between SDMA concentration and GFR was a quadratic relationship: [GFR in mL/min/kg] = 0.0031 [SDMA concentration in μg/dL]2 − 0.1703 [SDMA concentration in μg/dL] + 3.2444 (r = −0.79; P < .0001). Setting the upper limit of the normal reference interval for SDMA at 14 μg/dL corresponded to a GFR of 1.47 mL/min/kg, which represents an approximately 24% decrease from median GFR of healthy cats. Using a 30% decrease from median GFR as the gold standard for confirmed decrease in renal function, sensitivity (100%), specificity (91%), PPV (86%), and NPV (100%) were calculated for serum SDMA concentration. There were 2 false positives, whereby SDMA was >14 μg/dL (18 and 19 μg/dL), but GFR was not <1.36 mL/min/kg. Both cats had GFR of 1.45 mL/min/kg (25% decrease below the median).
The best fit equation for the relationship between sCr concentrations and GFR was an exponential relationship: [GFR in mL/min/kg] = 4.5501e−0.84 [sCr concentration in mg/dL] (r = −0.77; P < .0001). Setting the upper limit of the normal reference interval for sCr at 2.1 mg/dL corresponded to a GFR of 0.78 mL/min/kg, which represents approximately 60% decrease from the median. Using a 30% decrease from median GFR as the gold standard for confirmed decrease in renal function, the sensitivity (17%), specificity (100%), PPV (100%), and NPV (70%) were calculated for sCr concentration.
In healthy geriatric cats, the standard deviation and coefficient of variation for other variables measured over a 6‐month period were sCr (0.23 mg/dL; 19.85%), BUN (2.61 mg/dL; 12.99%), SDMA 1.54 μg/dL; 15.53%), and USG (0.01; 0.87%), respectively. Compared with healthy geriatric cats, cats with CKD at the time when serum SDMA was first detected as being >14 μg/dL (Table 1) were of similar age (P = .08) and body weight (P > .10), but had higher sCr, BUN, and SDMA concentrations (all P ≤ .001), more dilute urine based on USG (P < .001), and lower GFR (P < .001). The UPC ratios did not differ (P > .10). Cats with CKD at the time when sCr was first detected as being >2.1 mg/dL were older (P < .001), but still of similar body weight and with similar UPC ratios (both P > .10). Otherwise, cats with CKD continued to have higher sCr, BUN, and SDMA concentrations, more dilute urine based on USG, and lower GFR (all P < .001) compared with healthy geriatric cats.
Discussion
Using retrospective serum samples, we were able to show that serum SDMA increases before sCr in cats with CKD. Serum samples had been banked as part of annual examinations or as part of protocols for other studies. Thus, exact interval data were not available. Nonetheless, serum SDMA concentrations increased above the normal reference interval before sCr concentrations increased above the normal reference interval by a mean of 14.6 months (range, 0–48 months) in 21 cats diagnosed with CKD. Cats with CKD under consideration were IRIS Stage 1 (sCr < 1.6 mg/dL) or Stage 2 (sCr 1.6–2.8 mg/dL) and nonproteinuric (UPC ratio ≤ 0.2) or borderline proteinuric (UPC ratio 0.2–0.4) at the time of diagnosis. All healthy geriatric cats had serum SDMA and sCr concentrations in the normal reference interval. Thus, serum SDMA represents a promising biomarker for early detection of CKD in cats with compromised renal function. Early detection is desirable because it may be beneficial to initiate dietary or other interventions even earlier in order to slow progression of CKD, as was shown for cats with IRIS stages 2 and 3 CKD.2
A significant positive correlation (r = 0.72) was present between serum SDMA and sCr concentrations, similar to what has been reported previously in cats with CKD.12 These results support that SDMA is excreted primarily by the kidneys. Furthermore, our evidence suggests that nonazotemic cats with SDMA concentrations >14 μg/dL eventually progress to azotemic CKD.
Serum SDMA is a relatively new biomarker that allows early detection of CKD in a population of nonazotemic cats. Serum SDMA concentration was >14 μg/dL in all nonazotemic cats with GFR <1.36 mL/min/kg (median for healthy cats, 1.94 mL/min/kg). We chose GFR<1.36 mL/min/kg (30% decrease from median of healthy cats) as consistent with a diagnosis of CKD based on the lower 2.5 percentile of GFR measurements in healthy geriatric cats being 1.36 mL/min/kg. The normal reference interval for SDMA of < 14 μg/dL corresponded to a GFR of 1.47 mL/min/kg (approximately 24% decrease from median). Thus, 2 cats with CKD had SDMA concentrations ≥14 μg/dL, but GFR measured by iohexol clearance was not <1.36 mL/min/kg. Therefore, SDMA concentration indicated a false positive for decreased GFR in these 2 cats. Both cats had GFR of 1.45 mL/min/kg (25% decrease below median GFR). Our cut‐off (<1.36 mL/min/kg) for the diagnosis of CKD lowered the sensitivity of the SDMA assay to 91%.
Serum SDMA is a byproduct of intranuclear protein methylation. Although phosphorylation is the best understood post‐translational modification of proteins, arginine methylation also is important.13 Arginine methylation occurs when the nitrogens of arginine within polypeptides are post‐translationally modified to contain methyl groups. There are 3 main species of methylated arginine: MMA, ADMA, and SDMA.13 The methyl group is transferred from S‐adenosylmethionine to a nitrogen of arginine by 1 of several protein arginine methyltranferases (PRMT).13 Subsequent protein degradation of methylated proteins by hydrolysis yields individual methylated arginine amino acids, which are biologically active. ADMA functions as an endogenous nitric oxide synthase (NOS) inhibitor,8 preventing production of nitric oxide from l‐arginine. Although SDMA is not a direct inhibitor of NOS, it can compete with arginine for transport across membranes,8 and thus, indirectly decreases nitric oxide synthesis by limiting l‐arginine supply.8, 17 Asymmetric dimethylarginine is degraded primarily to dimethylamine and citrulline (≥80%) after metabolism by dimethylarginine dimethylaminohydrolase (DDAH) whereas only a small amount of intact ADMA (≤20%) is eliminated by the kidneys.12, 14 Symmetric dimethylarginine is eliminated primarily (≥90%) by renal clearance.14, 15 The presence of dimethylarginines in the urine has been recognized for over 40 years.18 Because SDMA is excreted by the kidneys, plasma concentrations are affected by changes in GFR. Asymmetric dimethylarginine accumulates in patients with renal dysfunction, although it is less abundant than SDMA, and its accumulation may be related to renal parenchymal damage, resulting in decreased DDAH expression and activity, rather than to decreased glomerular filtration of ADMA.14 In healthy human subjects from the Framingham offspring cohort, dietary components were not associated with plasma SDMA concentration.19, 20 Symmetric dimethylarginine concentrations were positively correlated with age and sCr concentration whereas body mass index, effective GRF, and diastolic blood pressure were inversely related to SDMA concentration.21
Glomerular filtration rate is directly related to functional renal mass. Early detection of a decrease in GFR may be important for initiating dietary or medical interventions in cats with CKD when sCr concentrations are still within the normal reference range (≤2.1 mg/dL).2 However, GFR testing remains an underused tool for diagnosis and management of kidney disease because it is difficult to measure, expensive, and requires multiple blood samples or a 24‐hour urine collection.6 Instead, serial sCr concentrations commonly are used to assess renal function because sCr is easily measured and less expensive. Yet, cats with IRIS stage 1 and 2 CKD may have normal sCr because sCr does not increase above the reference range until approximately 75% of nephrons are nonfunctioning. Other limitations of using sCr as a biomarker for CKD include increased concentrations that may occur with some types of assays, and there is additional sCr secretion into the tubules in male dogs. Because creatinine is a non‐enzymatic breakdown product of phosphocreatine in muscle, daily production of creatinine in the body is determined largely by muscle mass, such that total lean body weight may influence sCr concentration as well. Overall, it is less than ideal as an early biomarker of CKD.6
Normal GFR is poorly defined in dogs and cats such that recommendations are to use the reference range established by the laboratory measuring the injected GFR marker. This helps minimize the broad reference range because of variations in protocols.6 Markers used to measure GFR should be freely filtered through the glomerulus, not bound to plasma proteins, neither reabsorbed nor secreted by renal tubules, nontoxic, and cannot themselves alter GFR. Inulin is considered the gold standard for measuring GFR.6, 16 In cats, however, plasma clearance of iohexol using 2–4 plasma samples provides an accurate estimate of GFR.16 Using plasma clearance of iohexol to estimate GFR in healthy cats, we then selected the lower 2.5 percentile to establish the lower limit of normal GFR.
Serum SDMA concentrations varied inversely with GFR (r = −0.79), increasing in cats as GFR decreased. Because SDMA is thought to be excreted exclusively by the kidneys, SDMA has been proposed as a marker of renal function in humans.10, 14, 21 Symmetric dimethylarginine recently was reported in human volunteers to provide an accurate and precise estimate of GFR and to serve as a more sensitive biomarker of renal dysfunction than sCr.9
Assuming pre‐renal (volume responsive) causes of decreased GFR and causes of acute kidney injury have been eliminated, an increased serum SDMA concentration (positive test) as a single‐point measurement or screening test is moderately effective for confirming CKD (PPV = 86%), although further monitoring is needed. Increased SDMA, however, correctly identified all positive CKD cases (100% sensitivity; also called the true positive rate, which is the ratio of true positives divided by true positives plus false negatives). As a screening test, a negative result is excellent at reassuring the owner that the patient does not have CKD (NPV = 100%), but the initial screening test correctly identifies only 91% of cats that do not have CKD (91% specificity; also called the true negative rate, which is the ratio of true negatives divided by false positives plus true negatives). In comparison, using sCr as a screening test, an increased sCr (positive test) is excellent for confirming CKD (PPV = 100%). It does, however, only correctly identify 17% of all cases (sensitivity). This is consistent with the conclusion of others that plasma Cr is not sufficiently sensitive for early diagnosis of renal dysfunction in cats.22 As a screening test, a negative result is poor at reassuring the owner that the patient does not have CKD (NPV = 70%), although at the initial screening the test correctly identifies 100% of those that do not have CKD (specificity). Because nutritional intervention using appropriately formulated renal diets for cats with CKD is relatively safe, it could be argued that treating cats for CKD based on using SDMA as a biomarker, because of its higher sensitivity, is more logical than missing CKD cats by reliance on sCr, which has higher specificity for CKD.
There is considerable interest in finding new biomarkers to track the progression of renal disease in cats (eg, SDMA, ADMA, and fibroblast growth factor 23)12, 23, 24 and dogs (eg, homocysteine, cystatin C, and clusterin).25, 26 Because plasma SDMA and ADMA concentrations were shown to increase in cats with CKD and to correlate with plasma Cr, they were suggested as possible markers for renal disease although no correlation to GFR was studied in cats.12 In humans, SDMA is more abundant than ADMA in patients with CKD and the correlation between ADMA concentrations and GFR is considerably weaker than that between SDMA and GFR.14 Fibroblast growth factor 23 concentration in cats was shown to negatively correlate with GFR (r = −0.472) and to increase in the early nonazotemic stage of CKD.24 However, as in humans, independent factors such as phosphate, parathyroid hormone, and calcium concentrations may be more important predictors of plasma fibroblast growth factor 23 concentration when GFR is markedly decreased.23, 27
Feeding a renal diet to cats with IRIS CKD stage 2 or higher is considered the current standard of care with strong evidence supporting this recommendation.5 Dietary modifications include decreased protein, phosphorus, and sodium content; increased water soluble vitamins and fiber content; increased caloric density; and additional n‐3 fatty acids, antioxidants, and potassium.5 The kidney diet evaluated in 1 study was superior to an adult maintenance diet in minimizing uremic episodes and kidney‐related deaths in cats with spontaneous stage 2 or 3 CKD.2 The use of a biomarker to help identify cats with CKD earlier in the disease course, corresponding to sCr in the range of 1.6–1.9 mg/dL, may provide additional options for slowing progressive loss of kidney function in order to ameliorate clinical and biochemical consequences of CKD, while maintaining adequate nutrition.5
In summary, this study demonstrated the utility of using SDMA as an early indicator of compromised renal function in cats with CKD. Future studies are needed to demonstrate that early intervention improves the outcome of CKD in cats.
Acknowledgment
Conflict of Interest Declaration: Jean A. Hall has received research grant support from Hill's Pet Nutrition, Inc. in the past.
The work presented in this study was funded by and performed at the Pet Nutrition Center, Hill's Pet Nutrition, Inc., Topeka, KS. The funding decision makers had no role in study design, data collection and analysis, or preparation of the manuscript.
The work was funded by and performed at the Pet Nutrition Center, Hill's Pet Nutrition, Inc., 1035 NE 43rd Street, Topeka, KS 66617‐1587.
Presented at 2014 ACVIM Forum in Nashville, TN.
Footnotes
Roche Diagnostics, Cobas 6000 series, c501 module, Indianapolis, IN
Diagnostic Center for Population and Animal Health, Michigan State University, E. Lansing, MI
Calbiochem, Darmstadt, Germany
Fisher Scientific, Pittsburgh, PA
Shimadzu, Marlborough, MA
Waters XBridge, Milford, MA
Sigma‐Aldrich, St. Louis, MO
IDEXX Laboratories, Inc, Westbrook, ME
SAS Institute, Cary, NC
References
- 1. American Association of Feline Practitioners; Academy of Feline Medicine . American Association of Feline Practitioners/Academy of Feline Medicine Panel Report on Feline Senior Care. J Feline Med Surg 2005;7:3–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ross SJ, Osborne CA, Kirk CA, et al. Clinical evaluation of dietary modification for treatment of spontaneous chronic kidney disease in cats. J Am Vet Med Assoc 2006;229:949–957. [DOI] [PubMed] [Google Scholar]
- 3. Plantinga EA, Everts H, Kastelein AM, et al. Retrospective study of the survival of cats with acquired chronic renal insufficiency offered different commercial diets. Vet Rec 2005;157:185–187. [DOI] [PubMed] [Google Scholar]
- 4. International Renal Interest Society . IRIS 2009 Staging of CKD. http://www.iris-kidney.com. Accessed July 8, 2013.
- 5. Polzin DJ. Evidence‐based step‐wise approach to managing chronic kidney disease in dogs and cats. J Vet Emerg Crit Care (San Antonio) 2013;23:205–215. [DOI] [PubMed] [Google Scholar]
- 6. Von Hendy‐Willson VE, Pressler BM. An overview of glomerular filtration rate testing in dogs and cats. Vet J 2011;188:156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finco DR, Brown SA, Vaden SL, et al. Relationship between plasma creatinine concentration and glomerular filtration rate in dogs. J Vet Pharmacol Ther 1995;18:418–421. [DOI] [PubMed] [Google Scholar]
- 8. Bode‐Boger SM, Scalera F, Kielstein JT, et al. Symmetrical dimethylarginine: A new combined parameter for renal function and extent of coronary artery disease. J Am Soc Nephrol 2006;17:1128–1134. [DOI] [PubMed] [Google Scholar]
- 9. Dixon JJ, Lane K, Dalton RN, et al. Symmetrical dimethylarginine is a more sensitive biomarker of renal dysfunction than creatinine. Crit Care 2013;17:P423. [Google Scholar]
- 10. Kielstein JT, Salpeter SR, Bode‐Boeger SM, et al. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function—A meta‐analysis. Nephrol Dial Transplant 2006;21:2446–2451. [DOI] [PubMed] [Google Scholar]
- 11. Fliser D, Kronenberg F, Kielstein JT, et al. Asymmetric dimethylarginine and progression of chronic kidney disease: The mild to moderate kidney disease study. J Am Soc Nephrol 2005;16:2456–2461. [DOI] [PubMed] [Google Scholar]
- 12. Jepson RE, Syme HM, Vallance C, et al. Plasma asymmetric dimethylarginine, symmetric dimethylarginine, l‐arginine, and nitrite/nitrate concentrations in cats with chronic kidney disease and hypertension. J Vet Intern Med 2008;22:317–324. [DOI] [PubMed] [Google Scholar]
- 13. Bedford MT, Richard S. Arginine methylation an emerging regulator of protein function. Mol Cell 2005;18:263–272. [DOI] [PubMed] [Google Scholar]
- 14. Schwedhelm E, Boger RH. The role of asymmetric and symmetric dimethylarginines in renal disease. Nat Rev Nephrol 2011;7:275–285. [DOI] [PubMed] [Google Scholar]
- 15. Kielstein JT, Boger RH, Bode‐Boger SM, et al. Marked increase of asymmetric dimethylarginine in patients with incipient primary chronic renal disease. J Am Soc Nephrol 2002;13:170–176. [DOI] [PubMed] [Google Scholar]
- 16. Braselton WE, Stuart KJ, Kruger JM. Measurement of serum iohexol by determination of iodine with inductively coupled plasma‐atomic emission spectroscopy. Clin Chem 1997;43:1429–1435. [PubMed] [Google Scholar]
- 17. Closs EI, Basha FZ, Habermeier A, et al. Interference of l‐arginine analogues with l‐arginine transport mediated by the y+ carrier hCAT‐2B. Nitric Oxide 1997;1:65–73. [DOI] [PubMed] [Google Scholar]
- 18. Kakimoto Y. Methylation of arginine and lysine residues of cerebral proteins. Biochim Biophys Acta 1971;243:31–37. [DOI] [PubMed] [Google Scholar]
- 19. Kakimoto Y, Akazawa S. Isolation and identification of N‐G, N‐G‐ and N‐G, N'‐G‐dimethyl‐arginine, N‐epsilon‐mono‐, di‐, and trimethyllysine, and glucosylgalactosyl‐ and galactosyl‐delta‐hydroxylysine from human urine. J Biol Chem 1970;245:5751–5758. [PubMed] [Google Scholar]
- 20. Paiva H, Lehtimaki T, Laakso J, et al. Dietary composition as a determinant of plasma asymmetric dimethylarginine in subjects with mild hypercholesterolemia. Metabolism 2004;53:1072–1075. [DOI] [PubMed] [Google Scholar]
- 21. Schwedhelm E, Xanthakis V, Maas R, et al. Plasma symmetric dimethylarginine reference limits from the Framingham offspring cohort. Clin Chem Lab Med 2011;49:1907–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miyagawa Y, Takemura N, Hirose H. Assessments of factors that affect glomerular filtration rate and indirect markers of renal function in dogs and cats. J Vet Med Sci 2010;72:1129–1136. [DOI] [PubMed] [Google Scholar]
- 23. Geddes RF, Finch NC, Elliott J, et al. Fibroblast growth factor 23 in feline chronic kidney disease. J Vet Intern Med 2013;27:234–241. [DOI] [PubMed] [Google Scholar]
- 24. Finch NC, Geddes RF, Syme HM, et al. Fibroblast growth factor 23 (FGF‐23) concentrations in cats with early nonazotemic chronic kidney disease (CKD) and in healthy geriatric cats. J Vet Intern Med 2013;27:227–233. [DOI] [PubMed] [Google Scholar]
- 25. Tvarijonaviciute A, Ceron JJ, Holden SL, et al. Effect of weight loss in obese dogs on indicators of renal function or disease. J Vet Intern Med 2013;27:31–38. [DOI] [PubMed] [Google Scholar]
- 26. Wehner A, Hartmann K, Hirschberger J. Utility of serum cystatin C as a clinical measure of renal function in dogs. J Am Anim Hosp Assoc 2008;44:131–138. [DOI] [PubMed] [Google Scholar]
- 27. Westerberg PA, Linde T, Wikstrom B, et al. Regulation of fibroblast growth factor‐23 in chronic kidney disease. Nephrol Dial Transplant 2007;22:3202–3207. [DOI] [PubMed] [Google Scholar]


