Abstract
Background
Multiple cytological patterns occur in bronchoalveolar lavage fluid (BALF) of horses with inflammatory airway disease (IAD). Only few data on BALF cytokine profiles are available for horses with IAD, and are limited to mRNA expression.
Hypothesis/Objective
Cytological profiles of IAD are associated with different BALF immunological pathways. To investigate BALF cytokine concentrations in a large number of horses with neutrophilic IAD.
Animals
One hundred and thirty‐eight client‐owned Standardbred racehorses in active training.
Methods
Prospective observational study. BALF samples were obtained from left and right lungs. Interleukin (IL)‐4, interferon (IFN)‐γ, and tumor necrosis factor (TNF)‐α concentrations were determined by ELISA.
Results
Fourteen horses had normal BALF cytological profiles and 56 exhibited evidence of bilateral neutrophilic IAD. Twenty‐four horses showed BALF with, respectively, IAD‐ and CTL consistent cytology and were excluded; as were 44 horses because of evidence of pulmonary hemorrhage. TNF‐α (56 ± 115 pg/mL; P = .034) and IFN‐γ concentrations (104 ± 247 pg/mL; P = .044) were significantly higher for IAD horses, compared with controls (respectively 19 ± 41 and 80 ± 116 pg/mL). Horses with ‘neutrophil’ subtype had significantly higher IFN‐γ concentrations (110 ± 154 pg/mL), than ‘neutrophil/metachromatic’ (56 ± 54 pg/mL; P = .028) and ‘neutrophil/metachromatic/eosinophil’ subtypes (44 ± 23 pg/mL; P = .012).
Conclusions and Clinical Importance
Cytokine concentrations in BALF suggested that neutrophilic IAD is associated with activation of the innate immune system and a possible T‐helper (Th)‐1 polarized response. This study also suggested that immunological pathways vary according to cytological IAD subtypes.
Keywords: Airway inflammation, ELISA, Th1/Th2, Equine
Abbreviations
- BAL
bronchoalveolar lavage
- BALF
bronchoalveolar lavage fluid
- CTL
control
- EIPH
exercise‐induced pulmonary hemorrhage
- IAD
inflammatory airway disease
- IFN
interferon
- IL
interleukin
- RAO
recurrent airway obstruction
- Th
T‐helper
- TNF
tumor necrosis factor
Inflammatory airway disease (IAD) is a syndrome defined as a nonseptic lower airway condition affecting horses of any age, which usually exhibit subtle clinical signs at rest.1 Inclusion criteria for IAD comprise (1) poor performance, exercise intolerance, or coughing, with or without excess tracheal mucus; (2) pulmonary dysfunction or cytological evidence of inflammation on bronchoalveolar lavage fluid (BALF). Exclusion criteria are either increased respiratory effort at rest or evidence of systemic signs of infection.1 The cytological profile of horses with IAD might reveal an increased total nucleated cell count and a very heterogeneous cell population, with mild neutrophilia or lymphocytosis or, alternatively, increased mast cells or eosinophil counts.1, 2
Multiple BALF cytological patterns in horses with IAD are specifically associated with particular clinical or functional response of the airways. Coughing is associated with significantly higher neutrophil percentages in BALF, compared with noncoughing horses.3 Both mast cell3 and eosinophil4 percentages in BALF are correlated with airway hyperresponsiveness, as determined by histamine induced broncho‐provocation; these cells also are, respectively, associated with a reduced respiratory compliance and an increased respiratory resistance of the lower airways.5 The possibility of IAD subtype dependant biological responses warrants further investigations.
Unlike recurrent airway obstruction (RAO), a condition associated with severe neutrophilic airway inflammation,6 very little is known about the molecular pathways and the role of immune system in the pathogenesis of IAD. Characterizing the immunological mechanisms involved in IAD would allow both a more precised diagnosis and the development of appropriate therapeutic options in the future. Several studies have investigated cytokine mRNA expression in BALF of horses with IAD,7, 8, 9 with variable results in terms of T‐helper (Th) lymphocyte polarization. Cytokine mRNA profiles in BALF appear to vary according to the cell types characterizing IAD, particularly neutrophils and metachromatic (mast) cells.7, 9 All previous studies investigating immunological profiles of IAD were, however, performed on a limited number of horses and based only on mRNA expression,7, 8, 9, 10 which might be subjected to posttranscriptional regulation and then not truly reflect protein concentrations.
The aim of this study was to investigate the pattern of cytokine concentrations in BALF samples of a large number of horses with IAD. Both interleukin (IL)‐4 and interferon (IFN)‐γ were investigated, as being archetypical cytokines for, respectively, Th2‐ and Th1‐type responses, and tumor necrosis factor (TNF)‐α as pro‐inflammatory cytokine reflecting innate immune response. The trial was designed to (1) compare BALF cytokine concentrations in controls and horses with cytological evidence of neutrophilic IAD; (2) compare BALF cytokine concentrations among samples with various IAD cytological subtypes; and (3) determine correlations between cytokine concentrations and BALF cell proportions.
Materials and Methods
Horses
As previously described,11 138 Standardbred trotters (76 geldings, 58 females and 4 males), aged 4.0 (3.0–6.0) years, were included in this prospective, observational study. Horses were housed in 11 training stables, and were sampled during summer race meetings in 2011 (64 horses) and 2012 (74 horses). None of the horses exhibited clinical signs of respiratory disease at rest (ie, cough or increased respiratory effort) or had exercise intolerance. They were all involved in active training and had raced within less than 1 month before sampling. Before any procedure, each horse was submitted to a thorough clinical examination to insure that no obvious clinical abnormality was present. The study was approved by the regional Animal Ethic Committee (no. CEEA.2012.179) and all owners signed a consent form.
BAL Procedure
BAL was performed at rest using a flexible 3.2 m long, 12.8 mm tip diameter videoendoscope,1 which was randomly introduced within the left or the right lung, according to the previously published procedure.11 A total of 250 mL of sterile isotonic saline solution was instilled via the endoscope biopsy channel, which was prefilled with 20 mL of saline. Two boluses of 125 mL each were instilled, and aspiration was immediately performed manually after each one. The first 20 mL of aspired liquid, corresponding to the residual volume within the endoscope biopsy channel, were systematically discarded. Following the BAL procedure in one lung side, the endoscope channel was flushed with a 20 mL bolus of isotonic saline. The endoscope was then moved backup to the carina, introduced in the contralateral lung and lavage procedure was repeated. Aliquots of BALF were pooled and kept in EDTA tubes at room temperature for cytological evaluation within 24 hours. All BALF samples for ELISA were stored at −80°C until analysis.
BALF Cytology and Case Definition
All laboratory analyses were conducted in a blinded manner. At reception in the laboratory, 200 μL of fluid were immediately cytocentrifuged (80 × g, 10 minutes)2 and stained with May‐Grünwald‐Giemsa. Left and right samples were processed simultaneously for each horse. Differential cell count was performed on 300 cells, excluding epithelial cells.
Horses were considered as controls (CTL) when BALF cytological profiles from both lungs revealed ≤5% neutrophils amongst leukocytes.1, 2 Horses for which any BALF exhibited proportions above this cutoff value were considered to have cytological evidence of neutrophilic IAD. In addition, BALF proportions of metachromatic cells and eosinophils (respectively ≥2% and ≥1% among leukocytes) were also used for characterizing IAD cytological subtypes. Similarly, the ratio of BALF hemosiderophage/macrophage >20% was considered as cytological evidence of previous episode(s) of exercise‐induced pulmonary hemorrhage (EIPH).12
Quantification of BALF Cytokines and BALF Dilution
After concentrating the BALF 10‐fold,3 TNF‐α, IFN‐γ, and IL‐4 were measured by ELISA,4 according to the manufacturer's instructions with minor modifications. Briefly, the concentration of detection antibody for TNF‐α was set at 300 ng/mL instead of 200 ng/mL; incubation with Streptavidin‐HRP and TMB was also increased to 30 minutes instead of 20 minutes, as previously published.13 Measures were systematically performed in duplicates. According to previous recommendations,14 acellular BAL components were expressed as concentration per volume recovered. The range of quantification in BALF was 3.6–500 pg/mL for TNF‐α, and 31–2000 pg/mL for IL‐4 and IFN‐γ.
Urea concentrations were determined in left and right BALF and serum samples;5 and the ratio (BALF/serum) calculated. This served only as an indicator of BALF dilution.15 and was not employed for normalization (correction) of cytokine concentrations.14
Statistical Analyses
Arbitrary values corresponding to the lowest and the highest standard concentration were attributed to samples with, respectively, undetectable cytokines and optical density values exceeding the range of quantification. Continuous data distributions were not normally distributed as assessed by Shapiro‐Wilk W‐test;6 and nonparametric tests were then used for further investigations. Differences between groups for each variable were evaluated either by Mann‐Whitney or Kruskal‐Wallis with Dunn's posthoc tests. Correlations were evaluated by Spearman's correlation coefficient. Data are presented as median (1st–3rd quartile), unless stated otherwise. A difference of P < .05 was considered significant for all analyses.
Results
BALF Cytology, Characterization, and Sample Status
Left and right lungs were sampled in 138 horses. Among these, 44 horses met the cytological inclusion criteria for EIPH and were excluded from the present study, as were 24 horses showing BALF with, respectively, IAD‐ and CTL consistent cytology (Fig 1). A total of 70 horses (140 BALF samples) was then investigated for cytokine concentrations, among which 14 were CTL and 56 horses had cytological evidence of neutrophilic IAD from both sampled lungs.
Figure 1.
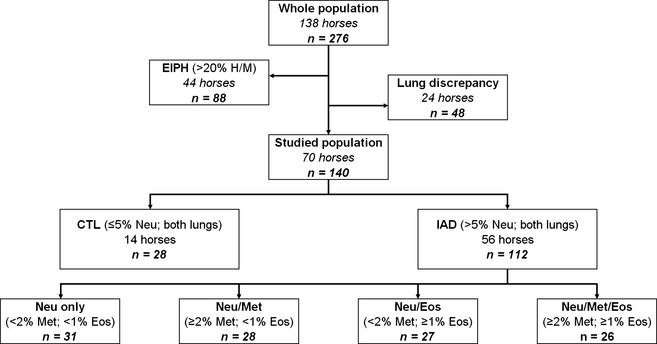
Diagram with breakouts for the 276 bronchoalveolar lavage fluid (BALF) samples according to their cytological profiles. CTL, control; IAD, inflammatory airway disease; EIPH, exercise‐induced pulmonary hemorrhage; H/M, haemosiderophages/macrophages; Neu, neutrophils; Met, metachromatic cells; Eos, eosinophils. ‘Lung discrepancy’ means horses with IAD consistent cytology from one lung and CTL consistent cytology from the contralateral lung.
Volume of BALF recovered, dilution factor and total cell counts were not significantly different among groups (Table 1). Variable combinations of abnormal BALF cytological profiles were present for the 56 horses of the IAD group (Fig 1). Among these 112 samples with >5% neutrophils, 31 had no other abnormality, 28 also had ≥2% metachromatic cells, 27 had ≥1% eosinophils and 26 samples had increased proportions of both metachromatic cells and eosinophils.
Table 1.
Characteristics of the 140 bronchoalveolar lavage fluid (BALF) samples, from horses classified as being controls (CTL), or having cytological evidence of inflammatory airway disease (IAD). Data are presented as median (1st–3rd quartile)
| Equine Status | Number of Samples | Volume Recovered (mL) | Dilution Factor | Total Cell Count (/mm3) |
|---|---|---|---|---|
| CTL | 28 | 117 (97–129) | 0.0160 (0.0123–0.0253) | 220 (150–300) |
| IAD | 112 | 109 (100–124) | 0.0173 (0.0121–0.0270) | 190 (150–255) |
BALF Cytokine Concentrations
BALF concentrations of TNF‐α (P = .034) and IFN‐γ (P = .044) were significantly higher for horses in the IAD group when compared with controls (Fig 2). IAD horses with ‘neutrophil’ subtype had significantly higher concentrations of IFN‐γ, when compared with horses with ‘neutrophil/metachromatic’ (P = .028) and ‘neutrophil/metachromatic/eosinophil’ (P = .012) subtypes (Fig 3). No significant difference was found for IL‐4 concentrations in any group comparisons.
Figure 2.
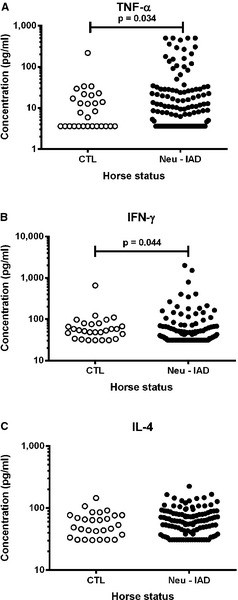
Concentration of (A) tumor necrosis factor (TNF)‐α, (B) interferon (IFN)‐γ and (C) interleukin (IL)‐4 in 140 bronchoalveolar lavage fluid (BALF) samples, from horses classified as being controls (CTL; cytologies with ≤5% neutrophils among leukocytes; n = 28) or having cytological evidence of inflammatory airway disease (IAD; >5% neutrophils; n = 112).
Figure 3.
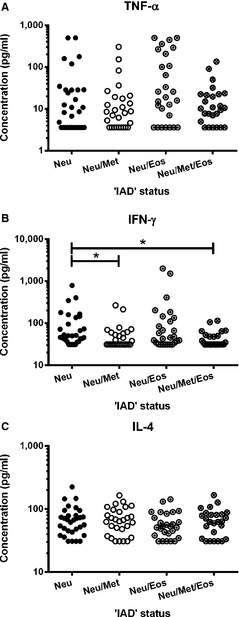
Concentration of (A) tumor necrosis factor (TNF)‐α, (B) interferon (IFN)‐γ and (C) interleukin (IL)‐4 in 112 bronchoalveolar lavage fluid (BALF) samples, from horses with cytological evidence of inflammatory airway disease (IAD), based on increased proportions of neutrophils only (Neu; >5% amongst leukocytes; n = 31), neutrophils and metachromatic cells (Met; ≥2%; n = 28), neutrophils and eosinophils (Eos; ≥1%; n = 27), or neutrophils and metachromatic cells and eosinophils (n = 26). Stars indicate significant differences (P < .05).
Correlations
No significant association was found between total or differential cell counts and any of the investigated cytokines, while IFN‐γ and TNF‐α concentrations in BALF were significantly correlated (r = 0.495, CI 0.353–0.615; P < .001).
Discussion
In this study, cytokine concentrations in BALF were investigated in non‐RAO horses with lower airway inflammation. Although a large overlap between groups was present, BALF concentrations of both IFN‐γ and TNF‐α were significantly higher in horses with neutrophilic ‘IAD’ compared to controls. Concentrations of IFN‐γ also differed between IAD subpopulations, the higher concentrations being observed for ‘neutrophil’ subtype compared to other cellular subtypes.
Horses Recruitment and Case Definitions
In this study, BALF samples were collected in left and right lungs of a large population of horses (n = 138). This allowed enlarging considerably the number of samples simultaneously analyzed, the final investigations being performed in a total of 70 horses. As a comparison, previous studies on BALF mRNA cytokine expression in IAD were based on overall 27–38 horses,7, 8, 9 hence limiting the statistical power of the tests, particularly when subpopulations of IAD horses were compared.
The cytological definition of IAD largely varies among studies, making comparisons difficult. We then decided for discriminating CTL and IAD groups, to use the most restrictive (consensual) definition.1, 2 The phenotype of IAD horses being investigated might also vary among studies, and partially explain some of the controversies among the published results. Horses were sampled in the field by the authors,7 or travelled before investigation, as referred to the veterinary hospital for abnormal respiratory noise, poor performance8 or exercise intolerance.9 BALF samples from several horses were collected after a strenuous treadmill exercise;8 while some other horses, even being sampled at rest, had red tinged BALF indicating recent bleeding.9 It was decided in this study to sample horses at rest, since the possibility of cytological modifications of BALF in response to exercise is still controversial,16, 17 and cytokine mRNA expression might also be influenced by the previous exercise.18 Similarly, all samples with any evidence of EIPH were excluded from this study, to avoid the uncharacterized immune response potentially induced by the ruptured alveolar capillary walls.
Airway immune response might notably involve T cells, alveolar macrophages, and bronchial epithelial cells (reviewed by Pirie).19 Systemic inflammatory changes in horses with heaves have, however, been recently described;20 warranting further local (airways) and systemic investigations in IAD affected horses. In this study, cytokine concentrations were indeed measured in BALF samples only. In the same manner, samples from left and right lungs were previously found not to be equivalent, according to linear regression between cell percentages from left and right lung BALF.11 Regarding the final diagnosis of IAD, a fair agreement only is also observed among lungs (κ = 0.30; CI 0.09–0.50).11 In this study, horses with discrepancy between lungs when considering cytological profiles were then excluded from the statistical analyses, to avoid BALF samples with CTL consistent cytology to be included in the IAD group of horses.
Main Cytokine Profiles
Similar to previous findings on RAO affected horses,21, 22, 23, 24 contradictory results are observed for IAD with regard to the Th1/Th2 polarized response being involved. Data from this study might suggest a predominant Th1‐cell response, while cytokine mRNA expression in previous studies revealed either no Th polarization,8 or a Th2‐cell predominance,7 or a mixed Th1/Th2 response.9
TNF‐α concentrations in BALF suggested the involvement of pulmonary innate immune response in the pathogenesis of IAD, as previously demonstrated by mRNA expression in horses with either IAD8, 9 or RAO.22 The statistically significant results from this study should however be interpreted with caution in a clinical setting, since large overlaps between groups were actually present for cytokine concentrations. Although increased concentrations of IFN‐γ also were coherent with previous data from both IAD9 and RAO,21, 22 IL‐4 concentration in BALF was surprisingly not modified by any condition. Since the horses being sampled might be at various stages of evolution for IAD, a possible hypothesis could be a temporal association with immune response obfuscating the role of the Th2 pathway. This cytokine is indeed significantly up‐regulated in RAO horses22, 24 as well as IAD horses with increased proportions of metachromatic cells in BALF.9 These studies however reported mRNA concentrations only, which might not be reflective of biologically active cytokine concentrations.
IAD Cytological Subtypes
No correlation was found in this study between either cell counts or percentages and BALF cytokine concentrations, which might be because of the multiple cellular sources of cytokines. On the other hand, mRNA expression of both IL‐4 and IFN‐γ correlates significantly with proportions of metachromatic cells.9 In another study, multiple significant correlations were also found between BALF cell types and cytokine expressions;7 correlation coefficients were however not provided.
Being present with increased proportions in at least one lung for 120 of the 138 horses with IAD, neutrophils were largely the predominant BALF cell population characterizing this syndrome in this study. No prominent clinical signs of respiratory disease have however been reported, unlike previous findings of an association between airway neutrophilia and chronic cough (>3 weeks).3 Since one of the inclusion criteria of this study was to be in active training and racing at the time of sampling, such chronically coughing horses were however unlikely to be investigated, somehow biasing the investigations toward a subclinical airway inflammation.
Protein concentrations of IFN‐γ in BALF were significantly lower when increased proportions of metachromatic cells and neutrophils were simultaneously present, compared to increased proportions of neutrophils only. These results might suggest a down‐regulation of Th1 response, possibly induced by Th2 cells. BALF concentrations of IL‐4, mainly secreted by mast cells,25 were however not significantly modified; while IL‐10, a potent inhibitor of IFN‐γ also secreted by mast cells,26 has not been investigated in this study. Conversely, IL‐4 mRNA is less expressed in BALF of ‘neutrophilic’ IAD compared to ‘metachromatic’ IAD, while IL‐10 mRNA expression is not significantly different among these IAD subtypes.7
A nonnegligible number of horses with neutrophilic IAD (65/120) also exhibited increased proportions of BALF eosinophils from at least one lung in this study. Being significantly associated with abnormal lung function,4, 5 these cells were suggested to be responsible for one of the IAD subtypes.1, 2 While not investigated in the previous studies on IAD,7, 8, 9 no significant influence of eosinophil proportions on BALF cytokine concentrations was detected in this study. Similarly, neither distinct clinical signs nor significant modifications of IL‐4 and IL‐5 mRNA expression in BALF occur in horses with transient pulmonary eosinophilia.27
Limitations of the Study
Quantitative polymerase chain reaction (qPCR) for mRNA expression was not performed, since BALF samples have not been appropriately conditioned for RNA preservation. Comparisons between mRNA expression and subsequent protein concentrations might however be relevant in such studies, as posttranscriptional regulation might apply. Indeed, inconsistencies have previously been reported in BALF of healthy horses, where IL‐4 mRNA expression was detected, while IL‐4 protein was not detected at any time point.28
Moreover, a few cytokines only were investigated, mainly because of the limited number of equine specific reagents currently available and methodological limitations. Using multiplex ELISA29 is largely warranted in the near future, especially when a large cohort of samples has to be investigated. As in previous similar studies,7, 8, 9 whole BALF with mixed cell populations have been investigated; also leading to undetectable cytokine concentrations in many of the samples. Cell isolation should be performed in the future, and cytokines productions analyzed separately for a better characterization of the Th driven response in IAD.
Conclusion
The results being obtained suggested neutrophilic IAD to be associated with a stimulation of the innate immune system and a possible Th1 polarized response. This study also suggested, at the protein level, that BALF immunological profiles might vary according to the IAD cytological subtypes. Further studies are required to determine the specific cytokine signatures associated with the different IAD phenotypes of athletic horses.
Acknowledgments
The authors thank Marie‐Pierre Toquet, Vincent Gennevieve, Ludovic Petit and Claudine Hary for their kind collaboration. This work was supported by ‘Hippolia’ Foundation.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
The work was done in LABÉO Frank Duncombe.
The study was supported by ‘County Council of Calvados’, ‘IFCE’ (National Studs) and ‘SECF’ (French Trotters Racing Association).
Footnotes
VO 320; Optomed, Les Ulis, France
Shandon Cytospin; Thermo Scientific, Waltham, MA
Amicon Ultra‐4 3K; Merck Millipore, Billerica, MA
DuoSet; R&D Systems, Minneapolis, MN
QuantiChrom Urea Assay; BioAssay Systems, Hayward, CA
Prism 6.04; GraphPad, La Jolla, CA
References
- 1. Couëtil LL, Hoffman AM, Hodgson J, et al. Inflammatory airway disease of horses. J Vet Intern Med 2007;21:356–361. [DOI] [PubMed] [Google Scholar]
- 2. Robinson NE. Inflammatory airway disease: Defining the syndrome. Conclusions of the Havemeyer Workshop. Equine Vet Educ 2003;15:61–63. [Google Scholar]
- 3. Bedenice D, Mazan MR, Hoffman AM. Association between cough and cytology of bronchoalveolar lavage fluid and pulmonary function in horse diagnosed with inflammatory airway disease. J Vet Intern Med 2008;22:1022–1028. [DOI] [PubMed] [Google Scholar]
- 4. Hare JE, Viel L. Pulmonary eosinophilia associated with increased airway responsiveness in young racing horses. J Vet Intern Med 1998;12:163–170. [DOI] [PubMed] [Google Scholar]
- 5. Richard EA, Fortier GD, Denoix JM, et al. Influence of subclinical inflammatory airway disease on equine respiratory function evaluated by impulse oscillometry. Equine Vet J 2009;41:384–389. [DOI] [PubMed] [Google Scholar]
- 6. Robinson NE. International workshop on equine chronic airway disease. Equine Vet J 2001;33:5–19. [DOI] [PubMed] [Google Scholar]
- 7. Beekman L, Tohver T, Léguillette R. Comparison of cytokine mRNA expression in the bronchoalveolar lavage fluid of horses with inflammatory airway disease and bronchoalveolar lavage mastocytosis or neutrophilia using REST software analysis. J Vet Intern Med 2012;26:153–161. [DOI] [PubMed] [Google Scholar]
- 8. Hughes KJ, Nicolson L, Da Costa N, et al. Evaluation of cytokine mRNA expression in bronchoalveolar lavage cells from horse with inflammatory airway disease. Vet Immunol Immunopathol 2011;140:82–89. [DOI] [PubMed] [Google Scholar]
- 9. Lavoie JP, Cesarini C, Lavoie‐Lamoureux A, et al. Bronchoalveolar lavage fluid cytology and cytokine messenger ribonucleic acid expression of racehorses with exercise intolerance and lower airway inflammation. J Vet Intern Med 2011;25:322–329. [DOI] [PubMed] [Google Scholar]
- 10. Ryhner T, Müller N, Balmer V, et al. Increased mucus accumulation in horses chronically affected with recurrent airway obstruction is not associated with up‐regulation of CLCA1, EGFR, MUC5AC, Bcl‐2, IL‐13 and IFN‐gamma expression. Vet Immunol Immunopathol 2008;125:8–17. [DOI] [PubMed] [Google Scholar]
- 11. Depecker M, Richard EA, Pitel PH, et al. Bronchoalveolar lavage fluid in Standardbred racehorses: Influence of unilateral/bilateral profiles and cut‐off values on lower airway disease diagnosis. Vet J 2014;199:150–156. [DOI] [PubMed] [Google Scholar]
- 12. Richard EA, Fortier GD, Lekeux PM, et al. Laboratory findings in respiratory fluids of the poorly‐performing horse. Vet J 2010;185:115–122. [DOI] [PubMed] [Google Scholar]
- 13. Lavoie‐Lamoureux A, Maghni K, Lavoie JP. Optimization of a procedure to accurately detect equine TNFα in serum samples. Vet Immunol Immunopathol 2010;138:118–123. [DOI] [PubMed] [Google Scholar]
- 14. Haslam PL, Baughman RP. Report of ERS Task Force: Guidelines for measurement of acellular components and standardization of BAL. Eur Respir J 1999;14:245–248. [DOI] [PubMed] [Google Scholar]
- 15. McGorum BC, Dixon PM, Halliwell RE, et al. Evaluation of urea and albumen as endogenous markers of dilution of equine bronchoalveolar lavage fluid. Res Vet Sci 1993;55:52–56. [DOI] [PubMed] [Google Scholar]
- 16. Clark SP, Lester GD, Vetro T, et al. Bronchoalveolar lavage in horses: Effect of exercise and repeated sampling on cytology. Aust Vet J 1995;72:249–252. [DOI] [PubMed] [Google Scholar]
- 17. Couëtil LL, Denicola DB. Blood gas, plasma lactate and bronchoalveolar lavage cytology analyses in racehorses with respiratory disease. Equine Vet J Suppl 1999;30:77–82. [DOI] [PubMed] [Google Scholar]
- 18. Davis MS, Williams CC, Meinkoth JH, et al. Influx of neutrophils and persistence of cytokine expression in airways of horses after performing exercise while breathing cold air. Am J Vet Res 2007;68:185–189. [DOI] [PubMed] [Google Scholar]
- 19. Pirie RS. Recurrent airway obstruction: A review. Equine Vet J 2014;46:276–288. [DOI] [PubMed] [Google Scholar]
- 20. Lavoie‐Lamoureux A, Leclere M, Lemos K, et al. Markers of systemic inflammation in horses with heaves. J Vet Intern Med 2012;26:1419–1426. [DOI] [PubMed] [Google Scholar]
- 21. Ainsworth D, Grünig G, Matychak MB, et al. Recurrent airway obstruction (RAO) in horses is characterized by IFN‐gamma and IL‐8 production in bronchoalveolar lavage cells. Vet Immunol Immunopathol 2003;96:83–91. [DOI] [PubMed] [Google Scholar]
- 22. Giguère S, Viel L, MacKay RJ, et al. Cytokine induction in pulmonary airways of horses with heaves and effect of therapy with inhaled fluticasone propionate. Vet Immunol Immunopathol 2002;85:147–158. [DOI] [PubMed] [Google Scholar]
- 23. Horohov DW, Beadle RE, Mouch S, et al. Temporal regulation of cytokine mRNA expression in equine recurrent airway obstruction. Vet Immunol Immunopathol 2005;108:237–245. [DOI] [PubMed] [Google Scholar]
- 24. Lavoie JP, Maghni K, Desnoyers M, et al. Neutrophilic airway inflammation in horses with heaves is characterized by a Th2‐type cytokine profile. Am J Respir Crit Care Med 2001;164:1410–1413. [DOI] [PubMed] [Google Scholar]
- 25. Khodoun MV, Orekhova T, Potter C, et al. Basophils initiate IL‐4 production during a memory T‐dependent response. J Exp Med 2004;200:857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galli SJ, Kalesnikoff J, Grimbaldeston MA, et al. Mast cells as “tunable” effector and immunoregulatory cells: Recent advances. Annu Rev Immunol 2005;23:749–786. [DOI] [PubMed] [Google Scholar]
- 27. Riihimäki M, Lilliehöök I, Raine A, et al. Clinical alterations and mRNA levels of IL‐4 and IL‐5 in bronchoalveolar cells of horses with transient pulmonary eosinophilia. Res Vet Sci 2008;85:52–55. [DOI] [PubMed] [Google Scholar]
- 28. Perkins GA, Viel L, Wagner B, et al. Histamine bronchoprovocation does not affect bronchoalveolar lavage fluid cytology, gene expression and protein concentrations of IL‐4, IL‐8 and IFN‐gamma. Vet Immunol Immunopathol 2008;126:230–235. [DOI] [PubMed] [Google Scholar]
- 29. Wagner B, Freer H. Development of a bead‐based multiplex assay for simultaneous quantification of cytokines in horses. Vet Immunol Immunopathol 2009;127:242–248. [DOI] [PubMed] [Google Scholar]


