Abstract
Background
A strong correlation between left atrial (LA) dysfunction and the severity of cardiac disease has been described in human patients with various cardiac diseases. The role of LA dysfunction in dogs with chronic mitral valvular heart disease (CMVHD) has not been addressed.
Objectives
To investigate the correlation between LA function and the prognosis of dogs with CMVHD.
Animals
Thirty‐eight client‐owned dogs with CMVHD.
Methods
Prospective clinical cohort study. Dogs were divided into 2 groups (survivors and nonsurvivors) based on the onset of cardiac‐related death within 1 year. Physical examination and echocardiographic variables were compared between the groups. For the assessment of the comparative accuracy in identifying patients with cardiac‐related death, receiver operating characteristic (ROC) curves and multivariate logistic analysis were used.
Results
The highest accuracy was obtained for the LA active fractional area change (LA‐FAC act), with an area under the ROC curve (AUC) of 0.95, followed by the left atrial to aortic root ratio (LA/Ao), with an AUC of 0.94; peak early diastolic mitral inflow velocity (E), with an AUC of 0.85; and LA total fractional area change (LA‐FAC total), with an AUC of 0.85. In the multivariate logistic regression analysis, LA‐FAC act emerged as the only independent correlate of cardiac‐related death within 1 year (odds ratio = 1.401, P = .002).
Conclusions and Clinical Importance
Regarding both the size and function, the LA has a strong correlation with the prognosis of dogs with CMVHD. The most significant independent predictor of mortality in this study was LA‐FAC act.
Keywords: Cardiac‐related death, Dog, Echocardiography, Left atrial emptying fraction
Abbreviations
- A
late diastolic mitral inflow velocity
- Am
late diastolic velocity of the septal mitral annulus
- AUC
area under the receiver operating characteristic curve
- CI
confidence interval
- CMVHD
chronic mitral valvular heart disease
- Em
early diastolic velocity of the septal mitral annulus
- E
peak early diastolic mitral inflow velocity
- FS
fractional shortening
- LA/Ao
left atrial to aortic root ratio
- LA‐FACact
left atrial active fractional area change
- LA‐FACpass
left atrial passive fractional area change
- LA‐FACtotal
left atrial total fractional area change
- LA
left atrial
- LAmax
maximum area of the left atrium at ventricular end‐systole
- LAmin
minimum area of the left atrium at ventricular end‐diastole
- LAp
left atrium at onset of the P wave on the ECG
- LVIDd inc%
percent increase in left ventricular diameter in diastole
- LVIDd
left ventricular diameter in diastole
- LVIDsinc%
percent increase in left ventricular diameter in systole
- LVIDs
left ventricular diameter in systole
- LV
left ventricular
- MR
mitral regurgitation
- ROC
receiver operating characteristic
Chronic mitral valvular heart disease (CMVHD) is the most common heart disease in dogs. This disease is caused by progressive myxomatous degeneration of the mitral valve, leading to incomplete coaptation of the leaflets and valvular regurgitation.1, 2 The reported prevalence of this disease in small dogs ranges from 14% to over 40%, depending on the breed.3, 4, 5 Although most dogs with CMVHD remain asymptomatic for years or even for life,6, 7, 8, 9 the progression of mitral regurgitation (MR) can cause severe congestive heart failure, leading to death in some dogs.
Predicting the onset of congestive heart failure or cardiac‐related death is of clinical importance. To date, many studies have investigated the prognostic factors for survival or disease worsening in dogs with CMVHD.9, 10, 11, 12 The high prognostic value of the degree of left atrial (LA) dilatation has been demonstrated in dogs with CMVHD.10, 11, 12 Chronic and hemodynamically significant MR results in volume overload, which is first characterized by LA enlargement. Echocardiography can be used to measure LA size noninvasively; traditionally, LA size is measured by using linear M‐mode or two‐dimensional (2D) methods. The left atrial to aortic root ratio (LA/Ao) determined from the right parasternal short axis view is the most commonly used method for detecting LA enlargement in veterinary clinical practice, and it has been demonstrated to be a reliable method to detect LA enlargement.13, 14
The left atrium plays a key role in cardiac performance by modulating left ventricular (LV) filling with its reservoir function (expansion associated with the inflow of blood from the pulmonary veins during ventricular systole), conduit function (passage of blood from the pulmonary veins to the left ventricle during ventricular diastole), and booster pump function (augmentation of LV filling during atrial contraction).15 LV diastolic dysfunction, elevated filling pressure, LV hypertrophy, and MR are all potential contributors to ongoing LA morphologic and pathologic changes. Increased LA volume may be accompanied morphologic and pathologic changes. Increased LA volume may be accompanied by a progressive impairment in LA function, and both may precede symptom development and adversely affect prognosis. A strong correlation between LA dysfunction and the severity of cardiac disease has been described in human patients with various cardiac diseases.16, 17, 18 The role of LA dysfunction in dogs with CMVHD has not been addressed.
The purpose of this study was to investigate the correlation between LA function and survival time in dogs with CMVHD.
Materials and Methods
Animals
Thirty‐eight client‐owned dogs were prospectively studied. Dogs with CMVHD were consecutively selected between April 2010 and December 2013 at the Hokkaido University Veterinary Teaching Hospital. The diagnosis of CMVHD was confirmed by the presence of a left systolic apical heart murmur and the echocardiographic findings of systolic MR by color Doppler imaging with irregularly thickened mitral valve leaflets.
All dogs included in this study had undergone physical examination, blood tests, thoracic radiographs, and echocardiography, and were classified according to the American College of Veterinary Internal Medicine consensus statement.19 Dogs with atrial flutter or fibrillation and other concurrent acquired cardiac diseases, such as cardiomyopathy or infective endocarditis, or congenital cardiac diseases were excluded.
Classification
Dogs were divided into 2 groups for statistical analysis based on whether they survived for more than 1 year after the first echocardiographic examination (Group A, “survivors”) or experienced cardiac‐related death within 1 year after the first echocardiographic examination (Group B, “nonsurvivors”). Cardiac‐related death was defined as death occurring because of progression of clinical signs of heart failure without any other identifiable cause of death.
Conventional Echocardiography and Doppler Examination
Conventional 2D and M‐mode echocardiographic and Doppler examinations were performed by an experienced veterinarian (KN) with an ultrasound unit (HI VISION Preirus1) equipped with a 3–7 MHz phased array sector probe (EUP‐S521) in all dogs. Dogs were unsedated and gently restrained in left and right lateral recumbency during the examination. Measurements were obtained using the 2D‐guided M‐mode with concomitant ECG registration for the ventricles according to the guidelines of the American Society of Echocardiography.20 The LA/Ao was obtained from the right parasternal short axis 2D view as previously described.14 The LV diameter in diastole (LVIDd) and LV diameter in systole (LVIDs) were measured from the M‐mode echocardiogram from the right parasternal short axis 2D view.21 M‐mode values were used to derive the fractional shortening (FS) and the percent increase in LVIDd (LVIDd inc%) and LVIDs (LVIDs inc%) according to the following equation22: % increase = 100 × (observed dimension − expected normal dimension)/expected normal dimension. Expected normal dimensions were calculated according to the following equations22: expected normal LVIDd = 1.53 × (body weight)0.294 and expected normal LVIDs = 0.95 × (body weight)0.315. From the left apical 4‐chamber view, pulsed Doppler was used to measure the peak early (E) and late (A) diastolic mitral inflow velocity, and tissue Doppler was used to measure the early diastolic (E m) and late diastolic (A m) velocity of the septal mitral annulus.
Left Atrial Function Analysis with 2D Speckle Tracking Echocardiography
For LA function analysis, images of the 3 consecutive cardiac cycles from the left apical 4‐chamber view were saved onto a hard drive for offline analysis. A frame corresponding to the time of the peak of the R wave on the ECG was selected as the indicator for left ventricular end‐diastole, and the endocardium of the left atrium was manually traced in that frame. The area of the left atrium was then automatically calculated by the software (Left Atrial Tracking1) in each subsequent frame throughout the cardiac cycle to derive a time‐left atrial area curve. The maximum area of the left atrium at ventricular end‐systole (LAmax), area of the left atrium at the onset of the P wave on the ECG (LAp), and minimum area of the left atrium at ventricular end‐diastole (LAmin) were determined by the same software. Indicators of left atrial phasic function (reservoir, conduit, and booster) were calculated with the following equations: total fractional area change (LA‐FACtotal) = 100 × (LAmax − LAmin)/LAmax; passive fractional area change (LA‐FACpass) = 100 × (LAmax − LAp)/LAmax; and active fractional area change (LA‐FACact) = 100 × (LAp − LAmin)/LAp.23
Statistical Analysis
Measurements are presented as the median and range. Variables were compared using the Mann‐Whitney U‐test for continuous variables and the chi‐squared test for categorical variables (Fisher's exact test for 2 categorical variables and the likelihood ratio test for more than 2 categorical variables). The relationships among different parameters were assessed by Spearman's correlation analysis.
For the assessment of the comparative accuracy of different echocardiography variables in identifying patients with cardiac‐related death, receiver operating characteristic (ROC) curves and the respective area under the ROC curve (AUC) were calculated for those variables with significance set at P < .05 in the Mann‐Whitney U‐test. Predictors of cardiac‐related death within 1 year were assessed using binary logistic regression analysis. Echocardiographic variables with a P < .01 in univariate analyses were included in the multivariate model. Hosmer‐Lemeshow statistics and accuracy rate were calculated to evaluate the fitness of the model. All statistical analyses were performed with commercially available statistical software.2 A two‐sided P‐value <.05 was considered significant.
Results
Table 1 shows the demographic data, physical examination results, and radiographic and echocardiographic characteristics of the study population. There were no significant differences between patients in Groups A and B with respect to age, sex, body weight, heart rate, and vertebral heart scale (P > .05). The distribution of American College of Veterinary Internal Medicine classes between the 2 groups was significantly different (P < .001).
Table 1.
Clinical and echocardiographic characteristics of dogs in Groups A and B. Group A included dogs surviving for more than 1 year after echocardiographic examination; Group B included dogs experiencing cardiac‐related death within 1 year
| Group A (n = 26) | Group B (n = 12) | P‐Value | |
|---|---|---|---|
| Age (years) | 12 (8–14) | 13 (7–15) | .053 |
| Sex (female/male) | 10/16 | 5/7 | .284 |
| Body weight (kg) | 6.05 (2.5–14.1) | 6.21 (2.95–9.8) | .588 |
| Heart rate (bpm) | 148 (88–186) | 158 (92–212) | .172 |
| ACVIM classa | |||
| B1 | 11 (42.3%) | 0 (0%) | <.001 |
| B2 | 13 (50%) | 2 (16.7%) | |
| C | 2 (7.7%) | 5 (41.7%) | |
| D | 0 (0%) | 5 (41.7%) | |
| Pulmonary edemaa | 2 (7.7%) | 7 (58.3%) | .002 |
| Vertebral heart scale | 11 (9.5–12.5) | 12.5 (9–13) | .067 |
| Medication | |||
| ACE inhibitora | 9 (34.6%) | 9 (75%) | .035 |
| Pimobendana | 5 (19.2%) | 7 (58.3%) | .026 |
| Loop diureticsa | 3 (11.5%) | 10 (83.3%) | <.001 |
| Conventional parameters | |||
| LA/Aoa | 1.70 (1.22–2.81) | 2.38 (1.92–3.75) | <.001 |
| LVIDd inc%a | 12.0 (−11.7 to 39.6) | 28.7 (−1.4 to 81.8) | .002 |
| LVIDs inc%a | −8.9 (−45.3 to 30.8) | 5.83 (−15.4 to 47.0) | .038 |
| FS | 45.2 (26.3–67.6) | 48.7 (41.2–50.8) | .283 |
| E (m/s)a | 0.84 (0.36–1.85) | 1.29 (0.82–1.93) | <.001 |
| A (m/s) | 0.74 (0.42–1.31) | 0.8 (0.53–1.17) | .566 |
| E/A a | 1.21 (0.54–3.78) | 1.52 (1.01–2.59) | .011 |
| E m (cm/s) | 6.45 (4.2–10.65) | 8.2 (5.5–10.8) | .081 |
| A m (cm/s) | 9.3 (4.5–13.7) | 7.9 (4.3–11) | .727 |
| E/E m a | 12.0 (7.9–31.9) | 15.6 (9.1–35.1) | .042 |
| LA function parameters | |||
| LA‐FACtotal a | 50.1 (35.5–64.7) | 37.1 (20.7–55.7) | .001 |
| LA‐FACpass | 26.6 (11.8–36.7) | 25.7 (15.5–38.1) | .914 |
| LA‐FACact a | 31.9 (13.9–50.0) | 14.9 (6.1–28.4) | <.001 |
Data are expressed as the median (range) or number (percentage).
A, late diastolic mitral inflow velocity; ACE, angiotensin‐converting enzyme; ACVIM, American College of Veterinary Internal Medicine; A m, late diastolic velocity of the septal mitral annulus; E, peak early diastolic mitral inflow velocity; E m, early diastolic velocity of the septal mitral annulus; FS, fractional shortening; LA, left atrial; LA/Ao, left atrial to aortic root ratio; LVIDd inc%, percent increase in left ventricular diameter in diastole; LVIDs inc%, percent increase in left ventricular diameter in systole; LA‐FACact, left atrial active fractional area change; LA‐FACpass, left atrial passive fractional area change; LA‐FACtotal, left atrial total fractional area change.
Values between Groups A and B differed significantly (P < .05).
The incidence of pulmonary edema was significantly higher in Group B (58.3%) than Group A (7.7%) (P = .002). The use of angiotensin‐converting enzyme (ACE) inhibitors (P = .035), pimobendan (P = .026), and loop diuretics (P < .001) was significantly different between the 2 groups.
Results of conventional echocardiographic parameters are also shown in Table 1. There were significant differences in LA/Ao, LVIDd inc%, E, E/A, and E/E m between the 2 groups. Two of the 3 LA function parameters, LA‐FACtotal [37.1% (20.7–55.7%) versus 50.1% (35.5–64.7%); P = .001] and LA‐FACact [14.9% (6.1–28.4%) versus 31.9% (13.9–50.0%); P < .001], significantly decreased in Group B compared to Group A.
The LA function parameters were significantly related to some of the assessed conventional echocardiographic parameters (Fig 1). Significant correlations were found between LA‐FACtotal and LA/Ao (r = −0.535, P = .001), LVIDd inc% (r = −0.332, P = .042), LVIDs inc% (r = −0.577, P < .001), and A m (r = 0.532, P = .001) (Table 2). The LA‐FACact had a significant correlation with age (r = −0.330, P = .043), LA/Ao (r = −0.771, P < .001), LVIDd inc% (r = −0.562, P < .001), LVIDs inc% (r = −0.500, P = .001), E (r = −0.551, P < .001), E/A (r = −0.437, P = .006), and A m (r = 0.461, P = .004) (Table 2).
Figure 1.
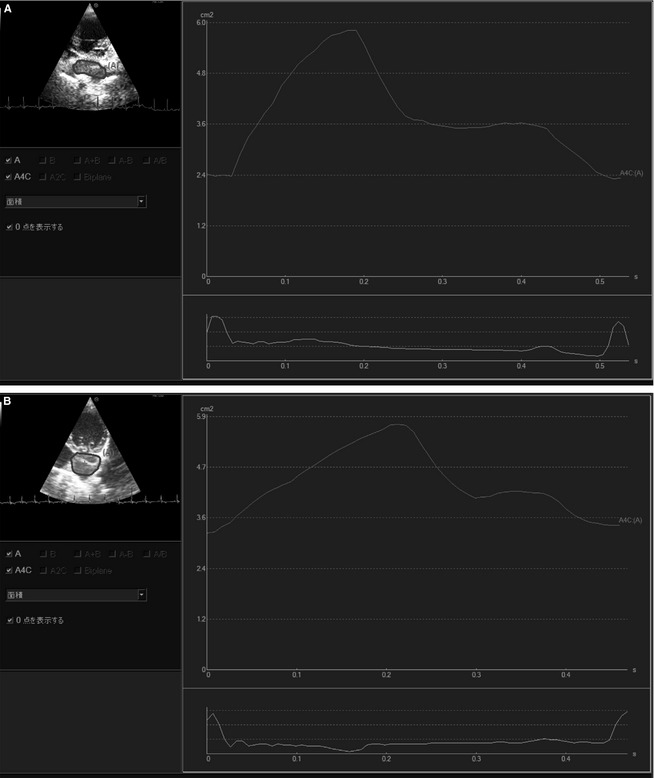
Time‐left atrial area curves of a dog in Group A (A) and Group B (B). The upper graph represents the time‐left atrial area curve and the lower represents the ECG tracing. The dog in Group B has a lower LA‐FACtotal and LA‐FACact than the dog in Group A.
Table 2.
Correlates of left atrial function parameters
| %LAEFtotal | %LAEFpass | %LAEFact | ||||
|---|---|---|---|---|---|---|
| P‐Value | r | P‐Value | r | P‐Value | r | |
| Age | .231 | .932 | .043 | −0.330 | ||
| Body weight | .205 | .264 | .659 | |||
| Heart rate | .530 | .023 | 0.378 | .873 | ||
| LA/Ao | .001 | −0.0535 | .962 | <.001 | −0.771 | |
| LVIDd inc% | .042 | −0.332 | .370 | <.001 | −0.562 | |
| LVIDs inc% | <.001 | −0.577 | .140 | .001 | −0.500 | |
| FS | .064 | .011 | 0.408 | .963 | ||
| E | .133 | .069 | <.001 | −0.551 | ||
| A | .482 | .976 | .634 | |||
| E/A | .136 | .197 | .006 | −0.437 | ||
| E m | .96 | .389 | .355 | |||
| A m | .001 | 0.532 | .127 | .004 | 0.461 | |
| E/E m | .611 | .124 | .051 | |||
| %LAtotal | <.001 | 0.586 | <.001 | 0.846 | ||
| %LApass | <.001 | 0.586 | .401 | |||
| %LAAact | <.001 | 0.846 | .401 | |||
A, late diastolic mitral inflow velocity; A m, late diastolic velocity of the septal mitral annulus; E, peak early diastolic mitral inflow velocity; E m, early diastolic velocity of the septal mitral annulus; FS, fractional shortening; LA/Ao, left atrial to aortic root ratio; LVIDd inc%, percent increase in left ventricular diameter in diastole; LVIDs inc%, percent increase in left ventricular diameter in systole; %LAEFact, left atrial active emptying fraction; %LAEFpass, left atrial passive emptying fraction; %LAEFtotal, left atrial total emptying fraction.
For the comparative assessment of the accuracy of conventional echocardiographic parameters and LA function parameters in identifying the dogs with short survival times, ROC curves and the corresponding AUC were calculated. As shown in Table 3, the highest accuracy was obtained for LA‐FACact, which had an AUC of 0.95, a sensitivity of 91%, and a specificity of 96%, followed by the LA/Ao, which had an AUC of 0.94, a sensitivity of 100%, and a specificity of 88%. The E/A had the lowest accuracy, with an AUC of 0.74, a sensitivity of 73%, and a specificity of 72%, whereas the LVIDs inc% had the second lowest accuracy, with an AUC of 0.77, a sensitivity of 73%, and a specificity of 76%. Although E, LA‐FACtotal, and LVIDd inc% had the same AUC of 0.85, they differed in sensitivity (82%, 82%, and 73%, respectively) and specificity (84%, 76%, and 84%, respectively).
Table 3.
Area under the receiver operating characteristic curve (AUC) and optimal diagnostic cutoffs between Groups A and B
| AUC | 95% CI | Se | Sp | Cutoff | P‐Value | |
|---|---|---|---|---|---|---|
| %LAEFact | 0.95 | 0.88–1.00 | 0.91 | 0.96 | 24.0 | <.0001 |
| LA/Ao | 0.94 | 0.85–1.00 | 1.00 | 0.88 | 2.06 | <.0001 |
| E | 0.85 | 0.71–0.98 | 0.82 | 0.84 | 1.17 | .001 |
| %LAEFtotal | 0.85 | 0.70–0.99 | 0.82 | 0.76 | 45.6 | .001 |
| LVIDd inc% | 0.85 | 0.72–0.98 | 0.73 | 0.84 | 26.2 | .001 |
| LVIDs inc% | 0.77 | 0.62–0.92 | 0.73 | 0.76 | −2.2 | .011 |
| E/A | 0.74 | 0.58–0.91 | 0.73 | 0.72 | 1.36 | .022 |
%LAEFact, left atrium active emptying fraction; LA/Ao, left atrial to aortic root ratio; E, peak early diastolic mitral inflow velocity; %LAEFtotal, left atrium total emptying fraction; LVIDd inc%, percent increase in left ventricular diameter in diastole; LVIDs inc%, percent increase in left ventricular diameter in systole; A, late diastolic mitral inflow velocity; Se, sensitivity; and Sp, specificity.
Based on the results of univariate analysis, the variables LA/Ao, E, LA‐FACtotal, and LA‐FACact were selected for multivariate logistic regression analysis (Table 4). In the multivariate logistic regression analysis, LA‐FACact emerged as the only independent correlate of cardiac‐related death within 1 year in our study population (odds ratio = 1.401; 95% confidence interval (CI), 1.132–1.735; P = .002) (Table 4).
Table 4.
Binary logistic regression analysis for cardiac‐related death within 1 year
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR | 95% CI | P‐Value | P‐Value | |
| LA/Ao | 0.956 | 0.929–0.984 | .002 | .372 |
| E | 0.019 | 0.001–0.326 | .006 | .705 |
| %LAEFtotal | 1.164 | 1.049–1.291 | .004 | .179 |
| %LAEFact | 1.401 | 1.132–1.735 | .002 | .002 |
CI, confidence interval; E, peak early diastolic mitral inflow velocity; LA/Ao, left atrial to aortic root ratio; OR, odds ratio; %LAEFact, left atrial active emptying fraction; %LAEFtotal, left atrial total emptying fraction.
Discussion
The findings of this study indicate that LA size and function are strongly correlated with early death in dogs with CMVHD. Although several echocardiographic parameters were significantly different between the 2 groups, LA‐FACact, the parameter corresponding to the booster pump function, was the most significant independent predictor of mortality in this study.
Many studies have reported the value of echocardiography for predicting survival time and providing prognostic indicators in dogs with CMVHD.9, 10, 11, 12, 24 Although the recent advancements in ultrasound equipment, such as tissue Doppler imaging and strain imaging, have facilitated more detailed analysis of cardiac ventricular function, assessment of LA size with LA/Ao using conventional B‐mode remains one of the most important echocardiographic methods to evaluate the severity and prognosis of CMVHD.24, 25, 26 To the best of our knowledge, this study is the first report demonstrating that LA function analysis could be the most reliable prognostic indicator in dogs with CMVHD.
In our study, significant increases in LA/Ao were observed in nonsurvivor dogs, and the LA/Ao had a moderate and strong correlation with the reservoir and booster pump functions, respectively. However, LA enlargement does not always result in its functional incompetence. During chronic MR, the left atrium enlarges in size and the LA chamber becomes more compliant. Thus, the enlarged left atrium appears to exert an important compensatory mechanism by buffering the rise in pressure in the atrium and by providing an adequate ventricular filling volume. Moreover, it has been demonstrated that the Frank‐Starling mechanism is also operative in the left atrium and that LA output increases as atrial volume increases, which contributes to maintaining a normal stroke volume.27 The increased atrial response to early‐stage LV filling impairment is characterized by augmented reservoir and booster pump functions according to the Frank‐Starling mechanism. At end‐stage LV dysfunction, however, the atrial reservoir and the booster pump functions decline.28 Therefore, the analysis of atrial function, as well as its size, is required to evaluate the severity and prognosis of dogs with CMVHD.
During LV systole, pulmonary venous inflow distends the left atrium, which acts as a reservoir by storing energy in the form of pressure.29 The LA reservoir function is determined mainly by LA compliance (stiffness) and LV systolic function (systolic apical motion of the LV base facilitates LA filling) and, to a lesser extent, by right ventricular systole (pulmonary venous inflow).15, 29 Decreased LA reservoir function has been described in human patients with severe MR.18 Similarly, this study showed that severe MR is associated with a significant reduction in LA reservoir function. This reduced function may be caused partly by reduced LV systolic function because %LVEFtotal had a mild correlation with the FS of the left ventricle in this study. However, the ultrastructural changes in the LA myocardium, including the presence of interstitial fibrosis and myocyte hypertrophy in patients with severe MR, may reduce LA compliance, resulting in reservoir dysfunction.30
The LA functions as a conduit after the reservoir phase, allowing a passive transfer of blood from the pulmonary veins toward the left ventricle. The LA conduit function is determined mainly by LA elasticity and afterload. Although impaired LA conduit function was observed in human patients with severe MR,31 there was no difference between the conduit function of survivors and nonsurvivors in this study. The clinical utility of evaluating LA conduit function in dogs with CMVHD is still uncertain.
Active LA contraction, the booster pump function, finalizes LV filling during late diastole. It depends on LA afterload (LV compliance and end‐diastolic filling pressure) and LA intrinsic contractility. In this study, impaired LA booster pump function was observed to a greater degree in nonsurvivor dogs, and it was the most significant independent predictor of mortality in this study; it may be related to the decreased LA contractility and increased LA afterload. These results are congruent with findings from human patients with hypertrophic cardiomyopathy and MR. In those studies, it was demonstrated that parameters of booster pump function had the highest correlation with the severity of the disease.16, 30
Mitral A m velocity with tissue Doppler imaging, another parameter of atrial function, had moderate correlations with LA‐FACtotal and LA‐FACact (reservoir and booster pump function, respectively). In humans, several studies have demonstrated that A m velocity can be used as a rapid and accurate marker of global atrial function.32, 33 The A m velocity correlates with other parameters of atrial function, including the peak A velocity, atrial ejection fraction, and atrial ejection force.33 Moreover, A m velocity has been shown to provide important prognostic information in patients with various cardiac diseases.34 In this study, there was no significant difference in A m velocity between the survivor and nonsurvivor dogs, reflecting a lower value for this prognostic predictor in dogs with CMVHD.
Some limitations of this study must be considered. First, no invasive assessment of LA mechanical properties or afterload was performed to prove the determinations of reduced LA function, which must be the focus of further specific studies. Second, the number of dogs studied was small, rendering the study underpowered to detect differences between groups. Last, it is possible that medication use influenced the echocardiographic parameters and survival time. The use of ACE inhibitors, pimobendan, and loop diuretics was significantly higher in the nonsurvivors. Because this study included dogs in various clinical stages, it was impossible to standardize the treatment.
In conclusion, both LA size and function are strongly correlated with the prognosis of dogs with CMVHD. Booster pump function was the most significant independent predictor of mortality in this study. The assessment of LA function can provide further insights about the pathophysiology and prognosis in dogs with CMVHD.
Acknowledgments
This study was partially supported by the Grants‐in‐Aid for Scientific Research (no. 25850203) from the Japanese Society for the Promotion of Science (KN) and the Grants‐in‐Aid from Kuribayashi Academic Scholarship Foundation.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
This study was performed at the Graduate School of Veterinary Medicine, Hokkaido University.
Footnotes
Hitachi Medical Corp., Chiba, Japan
IBM SPSS Statistics, version 21; IBM Corp., Chicago, IL
References
- 1. Häggström J, Kvart C, Pedersen HD. Acquired valvular disease In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 6th ed St. Louis, MO: Elsevier Saunders; 2005:1022–1039. [Google Scholar]
- 2. Pedersen HD, Kristensen Bø, Nørby B, Lorentzen KA. Echocardiographic study of mitral valve prolapse in Dachsunds. J Vet Med Ser A 1996;43:103–110. [DOI] [PubMed] [Google Scholar]
- 3. Beardow AW, Buchanan JW. Chronic mitral valve disease in Cavalier King Charles spaniels: 95 cases (1987–1991). J Am Vet Med Assoc 1993;203:1023–1029. [PubMed] [Google Scholar]
- 4. Serfass P, Chetboul V, Sampedrano CC, et al. Retrospective study of 942 small‐sized dogs: Prevalence of left apical systolic heart murmur and left‐sided heart failure, critical effects of breed and sex. J Vet Cardiol 2006;8:11–18. [DOI] [PubMed] [Google Scholar]
- 5. Chetboul V, Tissier R, Villaret F, et al. Epidemiological, clinical, echo‐Doppler characteristics of mitral valve endocardiosis in Cavalier King Charles in France: A retrospective study of 451 cases (1995 to 2003). Can Vet J 2004;45:1012–1015. [PMC free article] [PubMed] [Google Scholar]
- 6. Kvart C, Häggström J, Pedersen HD, et al. Efficacy of enalapril for prevention of congestive heart failure in dogs with myxomatous valve disease and asymptomatic mitral regurgitation. J Vet Intern Med 2002;16:80–88. [PubMed] [Google Scholar]
- 7. Atkins CE, Keene BW, Brown WA, et al. Results of the veterinary enalapril trial to prove reduction in onset of heart failure in dogs chronically treated with enalapril alone for compensated, naturally‐occurring mitral valve insufficiency. J Am Vet Med Assoc 2007;231:1061–1069. [DOI] [PubMed] [Google Scholar]
- 8. Pouchelon JL, Jamet N, Gouni V, et al. Effect of benazepril on survival and cardiac events in dogs with asymptomatic mitral valve disease: A retrospective study of 141 cases. J Vet Intern Med 2008;22:905–914. [DOI] [PubMed] [Google Scholar]
- 9. Chetboul V, Serres F, Tissier R, et al. Association of plasma N‐terminal pro‐B‐type natriuretic peptide concentration with mitral regurgitation severity and outcome in dogs with asymptomatic degenerative mitral valve disease. J Vet Intern Med 2009;23:984–994. [DOI] [PubMed] [Google Scholar]
- 10. Borgarelli M, Savarino P, Crosara S, et al. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med 2008;22:120–128. [DOI] [PubMed] [Google Scholar]
- 11. Serres F, Chetboul V, Tissier R, et al. Comparison of 3 ultrasound methods for quantifying left ventricular systolic function: Correlation with disease severity and prognostic value in dogs with mitral valve disease. J Vet Intern Med 2008;22:566–577. [DOI] [PubMed] [Google Scholar]
- 12. Serres F, Chetboul V, Tissier R, et al. Chordae tendinae rupture in dogs with degenerative mitral valve disease: Prevalence, survival, and prognostic factors (114 cases, 2001–2006). J Vet Intern Med 2007;21:258–264. [DOI] [PubMed] [Google Scholar]
- 13. Rishniw M, Erb HN. Evaluation of four 2‐dimensional echocardiographic methods of assessing left atrial size in dogs. J Vet Intern Med 2000;14:429–435. [DOI] [PubMed] [Google Scholar]
- 14. Hansson K, Häggström J, Kvart C, Lord P. Left atrial to aortic root indices using two‐dimensional and M‐mode echocardiography in Cavalier King Charles spaniels with and without left atrial enlargement. Vet Radiol Ultrasound 2002;43:568–575. [DOI] [PubMed] [Google Scholar]
- 15. Barbier P, Solomon SB, Schiller NB, Glantz SA. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation 1999;100:427–436. [DOI] [PubMed] [Google Scholar]
- 16. Roşca M, Popescu BA, Beladan CC, et al. Left atrial dysfunction as a correlate of heart failure symptoms in hypertrophic cardiomyopathy. J Am Soc Echocardiogr 2010;23:1090–1098. [DOI] [PubMed] [Google Scholar]
- 17. D'Andrea A, Caso P, Romano S, et al. Association between left atrial myocardial function and exercise capacity in patients with either idiopathic or ischemic dilated cardiomyopathy: A two‐dimensional speckle strain study. Int J Cardiol 2009;132:354–363. [DOI] [PubMed] [Google Scholar]
- 18. Cameli M, Lisi M, Giacomin E, et al. Chronic mitral regurgitation: Left atrial deformation analysis by two‐dimensional speckle tracking echocardiography. Echocardiography 2011;28:327–334. [DOI] [PubMed] [Google Scholar]
- 19. Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med 2009;23:1142–1150. [DOI] [PubMed] [Google Scholar]
- 20. Sahn DJ, De Maria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M‐mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978;58:1072–1083. [DOI] [PubMed] [Google Scholar]
- 21. Kienle RD, Thomas WP. Echocardiography In: Nyland TG, Mattoon JS, eds. Veterinary Diagnostic Ultrasound. Philadelphia, PA: WB Saunders; 1995:198–255. [Google Scholar]
- 22. Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Vet Intern Med 2004;18:311–321. [DOI] [PubMed] [Google Scholar]
- 23. To AC, Flamm SD, Marwick TH, Klein AL. Clinical utility of multimodality LA imaging: Assessment of size, function, and structure. JACC Cardiovasc Imaging 2011;4:788–798. [DOI] [PubMed] [Google Scholar]
- 24. Tidholm A, Ljungvall I, Höglund K, et al. Tissue Doppler and strain imaging in dogs with myxomatous mitral valve disease in different stages of congestive heart failure. J Vet Intern Med 2009;23:1197–1207. [DOI] [PubMed] [Google Scholar]
- 25. Zois NE, Tidholm A, Nägga KM, et al. Radial and longitudinal strain and strain rate assessed by speckle‐tracking echocardiography in dogs with myxomatous mitral valve disease. J Vet Intern Med 2012;26:1309–1319. [DOI] [PubMed] [Google Scholar]
- 26. Suzuki R, Matsumoto H, Teshima T, Koyama H. Clinical assessment of systolic myocardial deformations in dogs with chronic mitral valve insufficiency using two‐dimensional speckle‐tracking echocardiography. J Vet Cardiol 2013;15:41–49. [DOI] [PubMed] [Google Scholar]
- 27. Stefanadis C, Dernellis J, Toutouzas P. A clinical appraisal of left atrial function. Eur Heart J 2001;22:22–36. [DOI] [PubMed] [Google Scholar]
- 28. Prioli A, Marino P, Lanzoni L, Zardini P. Increasing degrees of left ventricular filling impairment modulate left atrial function in humans. Am J Cardiol 1998;82:756–761. [DOI] [PubMed] [Google Scholar]
- 29. Rosca M, Lancellotti P, Popescu BA, Piérard LA. Left atrial function: Pathophysiology, echocardiographic assessment, and clinical applications. Heart 2011;97:1982–1989. [DOI] [PubMed] [Google Scholar]
- 30. Cameli M, Lisi M, Righini FM, et al. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol 2013;111:595–601. [DOI] [PubMed] [Google Scholar]
- 31. Debonnaire P, Leong DP, Witkowski TG, et al. Left atrial function by two‐dimensional speckle‐tracking echocardiography in patients with severe organic mitral regurgitation: Association with guidelines‐based surgical indication and postoperative (long‐term) survival. J Am Soc Echocardiogr 2013;26:1053–1062. [DOI] [PubMed] [Google Scholar]
- 32. Sohn DW, Chai IH, Lee DJ, et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol 1997;30:474–480. [DOI] [PubMed] [Google Scholar]
- 33. Thomas L, Levett K, Boyd A, et al. Changes in regional left atrial function with aging: Evaluation by Doppler tissue imaging. Eur J Echocardiogr 2003;4:92–100. [DOI] [PubMed] [Google Scholar]
- 34. Yu CM, Sanderson JE, Marwick TH, Oh JK. Tissue Doppler imaging: A new prognosticator for cardiovascular diseases. J Am Coll Cardiol 2007;49:1903–1914. [DOI] [PubMed] [Google Scholar]


