Abstract
Background
Although a common neurological disorder in dogs, long‐term outcome of epilepsy is sparsely documented.
Objectives
To investigate risk factors for survival and duration of survival in a population of dogs with idiopathic epilepsy or epilepsy associated with a known intracranial cause.
Animals
One hundred and two client owned dogs; 78 dogs with idiopathic epilepsy and 24 dogs with epilepsy associated with a known intracranial cause.
Methods
A retrospective hospital based study with follow‐up. Dogs diagnosed with epilepsy between 2002 and 2008 were enrolled in the study. Owners were interviewed by telephone using a structured questionnaire addressing epilepsy status, treatment, death/alive, and cause of death.
Results
Median life span was 7.6 years, 9.2 years, and 5.8 years for all dogs, and dogs with idiopathic epilepsy or dogs with epilepsy associated with a known intracranial cause (P < .001), respectively. Survival time for dogs with idiopathic epilepsy was significantly (P = .0030) decreased for dogs euthanized because of epilepsy (median: 35 months) compared to dogs euthanized for other reasons (median: 67.5 months). Neutered male dogs with idiopathic epilepsy had a significant (P = .031) shorter survival (median: 38.5 months) after index seizure compared to intact male dogs (median: 71 months). Treatment with two antiepileptic drugs (AED′s) did not negatively influence survival (P = .056).
Conclusion and Clinical Importance
Dogs with idiopathic epilepsy can in many cases expect a life span close to what is reported for dogs in general. In dogs where mono‐therapy is not sufficient, the need for treatment with two AED′s is not linked to a poor prognosis.
Keywords: Canine, Life span, Mortality, Seizure
Epilepsy in dogs and people have been associated with an increased risk of premature death1, 2, 3, 4 and might in dogs lead to euthanasia3, 5, 6, 7 if seizure control is insufficient. Survival studies on epilepsy in dogs including both idiopathic and symptomatic cases have reported a median life span of 7.0 years.2, 3 Many factors might influence survival. Acute self‐sustaining seizures (status epilepticus) can be a direct cause of death. Furthermore, dogs experiencing status epilepticus or cluster seizures have a significantly decreased survival time compared to dogs not suffering from such phenomenon.5, 6, 7, 8, 9 Age at epilepsy debut affects survival in Border collies and Australian shepherds, where a significantly decreased survival time is associated with an onset of seizures before the age of 2 years.8, 9 Other studies, including both pure breeds and cross‐breeds, have however not shown any correlation between age at seizure onset and survival.3, 6
Sudden unexpected death in epilepsy (SUDEP) might occur in individuals with epilepsy. SUDEP is well recognized in humans10 with sudden death being 24 times more likely in people with epilepsy than in the general population.11 Although sparsely investigated, there are indications that SUDEP also occur in dogs.3, 6, 12
Although a condition which implicate a possible risk of premature death, epilepsy is also a potentially self‐limiting disorder and remission might occur either spontaneously or upon treatment.13 Remission of epilepsy has been documented in several studies of dogs ranging between 14 and 24% of the populations investigated.3, 6, 8, 9, 12, 14
Only few studies have provided information on mortality in populations of dogs, including purebreds and crossbreds, with idiopathic and symptomatic epilepsy. The aim of this study was to investigate survival and prognosis in such a population and to investigate selected risk factors possibly influencing survival time.
Materials and Methods
Study Design and Study Population
The study was designed as a retrospective hospital based study with follow‐up. The study population consisted of dogs diagnosed with idiopathic epilepsy or epilepsy associated with a known intracranial cause between 2002 and 2008 at the Small Animal Veterinary Teaching Hospital, University of Copenhagen. To be included in the study, the dogs should have a diagnosis of idiopathic epilepsy or epilepsy associated with a known intracranial cause from the hospital neurology clinic.
Definitions Applied in the Study
Epilepsy was defined in this study as recurrent seizures (two or more) of intracranial origin. To qualify for a diagnosis of idiopathic epilepsy, dogs had a normal interictal clinical and neurological examination and a complete blood count and biochemistry profile within reference range. Furthermore, a minimum follow‐up of 3 years after diagnosis, if the dog was still alive at the time of follow‐up, or until death, with no indication of progressive intracranial disease was available. This implies that the dogs could have died in the time between diagnosis and follow‐up. Diagnostic imaging was desirable. A diagnosis of epilepsy associated with a known intracranial cause required that a intracranial lesion was confirmed by computer tomography (CT), magnetic resonance imaging (MRI), cerebrospinal fluid (CSF) analysis, or post mortem histopathology.
Seizures were classified as primary generalized or focal with or without secondary generalization.
Cluster seizures were defined as >1 epileptic seizure within 24 hours. Status epilepticus was defined as “a single epileptic seizure event lasting more than 30 minutes or a series of seizures during which the normal level of brain function was not regained between ictal events in a period longer than 30 minutes.”15 SUDEP was defined as “sudden, unexpected, witnessed or unwitnessed, nontraumatic and nondrowning death, with or without evidence of a seizure and excluding documented status epilepticus.”4 Epilepsy‐related cause of death was defined as euthanasia motivated by circumstances directly associated with the epileptic condition. In cases where the epileptic condition was only one of more factors contributing to the decision of euthanasia death was registered as due to other causes. Dogs were registered as receiving antiepileptic treatment if they received treatment with a single drug (mono‐therapy) or a combination of two or more antiepileptic drugs (AED′s). Remission of epilepsy was defined as spontaneous remission (no seizure for ≥3 years without antiepileptic treatment) or remission upon treatment (no seizure for ≥3 years while receiving antiepileptic treatment).3, 12 Both definitions of remission implied seizure freedom at the time of follow‐up or at time of death.
Life span investigated time from date of birth and until time of death/time of follow‐up and survival time investigated time from date of index seizure and until time of death/time of follow‐up.
Telephone Follow‐up
A follow‐up investigation was conducted in January and February 2012. Owners of included dogs were contacted by telephone and interviewed using a structured questionnaire. The questionnaire consisted of three sections:
Section 1 confirmed the ID of the dog and investigated the status (dead or alive) of the dog.
Section 2 investigated the cause of death in deceased dogs and whether death had occurred spontaneously or the dog had been euthanized. In the case of euthanasia, owners were asked which factors had influenced the decision.
Section 3 investigated seizure history including date of index seizure, seizure semiology, information on antiepileptic treatment and seizure control, occurrence of status epilepticus, cluster seizures, or both and finally possible remission.
To identify possible recall bias, a comparison of the date of index seizure stated in the dogs' medical record was made with the date of index seizure stated by the owners in the follow‐up interview. To secure a standardized interview, one investigator (BCK) performed all the interviews.
Statistical Analysis
The study population was characterized by tabulating clinical variables stratified by epilepsy status. Data included information on sex, neutering status, breed, age at index seizure, seizure type, status epilepticus, cluster seizures, antiepileptic treatment, and remission of epilepsy. Median life span and median survival time for dogs with epilepsy (idiopathic or associated with a known intracranial cause) were estimated using Kaplan–Meier survival analysis.16 Dogs alive at the time of follow‐up were censored. Furthermore, Kaplan–Meier was used to estimate median life span and survival time in dogs with idiopathic epilepsy stratified by sex, neutering status, age at index seizure (≤24 months, >24 months), seizure type, treatment, and cause of death. Survival curves of the different strata were compared using a log‐rank test. Multivariable analysis of the above risk factors was carried out using Cox proportional hazard models.16 The analysis was carried out as a backward elimination procedure, where all variables where included and removed variables were reinserted one at a time in the final model.
All analyses were carried out using the survival package in R version 2.14.2.17 By using chi‐squared test and Fisher's exact test, the association between occurrence of cluster seizures and status epilepticus and the cause of death and neutering status (male dogs), respectively, was investigated. Statistical significance was defined as P ≤ .05 in all analyses.
Results
Descriptive Analysis
Total Population
One hundred and thirty‐one dogs met the inclusion criteria of the study. Twenty‐eight dogs were lost to follow‐up as the owners could not be reached and one owner declined to participate in the study. Thus, 102 dogs constituted the final study population, 78 dogs with idiopathic epilepsy and 24 dogs with epilepsy associated with a known intracranial cause (Table 1).
Table 1.
Characterization of the study population including dogs with idiopathic epilepsy and epilepsy associated with a known intracranial cause
| Idiopathic Epilepsy | Epilepsy Associated with a Known Intracranial Cause | |||
|---|---|---|---|---|
| n | % | n | % | |
| Overall (total population of 102 dogs) | 78 | 76 | 24 | 24 |
| Alive at follow‐up | 20 | 26 | 1 | 4 |
| Dead at follow‐up | 58 | 74 | 23 | 96 |
| Sex and neutering status | ||||
| Male | 62 | 79 | 20 | 83 |
| Intact | 38 | 61 | 15 | 75 |
| Neutered | 24 | 39 | 5 | 25 |
| Female | 16 | 21 | 4 | 17 |
| Intact | 8 | 50 | 3 | 75 |
| Neutered | 8 | 50 | 1 | 25 |
| Seizure type | ||||
| Focal | 15 | 19 | 7 | 29 |
| Focal with secondary generalization | 49 | 63 | 17 | 71 |
| Primary generalized | 11 | 14 | 0 | 0 |
| Unclassified | 3 | 4 | 0 | 0 |
| Antiepileptic treatment | 70 | 90 | 17 | 71 |
| Monotherapy | 41 | 59 | 14 | 82 |
| Two antiepileptic drugs | 28 | 40 | 3 | 18 |
| More than two drugs | 1 | 1 | 0 | 0 |
| Acute seizures | 61 | 78 | 24 | 100 |
| Cluster seizures | 47 | 77 | 18 | 75 |
| Status epilepticus | 14 | 23 | 6 | 25 |
| Remission | 10 | 13 | ||
| Spontaneously | 4 | 40 | 0 | 0 |
| Upon treatment | 6 | 60 | 0 | 0 |
| Death/Euthanasia | 58 | 74 | 23 | 96 |
| Motivated by epilepsy | 30 | 52 | 19a | 83 |
| Other causes | 28 | 48 | 4 | 17 |
6/19 dogs were euthanized because of a grave prognosis caused by the primary intracranial pathology.
Of the 102 dog owners who participated in the investigation, 84 (82%) were able to recall the time of their dog′s index seizure with a high accuracy (weeks proximity) at follow‐up when comparing to the date stated in the dog′s medical record at the first clinical consult at the hospital.
Dogs with Idiopathic Epilepsy
The group of dogs with idiopathic epilepsy consisted of 67 purebreds (distributed among 42 breeds) and 11 crossbreeds. Of these, 16 were females (8 intact, 8 neutered) and 62 were males (38 intact, 24 neutered). The median age at index seizure was 25 months (Q1–Q3: 12.25–50.75 months, range: 3–87 months), 2.1 years. The median number of months, the dogs had epilepsy (from index seizure to time of either death or follow‐up) was 61 months (range: 2–166 months). Three dogs had epilepsy for less than 10 months when they were euthanized. All three dogs had normal hematology and biochemistry profiles. Further testing in the three dogs, which did not reveal any abnormalities, was thyroid status and cardiac ultrasound (one dog), CSF examination (one dog) and dynamic bile acid testing, ACTH stimulation test and radiographs of the thorax and abdomen (one dog).
Fifteen dogs (19%) experienced focal seizures whereas 49 dogs (63%) experienced focal seizures with secondary generalization and 11 dogs (14%) experienced primary generalized seizures. Seizure type could not be classified in 3 dogs (4%). A history of cluster seizures was reported in 47 dogs (60%) and 14 dogs (18%) experienced status epilepticus (Table 1). In four of these dogs, status epilepticus occurred more than once. MRI was performed in 14 dogs, CT was performed in 16 dogs and 9 dogs had a CSF examination (6/9 dogs had also MRI performed and 2/9 had CT performed). No abnormalities were found on any of these specialized diagnostic tests.
At the time of follow‐up or time of death, 41 dogs (53%) received mono‐therapy with phenobarbital and 28 dogs (36%) received treatment with a combination of phenobarbital and potassium bromide. One dog (1%) received treatment with phenobarbital, potassium bromide, and levetiracetam. Eight dogs did not receive any antiepileptic treatment. Ten dogs (13%) met the criteria of remission of which 4 dogs had entered spontaneous remission while 6 dogs had entered remission upon treatment (5 mono‐therapy and 1 a combination of two AED′s) (Table 1). Of the 10 dogs, 3 dogs had a history of cluster seizures.
Dogs with Epilepsy Associated with a Known Intracranial Cause
The group of dogs with epilepsy associated with a known intracranial cause consisted of 22 purebreds (distributed among 19 breeds) and 2 crossbreeds. Of these, 4 were females (3 intact, 1 neutered) and 20 were males (15 intact, 5 neutered). Eleven dogs (46%) were diagnosed with intracranial neoplasia, nine dogs (38%) with intracranial inflammatory disease, three dogs (12%) with malformation (hydrocephalus) and one dog (4%) with hemorrhagic stroke. Upon presentation 6/24 dogs had a normal interictal neurological examination including a normal interictal behavior reported by the owner. Median age at index seizure was 47.5 months (Q1–Q3: 14.75–75.25 months, range: 1–138 months), 4 years. The median number of months the dogs had epilepsy (from index seizure to time of either death or follow‐up) was 8 months (range: 1–73 months).
Seven dogs (29%) experienced focal seizures while 17 dogs (71%) experienced focal seizures with secondary generalization. A history of cluster seizures was reported in 18 (75%) dogs and 6 dogs (25%) had experienced status epilepticus (Table 1). Seventeen dogs (7 with inflammatory disease, 8 with intracranial tumor, 1 with malformation, and 1 with hemorrhagic stroke) received treatment with AED′s. Treatment was either mono‐therapy with phenobarbital or with a combination of phenobarbital and potassium bromide. None of the dogs achieved remission.
Survival Analysis
Total Population
Twenty‐one of the 102 dogs that participated in the study were alive at the time of follow‐up while 81 dogs were deceased. A median life span of 91 months (95% CI: 83–119), 7.6 years, was estimated for the total study population (including both idiopathic cases and cases with epilepsy associated with a known intracranial cause) with a median survival time from index seizure being 56 months (95% CI: 37–67), 4.7 years.
Dogs with Idiopathic Epilepsy
At the time of follow‐up 20 (26%) dogs were alive while 58 (74%) dogs were dead. A median life span of 110 months (95% CI: 88–123), 9.2 years, was estimated with a median survival time after index seizure being 66 months (95% CI: 60–81), 5.5 years (Table 2).
Table 2.
Age at index seizure, time with epilepsy, life span, and survival time for the study population
| n | Age at Index Seizure (Months), Median (25–75%) | Life Span (Months), Median (95% CI) | Survival Time (Months), Median (95% CI) | |
|---|---|---|---|---|
| Idiopathic epilepsy | 78 | 25 (12.25–50.75) | 110 (88–123) | 66 (60–81) |
| Epilepsy associated with a known intracranial cause | 24 | 47 (14.75–75.25) | 69.5 (56–83) | 8 (3–21) |
| Cause of death | ||||
| Motivated by epilepsy | 30 | 16 (10.08–37.75) | 71 (52–91) | 35 (28–61)a |
As all dogs were dead, the median survival time represent the true median and not the estimated.
A significant association was found between survival time and neutering status for male dogs with neutered male dogs having a significantly (P = .031) shorter survival time (median: 38.5 months) than intact male dogs (median: 71 months). Furthermore, an association was shown between neutering status of male dogs and the occurrence of cluster seizures with neutered male dogs experiencing significant (P = .029, using Fisher′s exact test) more cluster seizures compared to intact male dogs. There was a strong significant effect of cluster seizures (P < .001) and status epilepticus (P < .001) on the survival time. No significant association was found in survival time when stratified by sex (P = .61), neutering status for females (P = .45), age at index seizure (P = .32), seizure type (P = .067), or between dogs receiving antiepileptic treatment, or no treatment (P = .67). Nor did testing mono‐therapy against a combination of two AED′s reveal any significant difference (P = .056). When the above variables were analyzed in a multivariable model using Cox proportional hazards for the survival time after seizure onset, only cluster seizures (P = .0027) and status epilepticus (P = .0098) remained in the model. Hazard ratio for cluster seizures and status epilepticus were 9.3 (95% CI; 2.2–40.0) and 2.7 (95% CI; 1.3–5.6) respectively. The dog that received phenobarbital, potassium bromide, and levetiracetam was excluded from calculation.
Epilepsy‐related cause of euthanasia was reported in 30/58 dogs (52%). One dog (a male) died spontaneously after a generalized tonic‐clonic seizure, which did not differ from previous seizures in the dog (possible SUDEP). The dog, an Old English sheepdog, experienced cluster seizures regularly, and the owner reported a poor response to antiepileptic treatment with seizure episodes every other week. The median life span for dogs euthanized because of epilepsy‐related causes were 71 months (Q1–Q3: 40.75–104.5) while the median survival time was 35 months (Q1–Q3: 18.75–63.25).
A significant association was found between cause of death, life span (P = .00021), and survival time (P = .0030) (Figs 1, 2). Dogs that died/were euthanized because of causes directly related to the epileptic condition had a significant shorter median life span and median survival time compared to dogs that died or were euthanized because of other causes. An association was also demonstrated between cause of death, the occurrence of cluster seizures, or both status epilepticus. The frequency of cluster seizures (P < .001) and status epilepticus (P = .003) were significantly increased in dogs in which epilepsy was the direct cause of euthanasia compared to dogs in which death was motivated by other causes.
Figure 1.
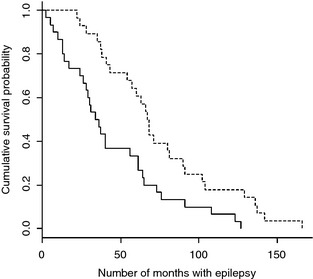
Kaplan–Meier plot of survival time for dogs with idiopathic epilepsy stratified by cause of death. Solid line = Death motivated by the epileptic condition (n = 30). Dashed line = Death because of other causes (n = 28).
Figure 2.
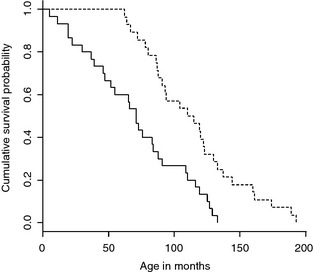
Kaplan–Meier plot of life span for dogs with idiopathic epilepsy stratified by cause of death. Solid line = Death motivated by the epileptic condition (n = 30). Dashed line = Death because of other causes (n = 28).
Dogs with Epilepsy Associated with a Known Intracranial Cause
At the time of follow‐up one dog (4%) was alive while 23 dogs (96%) were dead. The median life span was 69.5 months (95% CI: 56–83), 5.8 years, whereas the median survival time after index seizure was 8 months (95% CI: 3–21), 0.7 years (Table 2). Epilepsy‐related cause of euthanasia was reported in 19/23 dogs (83%). A significant association was found between survival time and the underlying etiology, with dogs diagnosed with inflammatory CNS disease having a significantly longer survival time (P = .0096) than dogs diagnosed with intracranial neoplasia, malformation, or hemorrhagic stroke (Fig 3).
Figure 3.
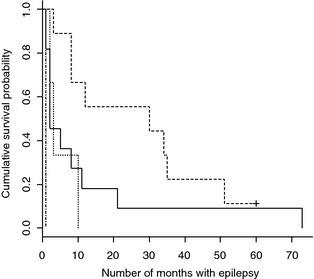
Kaplan–Meier plot of survival for dogs with epilepsy associated with a known intracranial cause stratified by intracranial etiology. Dashed line = inflammatory disease (n = 9), Solid line = neoplastic disease (n = 11), Dotted line = malformation (n = 3), and Dot‐dash line = hemorrhagic stroke (n = 1). Hash mark indicates censored data.
Comparison of the Two Groups
When comparing median survival time for the two strata idiopathic epilepsy and epilepsy associated with a known intracranial cause, dogs with idiopathic epilepsy had a significant (P < .001) longer survival time than dogs with epilepsy associated with a known intracranial cause (Fig 4).
Figure 4.
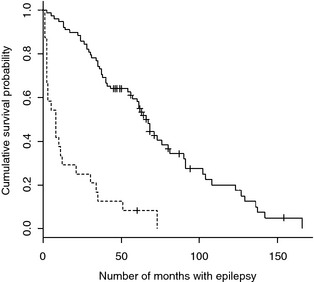
Kaplan–Meier plot of survival time in dogs with idiopathic epilepsy and epilepsy associated with a known intracranial cause. Solid line = dogs with idiopathic epilepsy (n = 78). Dashed line = dogs with epilepsy associated with a known intracranial cause (n = 24). Hash marks indicate censored data.
Discussion
This study shows that in many cases dogs with idiopathic epilepsy can expect a life span close to what is reported for dogs in general. The few studies which have investigated survival in populations of dogs consisting of both purebred and crossbreed dogs have estimated a median life span of 7.0 years when including both idiopathic and symptomatic cases.2, 3 This is in accordance with the median life span of 7.6 years found for the total study population in this study. When investigating survival according to etiology, however, our study interestingly showed that dogs with idiopathic epilepsy had a median life span close to the median life span of 10.0 years reported for dogs in general.2 This information is of interest for clinicians when discussing prognosis of idiopathic epilepsy with dog owners.
The study confirmed that dogs with epilepsy associated with a known intracranial cause have an increased risk of premature death when compared to dogs with idiopathic epilepsy. This result is not unexpected but is, however, of interest as no previous studies have provided statistical evidence for the association between intracranial lesions causing symptomatic epilepsy and a decreased survival time.
Our finding is consistent with human epilepsy studies on survival, which have documented that case fatality is highest in individuals with epilepsy associated with a known intracranial cause compared to idiopathic epilepsy.1, 4 A similar result, which found a significantly longer mean survival time in dogs with idiopathic epilepsy compared to dogs with epilepsy associated with a known intracranial cause, has previously been reported.6 However, the study only included dogs with seizure onset before the age of 1 year, and the results are therefore not directly comparable to this study.
Conflicting results exist in human medicine with regard to the question of premature death associated with idiopathic epilepsy. Some studies have reported a slightly increased mortality while others have found no increased risk compared to the general population.1, 4, 18, 19
In this study, dogs with symptomatic epilepsy caused by inflammatory disease had a significantly longer survival time compared to dogs with epilepsy caused by neoplasia, malformation, or hemorrhagic stroke. This finding might reflect that anti‐inflammatory treatment options are available.
When investigating risk factors for idiopathic epilepsy neutered male dogs had a significantly shorter survival time when compared to intact males. The fact that a significantly higher proportion of neutered male dogs experienced cluster seizures compared to intact males might explain this finding. Whether testosterone possesses pro‐ or anticonvulsive properties is an ongoing subject of investigation.20, 21, 22, 23 A seizure protective effect, through modulation of the GABA receptor driven by testosterone‐derived neurosteroid 3α‐androstanediol, has however been suggested in some in vivo rodent studies.20, 21
Although tempting to assume that treatment with a combination of two AED′s reflects more grave (difficult to control) epilepsy, an interesting finding of our study was, that dogs receiving a combination of two AED′s did not differ significantly from dogs receiving mono‐therapy with respect to survival. A similar result was reported in Australian shepherds with idiopathic epilepsy whose survival was also not influenced by poly‐therapy.9
Death/euthanasia was directly motivated by epilepsy in 52% of the dogs with idiopathic epilepsy, of which a significant proportion had a history of cluster seizures, status epilepticus or both. Both cluster seizures and status epilepticus have previously been reported as risk factors of survival in epileptic dogs.3, 5, 6, 7 Neither cluster seizures or status epilepticus are well tolerated from an owners perspective3, 24 which underlines that prompt treatment and continuous optimizing of seizure control is of a central point of attention for the clinician.
Genetic factors might influence the severity of epilepsy in certain breeds with idiopathic epilepsy and thus influence survival.8, 9, 12, 25 The median survival time (time from index seizure to death/follow‐up) for dogs with idiopathic epilepsy reported in this study is comparable to the median survival time of 4.25 years reported in Belgian shepherds12 experiencing a relatively mild form of focal idiopathic genetic epilepsy. A graver prognosis has been found in Border collies in which the median survival time was only 2.07 years.8 The fact that certain breeds might experience epilepsy subtypes, which are difficult to control, is important to discuss with owners bringing such pure breed epileptic dogs to the clinic.
One case of possible SUDEP occurred in a male dog with idiopathic epilepsy after an episode of a single generalized tonic‐clonic seizure. The dog experienced a poor response to AED′s with episodes of cluster seizures regularly and seizures every other week. In human epileptology, SUDEP is a well‐known phenomenon with young age, male gender, poor seizure control and episodes with generalized tonic‐clonic seizures being risk factors.10 SUDEP has previously been reported to occur in dogs3, 6, 12 and remains an area for further investigation.
This study documented a remission of epilepsy of 13% and thus confirmed that idiopathic epilepsy in dogs is not necessarily a chronic condition. Our result is supported by a previous study in using an identical definition for remission, and which found a remission rate of 15%.3 Three of the dogs that entered remission in this study had a history of one or more episodes of cluster seizures, indicating that remission can be achieved even in such severe cases. This has also been reported for Border collies and Australian shepherds diagnosed with idiopathic epilepsy.8, 9
Although desirable from a diagnostic perspective, some owners declined neuroimaging and imaging results were therefore not available for a proportion of dogs with idiopathic epilepsy. This is a weakness to the study as cases with an intracranial lesion might falsely have been included in the idiopathic epilepsy group. However, none of these dogs revealed interictal signs of intracranial lesions at inclusion, and neither did they develop such progressive signs until death or follow‐up after a minimum of 3 years, which support the diagnosis of idiopathic epilepsy. For the three dogs with a follow‐up period less than 10 months, no interictal neurological signs or behavioral changes were observed. This supports a diagnosis of idiopathic epilepsy although there is a possibility that this is not the case.
The fact that no abnormalities were detected in the 30 dogs with a CT‐ or MRI‐scan or in the 9 dogs that had a CSF examination also supports the validity of our case definition. It has previously been reported that only 2.2% of dogs under the age of six with a normal neurological examination had significant intracranial abnormalities on MRI.26 However, this result cannot be directly applied to the group of dogs with idiopathic epilepsy in this study, as inclusion criteria in the two studies are not identical.
A possible limitation when working with questionnaires and collecting historical information from owners is recalling bias. Yet, 82% of the owners in this study were able to state almost the exact date of the index seizure of their dogs compared with what was recorded in the dogs' medical record at their first hospital visit. This indicates that recall bias only played a minor role in this study.
Conclusion
Our study confirmed that dogs with epilepsy associated with a known intracranial cause have a grave prognosis, which is associated with the nature of the primary intracranial lesion. This study shows that dogs with idiopathic epilepsy in many cases can expect a median life span close to what is reported for dogs in general. Another encouraging finding was that receiving treatment with a combination of two AED′s did not affect survival negatively. However it is important, when advising owners, to include the information that certain breeds express epilepsy phenotypes that might be difficult to manage and that in each single patient, individual factors and decisions made by the clinician and the owner together will influence prognosis and survival.
Acknowledgments
The authors thank the Danish Animal Welfare Society for financial support.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
The study was conducted at the Small Animal Veterinary Teaching Hospital, University of Copenhagen, Dyrlaegevej 16, 1870 Frederiksberg C, Denmark.
Preliminary results were presented as a poster presentation at the 25th Annual Symposium of the European Society of Veterinary Neurology, Ghent, Belgium, 13–15th of September 2012.
References
- 1. Hauser WA, Annegers JF, Elveback LR. Mortality in patients with epilepsy. Epilepsia 1980;21:399–412. [DOI] [PubMed] [Google Scholar]
- 2. Proschowsky HF, Rugbjerg H, Ersbøll AK. Mortality of purebred and mixed‐breed dogs in Denmark. Prev Vet Med 2003;58:63–74. [DOI] [PubMed] [Google Scholar]
- 3. Berendt M, Gredal H, Ersbøll AK, Alving J. Premature death, risk factors, and life patterns in dogs with epilepsy. J Vet Intern Med 2007;21:754–759. [DOI] [PubMed] [Google Scholar]
- 4. Neligan A, Bell G, Johnson A, et al. The long‐term risk of premature mortality in people with epilepsy. Brain 2011;134:388–395. [DOI] [PubMed] [Google Scholar]
- 5. Saito M, Muñana KR, Sharp NJ, Olby NJ. Risk factors for development of status epilepticus in dogs with idiopathic epilepsy and effects of status epilepticus on outcome and survival time: 32 cases (1990–1996). J Am Vet Med Assoc 2011;219:618–623. [DOI] [PubMed] [Google Scholar]
- 6. Arrol L, Penderis J, Garosi L, et al. Aetiology and long‐term outcome of juvenile epilepsy in 136 dogs. Vet Rec 2012;170:335. [DOI] [PubMed] [Google Scholar]
- 7. Monteiro R, Adams V, Keys D, Platt SR. Canine idiopathic epilepsy: Prevalence, risk factors and outcome associated with cluster seizures and status epilepticus. J Small Anim Pract 2012;53:526–530. [DOI] [PubMed] [Google Scholar]
- 8. Hülsmeyer V, Zimmermann R, Brauer C, et al. Epilepsy in Border Collies: Clinical manifestation, outcome, and mode of inheritance. J Vet Intern Med 2010;24:171–178. [DOI] [PubMed] [Google Scholar]
- 9. Weissl J, Hülsmeyer V, Brauer C, et al. Disease progression and treatment response of idiopathic epilepsy in Australian Shepherd dogs. J Vet Intern Med 2012;26:116–125. [DOI] [PubMed] [Google Scholar]
- 10. Donner EJ. Explaining the unexplained; expecting the unexpected: Where are we with sudden unexpected death in epilepsy? Epilepsy Curr 2011;11:45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ficker DM, So EL, Shen WK, et al. Population‐based study of the incidence of sudden unexplained death in epilepsy. Neurology 1998;51:1270–1274. [DOI] [PubMed] [Google Scholar]
- 12. Gulløv CH, Toft N, Berendt M. A longitudinal study of survival in Belgian Shepherds with genetic epilepsy. J Vet Intern Med 2012;26:1115–1120. [DOI] [PubMed] [Google Scholar]
- 13. Kwan P, Sander JW. The natural history of epilepsy: An epidemiological view. J Neurol Neurosurg Psychiatry 2004;75:1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berendt M, Gredal H, Pedersen LG, et al. A cross‐sectional study of epilepsy in Danish Labrador Retrievers: Prevalence and selected risk factors. J Vet Intern Med 2002;16:262–268. [DOI] [PubMed] [Google Scholar]
- 15. Guidelines for epidemiologic studies on epilepsy. Commission on Epidemiology and Prognosis ILAE. Epilepsia 1993;34:592–596. [DOI] [PubMed] [Google Scholar]
- 16. Dohoo I, Martin W, Stryhn H. Veterinary Epidemiology Research, 2nd ed Charlottetown, PE: VER Inc.; 2009. [Google Scholar]
- 17. R Development Core Team . R: A Language anf Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. ISBN 3‐900051‐07‐0, http://www.R-project.org/. Accessed June 10, 2013. [Google Scholar]
- 18. Olafsson E, Hauser WA, Gudmundsson G. Long‐term survival of people with unprovoked seizures: A population‐based study. Epilepsia 1998;39:89–92. [DOI] [PubMed] [Google Scholar]
- 19. Lindsten H, Nyström L, Forsgren L. Mortality risk in an adult cohort with a newly diagnosed unprovoked epileptic seizure: A population‐based study. Epilepsia 2000;41:1469–1473. [DOI] [PubMed] [Google Scholar]
- 20. Frye CA, Reed TA. Androgenic neurosteroids: Anti‐seizure effects in an animal model of epilepsy. Psychoneuroendocrinology 1998;23:385–399. [DOI] [PubMed] [Google Scholar]
- 21. Reddy DS. Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3alpha‐androstanediol and 17beta‐estradiol. Neuroscience 2004;129:195–207. [DOI] [PubMed] [Google Scholar]
- 22. Pesce ME, Acevedo X, Bustamante D, et al. Progesterone and testosterone modulate the convulsant actions of pentylenetetrazol and strychnine in mice. Pharmacol Toxicol 2000;87:116–119. [DOI] [PubMed] [Google Scholar]
- 23. Mejías‐Aponte CA, Jiménez‐Rivera CA, Segarra AC. Sex differences in models of temporal lobe epilepsy: Role of testosterone. Brain Res 2002;944:210–218. [DOI] [PubMed] [Google Scholar]
- 24. Chang Y, Mellor DJ, Anderson TJ. Idiopathic epilepsy in dogs: Owners' perspectives on management with phenobarbitone and/or potassium bromide. J Small Anim Pract 2006;47:574–581. [DOI] [PubMed] [Google Scholar]
- 25. Casal M, Munuve R, Janis M, et al. Epilepsy in Irish wolfhounds. J Vet Intern Med 2006;20:131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith PM, Talbot CE, Jeffery ND. Findings on low‐field cranial MR images in epileptic dogs that lack interictal neurological deficits. Vet J 2008;176:320–325. [DOI] [PubMed] [Google Scholar]


