Abstract
Background
Idiopathic and acquired epilepsy are common in dogs. Up to 30% of these dogs are refractory to pharmacological treatment. Accumulating experimental evidence indicates that brain immune response and presence of inflammatory mediators decrease the threshold for individual seizures and contribute to epileptogenesis.
Hypothesis
Dogs with seizures have higher cerebrospinal interleukin‐6 (IL‐6) and tumor necrosis factor‐α (TNF‐α) concentrations compared to dogs with no seizures.
Methods
A prospective double blinded study; cerebrospinal fluid (CSF) and serum IL‐6, TNF‐α and total protein (TP) concentrations were measured by a blinded investigator for the study group and CSF IL‐6 and TNF‐α levels and TP concentrations were measured in the control group (CG).
Animals
Dogs presented with seizures that had enough CSF collected to allow analysis were included in the study group. Twelve apparently healthy, quarantined, stray dogs served as control (CG).
Results
Cerebrospinal fluid TNF‐α and IL‐6 concentrations were significantly higher (P = .011, P = .039) in dogs with seizures (0 ± 70.66, 0.65 ± 10.93 pg/mL) compared to the CG (0 ± 19, 0.73 ± 0.55 pg/mL). When assessing cytokine concentrations of specifically the idiopathic epilepsy (IE) dogs compared to the CG, only TNF‐α concentrations (8.66 ± 62, 0 ± 19 pg/mL) were significantly higher (P = .01). CSF TP concentrations were not significantly higher in the study dogs compared to the CG.
Conclusions and Clinical Importance
Higher TNF‐α and IL‐6 concentration in the CSF of dogs with naturally occurring seizures. The higher supports the hypothesis that inflammatory processes through certain mediators play a role in the pathogenesis of seizures in dogs.
Keywords: Antiinflammatory, Canine, Cytokine, Idiopathic epilepsy
Abbreviations
- AQ
albumin quota
- BBB
blood‐brain barrier
- CG
control group
- CNS
central nervous system
- CSF
cerebrospinal fluid
- CT
computed tomography
- HUVTH
Hebrew University Veterinary Teaching Hospital
- ICP
intracranial pressure
- IE
idiopathic epilepsy
- IL‐6
interleukin‐6
- IVDD
intervertebral disc disease
- ME
meningoencephalitis
- SD
standard deviation
- SE
status epilepticus
- TNCC
total nucleated cell count
- TNF‐α
tumor necrosis factor α
- TP
total protein
Idiopathic or acquired epilepsy are common, well‐described disorders in dogs. Idiopathic epilepsy (IE) is the most common etiology of epileptic seizures in dogs.1, 2 The onset of seizures with IE mostly occurs in dogs between the age of 1 and 5 years, and could present as generalized tonic‐clonic seizures.3 IE is diagnosed based on the signalment, history, normal neurological examination, and exclusion of other causes of recurrent seizures, including infection, inflammation, trauma, metabolic derangement (ie, hypoglycemia, hepatic encephalopathy and electrolyte imbalance) and neoplasia.2 Treatment of epilepsy in dogs is based on anticonvulsants, with phenobarbital and potassium‐bromide being the drugs of choice.1 In secondary epilepsy, treatment combines anticonvulsive treatment and specific treatment of the underlying primary process.1 Epilepsy in dogs shares many similarities with epilepsy in humans.4 In both species, as many as 30% of the cases treated pharmacologically are refractory to anticonvulsants, and are therefore susceptible to irreversible brain damage and death because of recurrent seizures and status epilepticus (SE).3 Thus, further exploration of novel therapeutic avenues for successful seizure management in cases failing to respond to conventional anticonvulsants is warranted.
Inflammation accompanies all types of brain insults, including immune‐mediated and infectious diseases, as well as traumatic, neurodegenerative, and epileptic disorders.5 Recent studies have suggested that increased brain inflammatory mediators decrease the threshold for individual seizures, thereby contributing to epileptogenesis.6, 7, 8 Brain parenchyma cells (ie, microglia, astrocytes, and neurons), blood‐brain barrier (BBB), and choroid plexus cells all produce inflammatory mediators. Brain inflammation may increase neuronal excitability, impair cell survival, and increase BBB permeability to blood‐borne molecules and cells.9, 10, 11, 12, 13
Communication between immune system cells occurs via direct, cell‐to‐cell interactions, or via cytokines.8 Transgenic IL‐6 or TNF‐α overexpressing mice were shown to be in a state of chronic inflammation.11 Chronic IL‐6 overexpression in mice resulted in spontaneous seizures, increased brain astrocyte and microglia cell number, higher CSF proinflammatory cytokine levels, BBB impairment, and various neurological deficits.8 TNF‐α induces γ‐aminobutyric acid‐A receptor endocytosis, resulting in decreased inhibitory strength.14, 15 Transgenic mice with low to moderate astrocyte TNF‐α overexpression have decreased number of seizure episodes, whereas those with high astrocyte TNF‐α expression developed signs of neurologic dysfunction.16, 17, 18 Proinflammatory cytokines concentrations, including TNF‐α, IL‐6, and IL‐1β, are increased during epileptic activity in rodents.11, 12, 13 Increased cytokines concentrations are reported in several central nervous system (CNS) diseases19, 20, 21, 22 Marked IL‐8 levels are found in chronic inflammatory canine distemper virus.20 Significant up‐regulation of IL‐6 is present in the early phase of spinal cord injury;21 pronounced IL‐4 production in the acute phase of SRMA,19 and increased IL‐6 levels occur in the CSF of dogs with SRMA when compared to epileptic dogs, dogs with intervertebral disc disease and dogs with brain tumors.22
In view of the above‐mentioned findings, we hypothesized that dogs with seizures express higher CSF, and possibly higher serum IL‐6 and TNF‐α concentrations compared to dogs with no apparent disease. In this study, CSF and serum IL‐6 and TNF‐α concentration were measured in dogs presenting seizures because of 3 different etiologies (ie, IE, meningoencephalitis, or neoplasia). Their CSF levels were compared to those of apparently healthy dogs.
Material and Methods
Selection of Dogs and Data Collection
This prospective study included dogs presented to the Hebrew University Veterinary Teaching Hospital (HUVTH) with epileptic seizures of neoplastic, inflammatory, or idiopathic etiology between 2006 and 2007. In addition, healthy dogs, euthanized by the municipal authority as part of a population control program, unrelated to this study, were used as control. Cisternal CSF samples obtained from these dogs immediately after euthanasia. The medical history of these dogs was unavailable, but they were kept in quarantine for 10 days at least, were examined daily by a veterinarian and appeared healthy. Dogs were included in this study only if sufficient CSF sample volume was obtained to allow analysis of the measured parameters.
In the study group, data were obtained from the medical records, including the signalment, medical history, physical and neurological examinations findings, laboratory test results (ie, complete blood count, serum chemistry and CSF analysis), and imaging findings.1 The history obtained included the age at onset of the first seizure episode, frequency and duration occurring seizures, and whether these are partial or generalized, the exact lag of time from the last seizure episode to presentation to the HUVTH, and type, dose and dosing interval of anticonvulsants used. Neurological examinations were performed by board‐certified neurologist in study group dogs and by the local veterinarian in the controls.
Collection of Samples and Laboratory Methods
Routine hematology1 , 2 and serum biochemistry (Cobas‐Mira3) (including TP and albumin) testing were performed by the HUVTH Diagnostic Laboratory from blood samples collected in potassium‐EDTA tubes and evacuated tubes with gel separators, respectively, in study group dogs. Leukocytosis was considered when a higher count than the reference range (6–17 × 109 cells/L) was noted. CSF samples were obtained via cisternal puncture under general anesthesia, as part of the routine diagnostic work up in the study dogs. CSF analysis included TP (Roche/Hitachi U/CSF total protein [TPUC3]3) and albumin4 concentration measurements, total nucleated cell count (TNCC), performed using a hemocytometer, and cytological evaluation of cytocentrifuged, stained5 smears. CSF was defined as inflammatory when CSF TP was >25 mg/dL or when total CSF TNCC was >5 cells/μL. Serum and CSF sample aliquots were stored at −80°C pending cytokine concentration measurement. The albumin quota (AQ) was calculated as CSF albumin concentration (mg/dL) divided by serum albumin concentration (g/dL), and further divided by 10.23 The reported AQ reference interval in dogs is 0.17–0.3, and an AQ > 0.3 was considered abnormally increased, suggesting BBB disruption.23 Serum and CSF IL‐6 and TNF‐α concentrations were measured using specific commercial canine ELISA tests (Quantikine IL‐6 canine immunoassay, CA6000 and Quantikine TNF‐α canine immunoassay, CATA00).6 Serum cytokines were not measured in the apparently healthy CG because of ethical considerations of obtaining ante‐mortem blood samples.
Statistical Analysis
The distribution pattern of continuous variables was assessed using the Shapiro–Wilk test. One‐way analysis of variance was applied to compare laboratory results of three groups or more, when data were distributed normally. When differences were significant, posthoc pair‐wise comparisons were made using Student's t‐test. The Kruskal–Wallis test was used to compare three groups or more when data were distributed nonnormally and when significant differences were recorded, pair‐wise comparisons were made using the Mann–Whitney test. The association between 2 continuous variables was analyzed using Spearman's correlation. Fisher's exact test was used to analyze the association between two categorical variables. All tests were two‐tailed, and a P ≤ .05 was considered statistically significant for all. Statistical analyses were performed by statistical software package SPSS 17.7
Results
The study group included 17 dogs, with 12 males (6 castrated) and 5 females (all neutered), with median age of 8.5 years (range 0.75–15), and median body weight of 27.5 kg (range 3.2–50). In the study group 7/17 dogs were of mixed breed, while other breeds included Boxer (2 dogs), Labrador Retriever (2), Border Terriers (2) and Yorkshire Terrier, Border Collie, English Bulldog, and Pekingese (1 each). The final diagnoses in this group included brain tumors (7 dogs), IE (7) and meningoencephalitis (ME, 3). The healthy CG included 12 dogs. Data regarding signalment were not recorded.
When assessing all seizure etiologies in the study group as a single group, TNF‐α and IL‐6 CSF concentrations were significantly higher compared to those of the CG (P = .011, P = .039, respectively; Table 1). Furthermore, there were significant positive correlations of CSF and serum IL‐6 (r = 0.57, P = .039) and TNF‐α (r = 0.56, P = .011).
Table 1.
Comparison of mean, median, and SD of TMF‐α and IL‐6 levels in the CSF and serum in the different study groups
| Study Group | TNF‐α CSF (pg/mL) | TNF‐α Serum (pg/mL) | IL‐6 CSF (pg/mL) | IL‐6 Serum (pg/mL) |
|---|---|---|---|---|
| Seizure | ||||
| Median | 24.33 | 7.33 | 1.23 | 1.1 |
| Range | 0–70.66 | 0–57.33 | 0.65–11.58 | 0–12.8 |
| N | 17 | 17 | 17 | 17 |
| Healthy | ||||
| Median | 5.16 | NC | 0.85 | NC |
| Range | 0–19 | NC | 0.73–1.28 | NC |
| N | 12 | NC | 12 | NC |
| P‐Value | .01 | .764 | .02 | .543 |
TMF‐α, tumor necrosis factor‐α; CSF, cerebrospinal fluid; IL‐6, interleukin‐6.
When subgroups of the different etiologies in the study group were separately compared with the CG, there was a significant higher CSF TNF‐α concentrations in dogs with IE than in the CG (P = .001) (Fig 1). In addition, higher albeit insignificantly CSF IL‐6 concentration were noted in dogs with IE than in the CG (P = .23) (Fig 2). In addition, CSF IL‐6 was significantly (P = .005) higher in study dogs with brain neoplasia compared to the CG. Both CSF IL‐6 and TNF‐α concentrations were significantly (P = .009 and P = .048) higher in study dogs with ME compared to the CG. No significant differences were noted between the CSF cytokine levels of the tumor and ME dogs.
Figure 1.
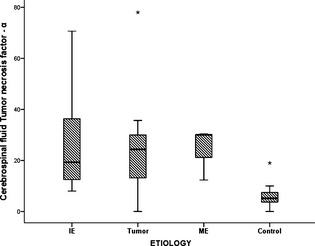
A box plot showing Tumor necrosis factor‐concentrations in the different etiologies causing seizures within the study group. IE, idiopathic epilepsy; ME, meningoencephalitis. Box and whiskers plots of the Tumor necrosis factor‐concentrations of the different etiologies causing seizures within the study group ie, idiopathic epilepsy (7 dogs), tumor (7 dogs), meningoencephalitis (3 dogs), and the control group (12 dogs). The line within the boxes represents the median, whereas the boxes represent the interquartile range, the whiskers represent the range and outliers are marked as asterisks. Posthoc pair‐wise comparisons were made using Student's t‐test.
Figure 2.
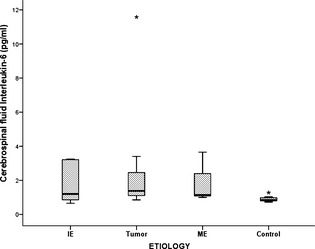
A box plot showing Interleukin‐6 concentrations in the different etiologies causing seizures within the study group. IE, idiopathic epilepsy; ME, meningoencephalitis. Box and whiskers plots of the Interleukin‐6 concentrations of the different etiologies causing seizures within the study group ie, idiopathic epilepsy (7 dogs), tumor (7 dogs), meningoencephalitis (3 dogs), and the control group (12 dogs). The line within the boxes represents the median, whereas the boxes represent the interquartile range, the whiskers represent the range and outliers are marked as asterisks. Posthoc pair‐wise comparisons were made using Student's t‐test.
Systemic inflammation, based on presence of leukocytosis was evident in 3 study dogs, 1 of which had concurrent inflammatory CSF (Table 2). There was no correlation between the blood leukocyte count and CSF cytokine levels.
Table 2.
Cytokine levels in dogs with seizures presented with information regarding type and duration of the seizures
| Diagnosis | Age (years) F/M | IL‐6 CSF (pg/mL) | IL‐6 Serum (pg/mL) | TNF‐α CSF (pg/mL) | TNF‐α Serum (pg/mL) | AQ | Inflammatory CSF | WBC (10×6/mL) | Time from Last Seizure (hours) 24, 48, >48 | Duration of Last Seizure (minutes) | Phenobarbital Antiepileptic Treatment before Admittance |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IE | 3/F | 3.23 | 0.88 | 8 | 40.33 | 0.135 | No | 13 | 3 | 5 | Yes |
| 1.5/M | 3.205 | 0.805 | 8.66 | 5 | 0.12 | No | 24.9 | 1 | 2 | No | |
| 9/M | 0.98 | 1.08 | 27.33 | 34 | 0.1 | NA | NA | 1 | NA | No | |
| 2.5/M | 0.73 | 1.605 | 16.33 | 0 | 0.076 | No | 25.4 | 1 | 6 | Yes | |
| 15/M | 0.655 | 1.08 | 19.33 | 43 | 0.14 | Yes | 8.88 | 2 | 7 | No | |
| 0.75/M | 3.255 | 0.78 | 45.33 | 0 | 0.056 | No | NA | 2 | 0.5 | No | |
| NA/M | 1.205 | 1.18 | 70.66 | 6.66 | 0.28 | NA | 6.92 | NA | NA | NA | |
| T | 8/M | 1.53 | 12.805 | 23.33 | 57.33 | NA | Yes | 21 | 1 | 1 | No |
| 15/M | 3.405 | 1.905 | 24.33 | 20.66 | 0.085 | NA | NA | NA | NA | NA | |
| 11/M | 0.855 | 1.28 | 3 | 24.66 | 0.1 | NA | 13.1 | 2 | 5 | No | |
| 10/M | 0.98 | 0.755 | 35.66 | 0 | 0.097 | Yes | 11.3 | 1 | NA | No | |
| 11/F | 11.58 | 1.08 | 78 | 34.33 | 0.5 | Yes | NA | 1 | 8 | No | |
| 3/M | 1.38 | 0 | 24.33 | 0 | 1.03 | Yes | 5.48 | 3 | 0.15 | No | |
| 9/M | 1.23 | 0.93 | 0 | 7.33 | 0.065 | No | 10.3 | 1 | NA | Yes | |
| ME | 9/F | 1.155 | 1.23 | 30 | 6 | 0.15 | Yes | 41 | 3 | NA | No |
| 7/F | 1.005 | 1.18 | 12.33 | 21.66 | NA | Yes | NA | 3 | 80 | No | |
| 6/F | 3.655 | 2.23 | 30.33 | 4.33 | 0.42 | Yes | 5.17 | 1 | NA | No |
Time from last seizure: 1; up to 24 hours, 2; up to 48 hours, 3; more than 48 hours.
IE, idiopathic epilepsy; T, tumor; ME, meningoencephalitis; IL‐6, interleukin 6; TNF‐α, tumor necrosis factor‐α; AQ, albumin quota; CSF, cerebrospinal fluid; WBC, white blood cell.
Inflammatory CSF was recorded in 9/14 dogs (64%). There was no association between blood leukocyte count or presence of an inflammatory CSF and CSF TNF‐α or IL‐6 concentrations (Table 2).
In 3/17 study dogs (18%), the AQ was abnormally high (0.42, 0.5, and 1.03), of which 2 had brain neoplasia, and 1 had ME. In all 3 dogs an inflammatory CSF was noted as well.
There were no associations (data not shown) between characteristics of the seizures ie, the lag of time from the last seizure episode to presentation, its type and duration, earlier phenobarbital treatment (Table 2) and CSF TP, albumin, TNF‐α, or IL‐6 concentrations, or with serum TNF‐α or IL‐6 concentrations.
Discussion
The significantly higher concentrations of both CSF TNF‐α and IL‐6 concentrations in the study group and of CSF TNF‐α in dogs with IE and of CSF IL‐6 concentration in dogs with brain tumors compared to CG are in agreement with previous studies.11, 12, 13, 22 Although CSF IL‐6 concentration in dogs with IE was not significantly higher compared to the control, it was significantly higher in the dogs with brain neoplasia, suggesting that IL‐6 could have a different role or reaction pattern in the inflammatory reaction of different CNS diseases. Furthermore, there were significant positive correlations CSF and serum IL‐6 and TNF‐α, suggesting that both cytokines increase systemically and within the CNS during an inflammatory seizure‐induced process.
Moreover, the limited number of dogs in each group might have reduced statistical power. The number of dogs with ME was small; however, it is noteworthy that in all three dogs with ME both CSF cytokines concentrations were higher compared to the controls, supporting a previous hypothesis that intrinsic CSF inflammatory conditions, such as SRMA, increase CSF cytokine production.22
The role of inflammation in epilepsy is incompletely understood, however, it was hypothesized that inflammatory reactions within the brain mediate some of the molecular and structural changes occurring during and after seizure activity.24, 25, 26 Production of proinflammatory cytokines in both epileptic human patients and animal models of epilepsy have been recorded.8, 27, 28 While expression of inflammatory cytokines in the normal brain is absent or minimal, markedly increased expression might be induced by acute epileptic events.8 Seizure activity in rodent models increases m‐RNA expression of TNF‐α, IL‐6, and IL‐1β, indicating that seizures trigger de novo cytokine synthesis.29 Such models have also showed that seizures trigger a rapid onset of marked reactive forebrain gliosis, resulting in high inflammatory mediators concentrations, including IL‐1β, IL‐6, and TNF‐α, which are involved in generation and propagation of epileptic activity.29, 30, 31
Neurons and glia cells express receptors for various cytokines such as IL‐1β and TNF‐α.32 Hence, dis‐regulation and excessive production of certain proinflammatory cytokines may potentiate neuronal degeneration and induction of seizures.8 Transgenic, IL‐6 and TNF‐α overexpressing mice have increased sensitivity to induction of seizures, indicating that chronic CNS inflammation predisposes to occurrence of seizures.33 All these studies suggest that proinflammatory cytokines play roles in both proepileptogenic mechanism as well as in the outcome of brain seizure activity. Nevertheless, further investigation is warranted to elucidate the roles of both IL‐6 and TNF‐α in epileptogenesis in both animal models and naturally occurring seizures.
Naturally occurring epileptic seizures in humans showed that increased CSF IL‐6 concentrations are positively associated with recent tonic‐clonic seizures, suggesting a role of cytokines in the formation of seizure activity,30 or a result of such event. Increased CSF cytokines concentrations might possibly account for occurrence and recurrences of seizures.8 In seizing dogs, an inflammatory “vicious cycle” might exist, in which seizures induce CNS proinflammatory mediator's production, thereby triggering seizure clusters and SE. In a study of cytokine expression in rat hippocampus specimens after induction of SE, IL‐6, TNF‐α and IL‐1β m‐RNA transcripts were increased at 2 hours postoccurrence of SE, reaching maximal levels 4 hours later, while afterward these slowly and progressively decreased.34 While IL‐6 and TNF‐α transcripts levels decreased to basal levels at 3 days postinduction of SE, levels of IL‐1β transcripts remained increased for 60 days.34 These findings could account for the lack of association between CSF IL‐6 and TNF‐α concentrations and duration of seizures or occurrence of seizure clusters in this study. Possibly due transient nature of the CNS expression of IL‐6 and TNF‐α, and with a relatively long lag of time from occurrence of seizures to presentation in these dogs, CSF levels of both cytokines decreased. Since postseizures IL‐1β expression is long term,31, 35 it would be interesting to measure the CSF concentration of this cytokine in addition to IL‐6 and TNF‐α in future studies of dogs with clusters seizures and SE, and probably CSF IL‐1β concentration should be followed up for a longer postseizure period to investigate its association with recurrent seizures in such dogs.
In humans with epilepsy, steroid use has been reported as early as 1942.36 In addition, seizures in humans were inhibited using nonsteroid antiinflammatory drugs (NSAIDs), including ibuprofen, paracetamol, and aspirin.26 Possibly, the antiepileptic effect of antiinflammatory drugs such as glucocorticoids and NSAIDs, occurs through inhibition of inflammatory reactions within the brain, mediated partly by proinflammatory cytokines. Furthermore, it was suggested that immuno‐modulating therapy should probably be initiated in each case of refractory SE of unknown cause.24 Following this same line, some forms of epilepsy in humans are sometimes treated, in addition to conventional antiepileptic drugs, with ACTH, prednisolone, or prednisone, as well as other immune‐modulating drugs such as intravenous human immunoglobulin, cyclophosphamide, rituximab, and plasmapharesis.24
It may be warranted to attempt such additional treatment in dogs that are refractory to conventional antiepileptic drugs.
Seizure‐mediated inflammatory reactions, IL‐1β affects BBB permeability via tight‐junction organization disruption in its cells, increased nitric‐oxide production and activation of endothelial cell matrix methaloproteinases.30, 31 Increased BBB permeability is associated with extravasations of circulating proteins and innate immune system cells as well as other inflammatory mediators, resulting in long‐lasting CNS inflammation.30 Disruption of the BBB is positively correlated with the frequency of spontaneous seizures in rats,30 and with posttraumatic epilepsy in traumatic brain injury in humans.37 In 3/17 of the study dogs (17%) herein, there was evidence of increased BBB permeability, based on increased AQ. Postseizure BBB disruption may persist for several weeks.38 This present finding should be further studied, and may have clinical implications; namely avoiding potentially neurotoxic medications, which would not have penetrated CNS when the BBB functions normally. Determining the AQ is a relatively simple method to assess BBB function, and should be considered in dogs with seizures, as part of the overall diagnostic work up. The AQ was abnormally high in only 3/17 study dogs, suggesting that the increased CSF cytokine concentrations levels in most cases with seizures herein were not a result of BBB disruption.
This study has several limitations. First, it included a limited number of cases, resulting in even smaller study subgroups, thereby weakening the statistical analyses and potentially leading to type‐II errors. Second, limited volume of CSF samples precluded analysis of certain parameters (eg, TNCC and cytokine concentrations), further limiting the statistical analyses. These limitations possibly contributed to the lack of association found between the presence of inflammatory CSF samples or the etiology of seizures and CSF cytokine concentrations. Third, the study group included dogs with seizures of various etiologies, potentially introducing variance. Fourth, serum cytokines were not measured in the apparently healthy controls, and thereby precluding comparison of serum IL‐6 and TNF‐α with negative controls and interpretation of the serum cytokines concentration in the study group. Fifth, the healthy control dogs were not screened for presence of sub‐clinical diseases, which if indeed present, might have affected the results. Lastly, the design of this study cannot exclude systemic inflammation processes as a cause for observed increased CSF cytokine levels. Nevertheless presently, there was no correlation between the blood leukocyte count and CSF cytokine levels or presence of an inflammatory CSF, in the study group as a whole, as well as in its subgroups (ie, tumor, ME, and IE subgroups). Future studies should include a larger, randomly selected population of dogs with seizures, as well as dogs with IE in particular, and efforts should be made to collect sufficient CSF volume to allow comprehensive analysis.
Acknowledgments
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
Footnotes
Elscint, Twin flash CT scanner, Elscint, Haifa, Israel
Arcus, Abacus, or Arcus Junior‐Vet, Hematology impedance analyzers, Diatron, Wien, Austria
Roche, Mannheim, Germany, at 37°C
Micro‐albumin, Pointe, Canton, MI
Modified Wright's stain, Bayer Hematek 2000 Slide Stainer, Bayer Diagnostics, Elkhart, IN
R&D Systems, Minneapolis, MN
SPSS 17, SPSS Inc., Chicago, IL
References
- 1. Chang Y, Mallor DJ, Anderson TJ. Idiopathic epilepsy in dogs: Owners' perspective on management with phenobarbital and/or potassium bromide. J Small Anim Pract 2006;47:574–581. [DOI] [PubMed] [Google Scholar]
- 2. Pákozdy A, Leschnik M, Tichy AG, et al. Retrospective clinical comparison of idiopathic versus symptomatic epilepsy in 240 dogs with seizures. Acta Vet Hung 2008;56:471–483. [DOI] [PubMed] [Google Scholar]
- 3. Klopmamm VT, Rambeck B, Tipold A. Prospective study of zonisamide therapy for refractory idiopathic epilepsy in dogs. J Small Anim Pract 2007;48:134–138. [DOI] [PubMed] [Google Scholar]
- 4. Löscher W. Animal models of intractable epilepsy. Prog Neurobiol 1997;59:239–258. [DOI] [PubMed] [Google Scholar]
- 5. Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. J Pharmacol 2006;147:232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gnatek Y, Zimmerman G, Goll Y, et al. Acetyl choline esterase loosens the brain's cholinergic anti‐inflammatory response and promotes epileptogenesis. Front Mol Neurosci 2012;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Librizzi L, Noè F, Vezzani A, et al. Seizure‐induced brain‐borne inflammation sustains seizure recurrence and blood‐brain barrier damage. Ann Neurol 2012;72:82–90. [DOI] [PubMed] [Google Scholar]
- 8. Vezzani A, Granata T. Brain inflammation in epilepsy: Experimental and clinical evidence. Epilepsia 2005;46:1724–1743. [DOI] [PubMed] [Google Scholar]
- 9. Becher B, Prat A, Antel JP. Brain‐immune connection: Immunoregulatory properties of CNS‐resident cells. Glia 2000;29:293–304. [PubMed] [Google Scholar]
- 10. Nguyen MD, Julien JP, Rivest S. Innate immunity: The missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci 2002;3:216–227. [DOI] [PubMed] [Google Scholar]
- 11. Siv M. Role of inflammation in epilepsy and treatment with IVIg. Intractable childhood epilepsy alliance drug information rotation, Wingate University School of Pharmacy, 5.17.2010. Internet sheet: http://www.ice-epilepsy.org/role-of-inflammation-in-epilepsy-and-treatment-with-ivig.html. Accessed January 30, 2014.
- 12. Billiau AD, Wouters CH, Lgae LG. Epilepsy and the immune system: Is there a link? Eur J Paediatr Neurol 2005;9:29–42. [DOI] [PubMed] [Google Scholar]
- 13. Van Rijckevorsel K. Immunological mechanisms in the aetiology of epilepsy. BioDrugs 1999;12:115–127. [DOI] [PubMed] [Google Scholar]
- 14. Beattie EC, Stellwagen D, Morishita W, et al. Control of synaptic strength by glial TNF‐alpha. Science 2002;295:2282–2285. [DOI] [PubMed] [Google Scholar]
- 15. Stellwagen D, Beattie EC, Seo JY, et al. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor‐alpha. J Neurosci 2005;25:3219–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Balosso S, Ravizza T, Perego C, et al. Tumor necrosis factor alpha inhibits seizures in mice via p75 receptors. Ann Neurol 2005;57:804–812. [DOI] [PubMed] [Google Scholar]
- 17. Cunningham AJ, Murray CA, O'Neill LA, et al. Interleukin‐1 beta (IL‐1 beta) and tumour necrosis factor (TNF) inhibit long term potentiation in the rat dentate gyrus in vitro. Neurosci Lett 1996;203:17–20. [DOI] [PubMed] [Google Scholar]
- 18. Probert L, Akassoglou K, Pasparakis M, et al. Spontaneous inflammatory demyelinating disease in transgenic mice showing central nervous system‐specific expression of tumor necrosis factor alpha. Proc Natl Acad Sci USA 1995;92:11282–11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwartz M, Puff C, Stein VM, et al. Pathogenetic factors for excessive IgA production: Th2‐dominated immune response in canine steroid‐responsive meningitis‐arteritis. Vet J 2011;187:260–266. [DOI] [PubMed] [Google Scholar]
- 20. Tipold A, Moore P, Zurbriggen A, et al. Early T cell response in the central nervous system in canine distemper virus infection. Acta Neuropathol 1999;97:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spitzbarth I, Bock P, Haist V, et al. Prominent microglial activation in the early proinflammatory immune response in naturally occurring canine spinal cord injury. J Neuropathol Exp Neurol 2011;70:703–714. [DOI] [PubMed] [Google Scholar]
- 22. Maiolini A, Otten M, Hewicker‐Trautwein M, et al. Interleukin‐6, vascular endothelial growth factor and transforming growth factor beta 1 in canine steroid responsive meningitis‐arteritis. BMC Vet Res 2013;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chrisman CL. Cerebrospinal fluid analysis, Moore, M.P.: Diseases of the spine. Vet Clin North Am Small Anim Pract. 1992;22:796–800. [DOI] [PubMed] [Google Scholar]
- 24. Ozkara C, Vigevano R. Immuno and anti‐inflammatory therapies in epileptic disorders. Epilepsia 2011;52:45–51. [DOI] [PubMed] [Google Scholar]
- 25. Marchi N, Granata T, Freri E, et al. Efficacy of anti‐inflammatory therapy in a model of acute seizures and in a population of pediatric drug resistant epileptics. PLoS One 2011;6:e18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wallenstein MC. Attenuation of penicillin models of epilepsy by nonsteroidal anti‐inflammatory drugs. Exp Neurol 1987;98:152–160. [DOI] [PubMed] [Google Scholar]
- 27. Minami M, Kuraishi Y, Yamaguchi T, et al. Convulsants induce interleukin‐1 beta messenger RNA in rat brain. Biochem Biophys Res Commun 1990;171:832–837. [DOI] [PubMed] [Google Scholar]
- 28. Sonmez FM, Serin HM, Alver A, et al. Blood levels of cytokines in children with idiopathic partial and generalized epilepsy. Seizure 2013;22:517–521. [DOI] [PubMed] [Google Scholar]
- 29. Sinha S, Patil SA, Jayalekshmy V, et al. Do cytokines have any role in epilepsy? Epilep Res 2008;82:171–176. [DOI] [PubMed] [Google Scholar]
- 30. Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun 2008;22:797–803. [DOI] [PubMed] [Google Scholar]
- 31. Vezzani A, Ravizza T, Balosso S, et al. Glia as a source of cytokines: Implications for neuronal excitability and survival. Epilepsia 2008;49:24–32. [DOI] [PubMed] [Google Scholar]
- 32. Tutuncuoglu S, Kutukculer N, Kepe L, et al. Proinflammatory cytokines, prostaglandins and zinc in febrile convulsions. Pediatr Int 2001;43:235–239. [DOI] [PubMed] [Google Scholar]
- 33. Samland H, Huitron‐Resendiz S, Masliah E, et al. Profound increase in sensitivity to glutamatergic but not cholinergic agonistinduced seizures in transgenic mice with astrocyte production of IL‐6. J Neurosci Res 2003;73:176–187. [DOI] [PubMed] [Google Scholar]
- 34. De Simoni MG, Perego C, Ravizza T, et al. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur J Neurosci 2000;12:2623–2633. [DOI] [PubMed] [Google Scholar]
- 35. Ravizza T, Rizzi T, Perego M, et al. Inflammatory response and glia activation in developing rat hippocampus after status epilepticus. Epilepsia 2005;46:113–117. [DOI] [PubMed] [Google Scholar]
- 36. Mcquarrie I, Anderson JA, Ziegler MR. Observations on the antagonistic effects of posterior pituitary and cortico‐adrenal hormones in the epileptic subject. J Clin Endocrinol Metabol 1942;2:406–410. [Google Scholar]
- 37. Tomkins O, Kaufer D, Korn A, et al. Frequent blood‐brain barrier disruption in the human cerebral cortex. Cell Mol Neurobiol 2001;21:675–691. [DOI] [PubMed] [Google Scholar]
- 38. Korn A, Golan H, Melamed I, et al. Focal cortical dysfunction and blood‐brain barrier disruption in patients with post‐concussion syndrome. J Clin Neurophysiol 2005;22:1–9. [DOI] [PubMed] [Google Scholar]


