Abstract
Background
Although gastroesophageal reflux (GER) often is assumed to be causative for upper gastrointestinal and respiratory signs in dogs, no attempts have been made to verify this assumption.
Objectives
To monitor esophageal pH with the Bravo pH system in healthy dogs and client‐owned dogs displaying signs commonly attributed to GER.
Animals
Seven healthy and 22 client‐owned dogs.
Methods
After routine esophagogastroduodenoscopy, radiotelemetric pH capsules were placed in distal esophagus for continuous pH recording. Reflux was defined as single pH measurement <4. At discharge, owners were instructed to press individually predefined clinical sign‐buttons on the receiver whenever indicated. Results between groups were compared using Mann–Whitney U‐test.
Results
The median (range) number of refluxes in client‐owned and healthy dogs, respectively, was 17 (1–205) and 10 (1–65), the number of refluxes >5 minutes in duration was 1 (0–14), and 1 (0–4), duration of longest reflux (min) was 10 (0–65) and 8 (0–27), and fractional time pH <4 (%) was 0.76% (0.01–6.28), and 0.3% (0–3.1). No differences were found between groups. The median of 7 (1–35) clinical sign‐button pushes were recorded in 21 dogs. Median of 12.5% (2.8% [1/35]–50% [2/4]) reflux‐positive clinical sign‐button pushes was found in 10 dogs with reflux‐positive pushes. Five (22.7%) dogs had increased esophageal acid exposure, and mild esophagitis was noted in 1 dog.
Conclusion and Clinical Importance
Despite evidence of increased GER in some dogs, the clinical sign‐reflux association remained poor. Future investigation should focus on dogs with esophagitis.
Keywords: Acid, Canine, Esophagitis, Esophagus
Abbreviations
- GER
gastroesophageal reflux
- GERD
gastroesophageal reflux disease
In people, gastroesophageal reflux disease (GERD) results from a failure of the normal antireflux barrier to protect against frequent and abnormal amounts of gastroesophageal reflux (GER). Although GER itself is a normal physiological process occurring multiple times a day, GERD is regarded as a multifactorial process usually producing clinical signs of acid regurgitation such as heartburn, dysphagia, belching, globus sensation, and nausea, as well as extraesophageal clinical signs (ie, laryngitis, pulmonary disease).1 These clinical signs can be caused by GER even when there is no evidence of mucosal pathology during endoscopy, and acidic regurgitation with normal esophageal mucosal appearance has been named nonerosive reflux disease.2, 3 Consequently, ambulatory intraesophageal pH monitoring has become the gold standard for establishing pathological reflux, not only providing information on distal esophageal acid exposure but also evaluating clinical signs associated with acid reflux episodes. Because catheter‐based pH recording systems were not tolerated by all patients, catheter‐free devices have become established.4 The oblong Bravo capsule1 (6 × 5.5 × 25 mm) has an antimony pH electrode and a reference electrode located at its distal tip, with an internal battery and transmitter. It is mounted onto the end of a delivery catheter as part of a prepackaged assembly for oral intubation.5 In people, the main indication is the monitoring of distal esophageal pH for diagnostic purposes, particularly in patients after a normal endoscopic examination. In veterinary medicine, this device has been recently used for prolonged measurement of intragastric pH in healthy dogs.6
Although GER also has been implicated as an underlying cause of presumed esophagitis in dogs,7, 8, 9, 10, 11, 12 it currently is unclear whether GER represents a clinically relevant problem, and, if so, what its clinical signs would be. The diagnoses of all reported cases of esophagitis secondary to presumed GER in small animals have been made based on a combination of presumably typical historical and clinical signs, as well as radiographic, endoscopic, or histopathological findings without the actual demonstration of pathologic esophageal pH.7, 10, 13, 14, 15 Wireless intraesophageal pH monitoring has not been performed in dogs so far, and little is known about the physiologic esophageal canine pH range or how physiologic reflux episodes are characterized. Therefore, the aims of this study were to (1) examine the esophageal pH milieu of healthy dogs, (2) monitor the intraesophageal pH in dogs with clinical signs commonly attributed to reflux esophagitis in the veterinary literature,6, 7, 8, 9, 10, 11 and (3) examine potential temporal associations between displayed clinical signs and recorded GER episodes. Our fourth objective was to evaluate the feasibility of the Bravo system as a means of monitoring continuous intraesophageal pH in dogs.
Materials and Methods
Healthy Dogs
Seven healthy dogs (6 Beagles, 1 mixed‐breed dog; 3 females, 4 males), aged 1–4 years (median, 2 years), weighing 11–16.2 kg (median, 13.8 kg; median body condition score, 5/9; range 4–5), were the subjects of the first part of the study. All dogs lacked clinical signs of gastrointestinal disease and were deemed healthy based on physical examination, as well as results of CBC, serum biochemistry profile, and urinalysis. The Cantonal Institutional Animal Care and Use Committee of the Canton of Zurich, Zurich, Switzerland approved the protocol for this study (no. 102/2010).
Client‐owned Dogs with Clinical Signs Interpreted as GER
The decision to monitor intraesophaeal pH in individual patients was made when presenting clinical signs were suspicious for esophageal disease based on what has been published in the veterinary literature, and could not be explained by results of other tests. All dogs underwent a comprehensive clinicopathologic evaluation (physical examination including thorough examination of the oral cavity, CBC, serum biochemistry profile including lipase activity,16 Spec cPL,2 urinalysis, thoracic radiography, and abdominal ultrasonography) before placement of the Bravo capsule was considered. Parasites (helminths and giardia) were ruled out in 2 dogs where intermittent diarrhea was also part of the presenting problems.
Exclusion criteria were recent (<2 weeks) or current medication with gastric acid suppressants, as well as prokinetic medication. Gastrointestinal biopsies were assessed by a board‐certified pathologist (MR) according to WSAVA guidelines.17
The following breeds were represented: Labrador Retriever (5), mixed‐breed dog (5), Beauceron (1), Boxer (1), Czechoslovakian Wolfdog (1), Coton de Tulear (1), Entlebucher Mountaindog (1), French Bulldog (1), Hovawart (1), Malinois (1), Miniature Schnauzer (1), Shar Pei (1), Weimeraner (1), and West Highland White Terrier (1). The dogs had a median body weight of 21.3 kg (range, 6–47) and a median age of 5 years (range, 1–13).
The presenting anamnestic and clinical signs ultimately leading to the decision to monitor esophageal pH in these dogs were: repetitive lip smacking (15), increased empty swallowing motions (9), chronic intermittent vomiting (7; 2/7 dogs showed only early morning vomiting), sudden unexplained discomfort (6), belching (3), drooling (3), excessive grass eating (3), presumed postprandial pain (3), refusal to eat despite interest (3), regurgitation (2), retching (2), chronic mild intermittent cough (1), halitosis (2), and excessive surface licking (2). Additional findings on physical examination were laryngitis (1) and tonsillitis (1). Concurrent intermittent small bowel diarrhea was an additional, but not prevailing, complaint in 2 dogs. The 22 dogs presented with a median of 3 of the above‐mentioned clinical signs (range, 1–6), and 3 dogs presented with only 1 of the following clinical signs: chronic vomiting, excessive lip smacking, and excessive grass eating, respectively. The dog with mild intermittent cough as part of the presenting complaints also displayed the following clinical signs: increased swallowing motions, lip smacking, belching, and halitosis. Thoracic radiographic abnormalities were found only in a West Highland White Terrier presenting with regurgitation and vomiting and radiography disclosed mild to moderate esophageal dilatation.
Intraesophageal and Gastric pH Monitoring in Healthy Dogs
After a 12‐hour fast, dogs were anesthetized for endoscopy‐assisted placement of a pH capsule. Dogs were premedicated with acepromazine (0.03 mg/kg IM) and buprenorphine (0.014 mg/kg IM), an IV catheter was placed, and general anesthesia was induced with propofol and maintained with isoflurane. After routine esophagogastroduodenoscopy, pH capsules were placed under direct endoscopic guidance. All capsule placements were performed by an investigator (PHK) skilled in endoscope handling to ensure consistency in capsule positioning. The capsule and receiver were calibrated with commercial buffer solutions (pH 1.07 and 7.01) according to manufacturer's instructions, and only postcalibration values within a 0.05 pH unit range of the buffer values were considered adequate. To be able to record and monitor both continuous gastric as well as esophageal pH values, an additional intragastric pH capsule was first placed in the gastric fundus. The approach for gastric capsule placement was similar to what has been described recently.6 The esophageal capsule subsequently was positioned approximately 4–5 cm above the Z‐line. Mucosal attachment of the pH capsule was achieved according to manufacturer's instructions by the use of vacuum suction and a lock and pin mechanism.3 After capsule placement, pH recordings were obtained telemetrically at 6‐second sampling intervals. The immediate postanesthesia recovery (defined as time until dogs were ambulatory) was excluded from final pH analysis. Esophageal reflux was defined as a single pH measurement <4. The following parameters were calculated by the computer software4 : the total number of refluxes, number of refluxes >5 minutes, duration of longest reflux (minutes), and the percentage time pH < 4 (fraction time pH < 4; %). Data receivers were kept in close proximity to the dogs by attachment to the canine's collar or by attaching the receiver to the canine's cage when hospitalized. Seven days after capsule placement, a left lateral abdominal radiograph was obtained in all dogs to verify capsule detachment.
Intraesophageal pH Monitoring in Client‐owned Dogs
The same approach (anesthesia, esophageal capsule placement procedure) was used in client‐owned dogs. The pH receiver was attached either to a harness or collar, depending on the canine's size. In addition to the routine pH parameters, a clinical sign‐reflux correlation was assessed. The receiver has 3 so‐called “symptom buttons” that can be individually programmed. At discharge, owners were familiarized with the receiver and instructed to press the appropriate buttons if the dog showed the corresponding clinical signs. Assignment of buttons (depending on predominant presenting complaints) was determined together with the owner. A “clinical sign association” was considered positive if the clinical signs occurred within 2 minutes of the reflux event.17 Owners were instructed to maintain the usual daily routine (eg, feeding regimen, walking) and to fill out an activity diary to record the dogs' clinical signs, food intake and sleeping patterns. After 48 hours of pH data acquisition, owners returned to the hospital for data download. When possible, the receiver was reset to obtain a second 48 hours of data.
Statistical Analysis
Results are reported using descriptive statistics. Data were tested for normality by the Shapiro–Wilk test. Bravo esophageal pH data (healthy dogs versus patients) were compared using the nonparametric Mann–Whitney U‐test to evaluate statistical differences between groups.5 The level of significance was set at P < .05.
Results
Experience with the Bravo System
Healthy Control Dogs
Overall, 14 Bravo capsules were successfully attached to the distal esophageal mucosa and gastric mucosa; no technical problems were encountered. Total procedure times for endoscopy‐assisted capsule placement (gastric and esophageal) ranged from 15 to 30 minutes, with most procedures taking <20 minutes. Technically, the procedure was simple: the intragastric capsule fitted snugly between the gastric folds and the esophageal capsule fixed well to the esophageal mucosa. Premature capsule dislodgement was not noticed. Mucosal detachment and passing of the pH capsule through the digestive tract was verified radiographically in all dogs on day 7.
Client‐owned Dogs with Suspicion of GER
Three failures because of premature capsule detachment evidenced by a sudden drop to acidic gastric pH values lasting several hours followed by a sharp rise to slightly alkaline duodenal pH occurred after 180, 249, and 352 minutes in a Bull Terrier, Golden Retriever, and Labrador Retriever, respectively. Not a single reflux was recorded during these brief pH recording periods, but the pH data were excluded, leaving a total of 22 dogs for analysis. The overall failure rate of capsule placement therefore was 12% (3/25).
Esophageal and Gastric pH Recordings in healthy Dogs
The median pH monitoring time was 45.13 hours (range, 37.43–65.05). The median total number of refluxes was 10 (range, 1–65), the median number of refluxes >5 minutes was 1 (range, 0–4), the median duration of longest reflux was 8 minutes (range, 0–27). The median time pH < 4 was 10 minutes (range, 0–86), whereas the median esophageal fraction time of pH < 4 was 0.3% (range, 0–3.1). None of the dogs had reflux episodes detected during the immediate recovery period after anesthesia. Postprandial reflux was detected after 5 of 29 (17.2%) administered meals in 3 of 7 dogs; their 5 reflux episodes lasted 1 and 6 minutes, 5 and 4 minutes, and 9 minutes, respectively. The median gastric pH was 1.1, the median percentage of the investigation time that the gastric pH oscillated between 0.5 and 2.5 was 90.32% (range, 78–97.4%). When comparing nighttime to daytime pH values, no differences were found.
Endoscopic and Histopathologic Assessment of Gastrointestinal Biopsies in healthy Dogs
Esophagogastroduodenoscopy, as well as endoscopic gastric and duodenal biopsy results were unremarkable in all dogs. Two gastric biopsies disclosed evidence of spiral‐shaped organisms attached to the mucosal surface without concurrent pathologic mucosal or lamina propria histopathologic changes.
Esophageal pH Recordings in Client‐owned Dogs with Suspicion of GER
The median pH monitoring time was 60.45 hours (range, 37.24–91.45). The median total number of refluxes was 17 (range, 1–205), the median number of longest (>5 minutes) refluxes was 1 (0–14), the median duration of longest reflux was 10 minutes (range, 0–65). The median time of pH < 4 was 29 minutes (range, 1–292), whereas the median fraction time pH < 4 was 0.76% (range, 0.01–6.28). Neither the total pH monitoring time, nor any of the pH parameters compiled, differed statistically between healthy control dogs and client‐owned dogs (Figs 1, 2, 3, 4). None of the dogs had reflux episodes detected during the immediate recovery period from anesthesia. A median of 8 meals (range, 4–17) per dog was given during the pH recording time. Postprandial reflux was detected after 17 of 201 (8.4%) meals in 8 of 22 dogs; none of the reflux episodes lasted longer than 2 minutes. A median of 7 (range, 0–35) clinical sign‐button pushes per dog was recorded. One owner did not use the clinical sign‐buttons at all during the pH monitoring phase. In 13 dogs, all of the clinical sign‐button pushes were reflux‐negative, whereas a range of 1–3 reflux‐positive pushes was recorded in 10 dogs. When considering only the 10 dogs with at least 1 reflux‐positive push, a median of 12.5% (range, 2.8% [1/35 pushes]–50% [2/4 pushes]) of the clinical sign‐button pushes actually were reflux‐positive. When comparing recorded pH values between daytime and nighttime, no differences were found.
Figure 1.
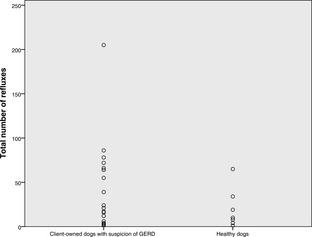
Dot plot showing the total number of refluxes in 22 dogs with suspicion of GERD and in 7 healthy control dogs.
Figure 2.
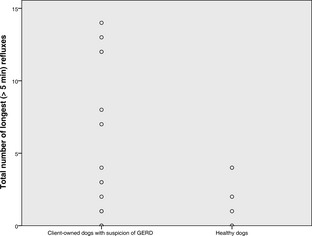
Dot plot showing the number of refluxes >5 minutes in 22 dogs with suspicion of GERD and in 7 healthy control dogs.
Figure 3.
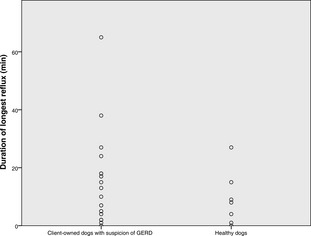
Dot plot showing the duration of longest reflux (minutes) in 22 dogs with suspicion of GERD and in 7 healthy control dogs.
Figure 4.
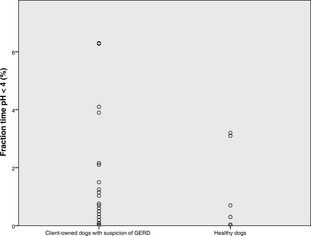
Dot plot showing the fraction time pH <4 (%) in 22 dogs with suspicion of GERD and in 7 healthy control dogs.
Endoscopic and Additional Diagnostic Findings in Client‐owned Dogs with Suspicion of GER
Esophagogastroduodenoscopy was performed in 20 dogs, whereas complete enteroscopy (retrograde and antegrade approach) using a single‐balloon enteroscope6 was performed in 2 dogs in which intermittent diarrhea also was part of the problem list. Mild distal esophagitis (mild diffuse erythema and mildly increased granularity above the lower esophageal sphincter) was noted in a Labrador Retriever later diagnosed with metastatic mast cell disease. A flaccid and dilated tubular esophagus was noted in the dog with radiographic evidence of esophageal dilatation. No specific endoscopic findings were recorded in the remaining 20 dogs. Additional diagnostic examinations performed either in conjunction with endoscopy and pH capsule placement or later were: measurement of anti‐acetylcholine receptor antibodies (2), treatment trial with phenobarbital for presumed limbic epilepsy (2), tracheobronchial cytology and bacteriology (1), tonsillectomy (1), fine needle aspiration cytology of liver, spleen, and mesenterial lymph nodes (1), and fine needle aspiration cytology of salivary glands and retropharyngeal lymph nodes (1).
Histopathologic Assessment of Gastrointestinal Biopsies in Client‐owned Dogs with Suspicion of GER
Histopathologic assessment of gastric biopsies indicated the following diagnoses: unremarkable biopsies (8), spiral‐shaped bacteria on the mucosal surface without gastric abnormalities (5), and mild edema (1). Mild (4) to moderate (4) gastritis was diagnosed in the remaining 8 dogs and the following diagnoses were made: mild lymphoplasmacytic gastritis (2), mild lymphoplasmacytic and eosinophilic gastritis (1), mild neutrophilic gastritis with moderate fibrosis (1), moderate lymphoplasmacytic gastritis (2), moderate lymphoplasmacytic gastritis and mild fibrosis (1), and moderate lymphoplasmacytic and neutrophilic gastritis (1). Histopathologic assessment of intestinal biopsies indicated the following diagnoses: mild lymphoplasmacytic duodenitis (12), mild lymphoplasmacytic and eosinophilic duodenitis (2), mild lymphoplasmacytic duodenitis, jejunitis and ileitis (1), unremarkable duodenal biopsies (6), and unremarkable duodenal, jejunal, and ileal biopsies (1).
Final Diagnoses and Follow‐up of Client‐owned Dogs with Suspicion of GER
Initially, all dogs were treated with omeprazole (1–1.5 mg/kg body weight PO q24h) for 2 weeks. Clinical follow‐up evaluation was available for all dogs, and the final diagnoses were made on the basis of diagnostic tests and response to treatment. Food‐responsive disease (hypoallergenic diet [4], novel protein diet [4], home‐cooked diet [2]) was diagnosed in 10 dogs. Two of the food‐responsive dogs also had intermittent diarrhea and initially needed additional treatment with budesonide (1) and cyclosporine (1) but eventually could be controlled with diet only. Three dogs needed a combination of diet and continuous antisecretory medication (hypoallergenic diet and omeprazole [2], novel protein diet and ranitidine [1]). The final diagnoses in the remaining dogs were: atopic skin disease (1), idiopathic megaesopha‐gus (1), salivary (parotid) gland carcinoma (1), chronic laryngeotracheobronchitis (1), chronic tonsillitis and laryngitis (1), brachycephalic syndrome and syringomyelia grade 2 (1), and splenic mast cell disease with lymph node involvement (1). No underlying disease could be found in 2 dogs with fraction times of pH < 4 of 0.2 and 2.1%. Individual treatment of the underlying disease eliminated (13) or improved (5) clinical signs initially suspected of representing GER, except for 3 dogs that were diagnosed with idiopathic megaesophagus, salivary gland neoplasia (diagnosed 3 months after the pH study, after which the tumor had grown substantially) and mast cell disease.
The 5 dogs with the highest total fraction times (%) of pH < 4 (ranging from 6.3 to 4.1%) had the following clinicopathologic findings, diagnoses and outcome: A total fraction time pH < 4 of 6.3% was found in a 12‐year‐old male neutered Shar Pei presenting with intermittent excessive grass eating and lip smacking. Endoscopic evaluation and gastroduodenal biopsies were normal. A 12‐month follow‐up evaluation indicated clinical improvement with a combination of hypoallergenic diet7 and standard dosage of omeprazole. A total fraction time pH < 4 of 6.28% was found in a 4‐year‐old male neutered Beauceron presenting with lip smacking, early morning vomiting, and intermittent inappetence. Mild lymphoplasmacytic gastritic and moderate lymphoplasmacytic duodenitis were found on endoscopic biopsies. A 16‐month follow‐up evaluation showed full clinical recovery with a grain‐free home‐cooked diet and 1 ranitidine (2 mg/kg) dose in the evening. These 2 dogs with the highest fraction times of pH < 4 also had the longest duration of a single reflux (65 minutes in the Shar Pei, 38 minutes in the Beauceron) as well as the highest total number of refluxes >5 minutes (n = 14 in the Shar Pei, n = 13 in the Beauceron). A total fraction time of pH < 4 of 4.1% was found in a 5‐year‐old male, neutered Boxer presenting for lip smacking, retching, drooling, and frequent empty swallowing. A 13‐month follow‐up evaluation indicated improvement in the canine's clinical signs with a combination of hypoallergenic diet6 and omeprazole. A total fraction time of pH < 4 of 4.1% was found in a 9‐year‐old male, neutered Labrador Retriever presenting for empty swallowing and lip smacking. Endoscopy identified mild distal esophagitis; gastroduodenal biopsies disclosed mild lymphoplasmacytic duodenitis. No improvement was seen with omeprazole treatment. Repeated diagnostic evaluation 2 months later identified undifferentiated mast cell neoplasia in the spleen and mesenteric lymph nodes of unknown primary origin. A total fraction time pH < 4 of 4% was found in a 1‐year‐old male neutered Malinois presenting for retching, belching, grass eating, and intermittent sudden discomfort. Endoscopy and gastroduodenal biopsy results were normal. The dog responded fully to a hypoallergenic diet.7
Discussion
We were able to show that the Bravo pH capsule is a useful diagnostic tool for extended ambulatory catheterless esophageal pH monitoring in dogs. Capsule placement was quick and easy, attached capsules stuck sufficiently long to the esophageal mucosa in the majority of dogs, and none of the dogs seemed painful or showed clinical signs such of inappetence, or vomiting. Regarding the total number of refluxes, duration of longest refluxes, as well as fraction times of pH < 4, considerable variation existed in healthy dogs as well as in patients presenting with unclear clinical signs often believed to reflect pathological acidic reflux. When the results from both groups were compared, no statistically significant differences could be found. Our normal ambulatory esophageal pH values correlated well with those published so far in dogs.18, 19 To the authors' knowledge, ambulatory esophageal pH monitoring in awake dogs has only been performed with a catheter‐based system. In that study, esophageal pH values were recorded every minute for 8 hours. Not a single reflux episode was observed in any of the 10 awake female dogs during the observation period, and the esophageal pH remained continuously in a weakly alkaline range.18 Similarly, in a study establishing a canine GERD model, the distal esophageal pH environment of 21 awake female mongrel dogs with cervical esophagopexy and in‐dwelling pH catheters was documented over the course of 24 hours, and the fractional time pH < 4 also was low, with a median of 0.1%, and a mean of 0.43% (range, 0–3.59%).19 Our noninvasively recorded pH data are similar to McMahon's catheter‐based results,19 although we demonstrated a higher total number of reflux episodes ([10; range, 1–65] versus [2; range, 0–46])18 and overall longer single reflux episodes ([8 minutes; range, 0–27] versus [1 minutes; range, 0–16]).18 Concurrent investigation of both gastric and esophageal pH values in healthy dogs was performed because conflicting data exist on canine gastric pH, suggesting higher pH values compared to humans,20, 21 and results of a recent study using the Bravo system for monitoring canine gastric pH6 were not available at the time of investigation.
In human medicine, current consensus is that the total percentage of time of pH is <4 is the most useful single discriminator between physiologic and pathologic reflux.22 An abnormal test is described as a value greater than an established threshold, which typically is >2.0 SD above the mean or 95th percentile of normal controls.22 Our control values lie well within what has been established as normal in healthy humans. However, this also would apply to results of most of the evaluated 22 patients (Table 1) from this study (depending on the human reference range used) because the 2 most referenced thresholds for abnormal acid exposure using catheter‐free pH systems in humans have reported similar reference ranges of up to 4.4 and 5.3% fractional time of pH < 4 for a 48‐hour period.23, 24
Table 1.
Median, and 95th percentile values for the % total time esophageal pH < 4 measured with the Bravo pH Capsule
| % Total Time pH < 4 | Median | 95th Percentile |
|---|---|---|
| Healthy dogs (n = 7) | 0.30 | 3.20 |
| Client‐owned dogs (n = 22) | 0.76 | 6.29 |
Another critical clinical question is whether a patient's clinical signs are truly because of reflux or not. A so‐called “symptom event” is traditionally considered to be associated with a reflux episode if it occurs within 2 minutes of the reflux episode.25 A commonly used method to evaluate the temporal association between clinical signs and reflux is the symptom index. The symptom index is defined as the percentage of symptom events that are temporally related to a reflux episode. A symptom index of 50% usually is considered positive.22 In our study, only 1 Labrador Retriever presenting for intermittent drooling, lip smacking, and empty swallowing reached this threshold, but the total number of button pushes was very low in this case with 2 of 4 reflux‐positive button pushes during 67.22 hours of monitoring. Moreover, symptom index assessment is only considered worthwhile in the context of high esophageal acid exposure, but this canine's fractional time pH < 4 was 2.16%.
Our results challenge the validity of reported historical and clinical signs of GER. The difficulty here certainly is the absence of patient—clinician communication that is crucial for the perception of reflux events in humans. Instead, we depended on the attentiveness of canine owners, which is naturally confounded by inherent subjectivity when interpreting these clinical signs. Perhaps not surprisingly, these results suggest that clinical signs such as lip smacking are nonspecific. Nevertheless, we deliberately included patients presenting with a wider range of clinical signs than have either been reported in dogs,7, 10, 11, 12 or have been associated with GERD in people, such as chronic upper respiratory problems,1 in order not to overlook potential reflux patients.
A large proportion of included dogs examined for GER had histopathological evidence of inflammatory enteropathy and ultimately responded to a diet change. Similarly, both dogs in which the medical history included excessive licking of surfaces and objects among other clinical signs ultimately were diagnosed with food‐responsive disease. A recent study evaluating gastrointestinal disorders in dogs with excessive licking of surfaces found similar courses of disease, with dogs responding to the combination of diet and famotidine.26 In retrospect, it would have been desirable to exclude food‐responsive enteropathy first, but it is the authors' experience that dietary compliance increases considerably in cases in which a comprehensive investigation of the animal's problems has been performed. Secondly, because all patients underwent upper gastrointestinal endoscopy for evaluation of their clinical signs, we included capsule placement in the diagnostic evaluation to avoid a second anesthesia. Our results indicate that dietary trials should be pursued more vigorously in dogs presenting with signs such as lip smacking, chronic vomiting, surface licking, and excessive grass eating before embarking on more advanced diagnostic testing such as esophageal pH monitoring.
Mild upper respiratory signs (eg, cough, laryngitis) also were noted among repeated swallowing motions, lip smacking, belching, and retching in 2 dogs. According to some authors, development of recurrent cough without obvious involvement of the respiratory tract can be the only sign of GER in dogs.7, 9 Microaspirations of gastric contents are discussed as a cause of chronic respiratory disease in humans.1 We could not prove this assumption, as none of the dogs with the highest fraction times of pH < 4 had upper respiratory clinical signs, but we cannot exclude GER of nonacidic contents with our approach.
The presented esophageal pH results relate to the question of whether or not pathological reflux exists in dogs. Because our patients cannot talk to us, we cannot diagnose it based on history and observed clinical signs as often is done in human medicine. When looking at our heterogeneous group of dogs presenting with diverse clinical signs supposedly representing GER, it becomes obvious that the crux of the problem is how to identify candidates with relevant GER. Because nothing is known about esophageal pH profiles in dogs with potentially suspicious signs, we speculated that, like humans, dogs with normal esophagoscopy still could suffer from increased esophageal acid exposure. An inherent problem in this context is the lack of agreement in the endoscopic assessment of esophageal mucosal lesions in small animals. Although standardized severity scores for endoscopic esophageal lesions exist in humans (eg, Los Angeles Classification of Esophagitis), reliable endoscopic criteria for diagnosing esophagitis as well as grading its severity have not yet been established in veterinary medicine. In fact, no descriptive endoscopic study on canine esophagitis other than case reports has been published. Thus, the endoscopic diagnosis “esophagitis” in this study also was based on subjective criteria, and subtle reflux‐associated mucosal changes such as intrapapillary capillary loop lesions27 might have gone undetected with conventional state‐of‐the‐art endoscopy. Despite this, it is interesting to note that 3 of 5 dogs with the highest esophageal acid exposure profile (and endoscopically normal esophagus) could only be clinically controlled if they received their daily antacid medication. In all 3 cases, owners repeatedly tried to discontinue antacid drug administration, but clinical signs would recur. One of these dogs was clinically controlled with ranitidine, a drug with only weak antacid action.28 The drug's prokinetic action may have had beneficial effects in this dog.
Our study had some limitations. A relatively small number of healthy dogs was enrolled. However, our established normal ambulatory esophageal pH values agreed well with those published so far for dogs.19 The chosen cutoff “return to ambulatory after anesthesia” for starting esophageal pH monitoring after capsule placement was somewhat arbitrary, and we cannot definitely ascertain whether recent anesthesia had any sustained effect on GER beyond return to ambulation. However, postanesthetic GER was not recorded in any of the examined dogs. This may have been because of the chosen anesthesia protocol as we recently showed that the same sedation using acepromazine and buprenorphine had no significant effect on manometrically measured lower esophageal sphincter pressures compared to the awake state.29 Similarly, in humans it has been shown that Bravo capsule placement under sedation did not significantly increase the fraction time of abnormal test results when compared to unsedated capsule placement.30, 31
Lastly, the reliance of clients to detect clinical signs of GER may vary and not all dogs were continually observed. A study design involving confinement and video recording the dogs' behaviors which can be standardly assessed may be a more accurate method of documenting the relationship between reflux and clinical signs. With this, subtle clinical signs not yet associated with GER also may be identified.
In conclusion, although increased esophageal acid exposure can be found in some dogs presenting with signs presumably suspicious of GER, the clinical sign‐reflux association did not support the validity of these signs. The majority of examined dogs had no evidence of acidic reflux, although GER appears to cause clinical signs in a small subset of dogs. To bypass highly subjective and difficult to assess clinical signs, future studies should focus on patients with esophagitis. Thus, a causal relationship between esophagitis and gastric acid exposure can be investigated.
Acknowledgments
The study was performed at the Clinic for Small Animal Internal Medicine, Vetsuisse Faculty, University of Zurich. The study was not supported by a grant or otherwise.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
This study was presented in part as an oral abstract at the Congress of the European College of Veterinary Internal Medicine‐Companion Animals, Maastricht, the Netherlands, September 2012.
Footnotes
Bravo pH monitoring system, Given Imaging, Yoqneam, Israel
IDEXX GmbH, Ludwigsburg, Germany
Polygram Net Software, Given Imaging, Yoqneam, Israel
GraphPad Prism 5.0, Inc, La Jolla, CA, USA
Kook PH. Marching Down the Gut with Push‐Pull Enteroscopy. Proceedings of the 2011 ACVIM Forum Denver, Colorado, June 15–18
Hills z/d Provet AG, Lyssach, Switzerland
References
- 1. Richter JE, Friedenberg FK. Gastroesophageal reflux disease In: Feldman M, Friedman LS, Brandt LJ, ed. Sleisenger and Fordtran's Gastrointestinal and Liver Disease, 9th ed Philadelphia, PA: WB Saunders; 2010:705–733. [Google Scholar]
- 2. Karamanolis GP, Tutuian R. Role of non‐acid reflux in patients with non‐erosive reflux disease. Ann Gastroenterol 2013;26:100–103. [PMC free article] [PubMed] [Google Scholar]
- 3. Winter JW, Heading RC. The nonerosive reflux disease‐gastroesophageal reflux disease controversy. Curr Opin Gastroenterol 2008;24:509–515. [DOI] [PubMed] [Google Scholar]
- 4. Wenner J, Johnsson F, Johansson J, et al. Wireless esophageal pH monitoring is better tolerated than the catheter‐based technique: Results from a randomized cross‐over trial. Am J Gastroenterol 2007;102:239–245. [DOI] [PubMed] [Google Scholar]
- 5. Tutuian R. Reflux monitoring: Current status. Curr Gastroenterol Rep 2008;10:263–270. [DOI] [PubMed] [Google Scholar]
- 6. Tolbert K, Bissett S, King A, et al. Efficacy of oral famotidine and 2 omeprazole formulations for the control of intragastric pH in dogs. J Vet Intern Med 2011;25:47–54. [DOI] [PubMed] [Google Scholar]
- 7. Lux CN, Archer TM, Lunsford KV. Gastroesophageal reflux and laryngeal dysfunction in a dog. J Am Vet Med Assoc 2012;240:1100–1103. [DOI] [PubMed] [Google Scholar]
- 8. Tams TR. Handbook of Small Animal Gastroenterology, 2nd ed St Louis, MO: WB Saunders; 2003. [Google Scholar]
- 9. Lecoindre P. Diseases of the Esophagus In: Lecoindre P, Gaschen F, Monnet E, ed. Canine and Feline Gastroenterology, 1st ed Rueil‐Malmaison Cedex, France: Point Vétérinaire Publications; 2012:173–201. [Google Scholar]
- 10. Münster M, Hörauf A, Lübke‐Becker A, et al. Idiopathic esophagopathies resembling gastroesophageal reflux disease in dogs. Tierarztl Prax Ausg K Kleintiere Heimtiere 2013;41:173–179. [PubMed] [Google Scholar]
- 11. Han E. Diagnosis and management of reflux esophagitis. Clin Tech Small Anim Pract 2003;18:231–238. [DOI] [PubMed] [Google Scholar]
- 12. Glazer A, Walters P. Esophagitis and esophageal strictures. Compend Contin Educ Vet 2008;30:281–292. [PubMed] [Google Scholar]
- 13. Han E, Broussard J, Baer KE. Feline esophagitis secondary to gastroesophageal reflux disease: Clinical signs and radiographic, endoscopic, and histopathological findings. J Am Anim Hosp Assoc 2003;39:161–167. [DOI] [PubMed] [Google Scholar]
- 14. Gualtieri M, Olivero D. Reflux esophagitis in three cats associated with metaplastic columnar esophageal epithelium. J Am Anim Hosp Assoc 2006;42:65–70. [DOI] [PubMed] [Google Scholar]
- 15. Lobetti R, Leisewitz A. Gastroesophageal reflux in two cats. Feline Pract 1996;24:5–9. [Google Scholar]
- 16. Kook PH, Kohler N, Hartnack S, et al. Agreement of Serum Spec cPL with the 1,2‐o‐Dilauryl‐Rac‐Glycero Glutaric Acid‐(6′‐methylresorufin) Ester (DGGR) Lipase Assay and with Pancreatic Ultrasonography in Dogs with Suspected Pancreatitis. J Vet Intern Med 2014;28:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Day MJ, Bilzer T, Mansell J, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: A report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol 2008;138(Suppl 1):S1–S43. [DOI] [PubMed] [Google Scholar]
- 18. Favarato ES, de Souza MV, Costa PR, et al. Ambulatory esophageal pHmetry in healthy dogs with and without the influence of general anesthesia. Vet Res Commun 2011;35:271–282. [DOI] [PubMed] [Google Scholar]
- 19. McMahon RL, Ali A, Chekan EG, et al. A canine model of gastroesophageal reflux disease (GERD). Surg Endosc 2002;16:67–74. [DOI] [PubMed] [Google Scholar]
- 20. Sagawa K, Li F, Liese R, et al. Fed and fasted gastric pH and gastric residence time in conscious beagle dogs. J Pharm Sci 2009;98:2494–2500. [DOI] [PubMed] [Google Scholar]
- 21. Akimoto M, Nagahata N, Furuya A, et al. Gastric pH profiles of Beagle dogs and their use as an alternative to human testing. Eur J Pharm Biopharm 2000;49:99–102. [DOI] [PubMed] [Google Scholar]
- 22. Richter JE, Pandolfino JE, Vela MF, et al. Utilization of wireless pH monitoring technologies: A summary of the proceedings from the Esophageal Diagnostic Working Group. Dis Esophagus 2013;26:755–765. [DOI] [PubMed] [Google Scholar]
- 23. Pandolfino JE, Richter JE, Ours T, et al. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol 2003;98:740–749. [DOI] [PubMed] [Google Scholar]
- 24. Wenner J, Johnsson F, Johansson J, et al. Wireless oesophageal pH monitoring: Feasibility, safety and normal values in healthy subjects. Scand J Gastroenterol 2005;40:768–774. [DOI] [PubMed] [Google Scholar]
- 25. Slaughter JC, Goutte M, Rymer JA, et al. Caution about overinterpretation of symptom indexes in reflux monitoring for refractory gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2011;9:868–874. [DOI] [PubMed] [Google Scholar]
- 26. Bécuwe‐Bonnet V, Belanger M‐C, Frank D, et al. Gastrointestinal disorders in dogs with excessive licking of surfaces. J Vet Behav 2012;7:194–204. [Google Scholar]
- 27. Sharma P, Wani S, Bansal A, et al. A feasibility trial of narrow band imaging endoscopy in patients with gastroesophageal reflux disease. Gastroenterology 2007;133:454–464. [DOI] [PubMed] [Google Scholar]
- 28. Bersenas AM, Mathews KA, Allen DG, et al. Effects of ranitidine, famotidine, pantoprazole, and omeprazole on intragastric pH in dogs. Am J Vet Res 2005;66:425–431. [DOI] [PubMed] [Google Scholar]
- 29. Kempf J, Heinrich H, Reusch CE, et al. Evaluation of esophageal high‐resolution manometry in awake and sedated dogs. Am J Vet Res 2013;74:895–900. [DOI] [PubMed] [Google Scholar]
- 30. Nusrat S, Roy PM, Bielefeldt K. Wireless ambulatory pH studies: Manometric or endoscopic guidance? Dis Esophagus 2012;25:26–32. [DOI] [PubMed] [Google Scholar]
- 31. Korrapati V, Babich JP, Balani A, et al. Does deep sedation impact the results of 48 hours catheterless pH testing? World J Gastroenterol 2011;17:1304–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]


