Abstract
Background
Cyclooxygenase‐2 (COX‐2) is a key enzyme in the synthesis of pro‐inflammatory prostaglandins and 5‐lipoxygenase (5‐LO) is the major source of leukotrienes. Their role in IBD has been demonstrated in humans and animal models, but not in dogs with chronic enteropathies (CCE).
Hypothesis
COX‐2 and 5‐LO are upregulated in dogs with CCE.
Animals
Fifteen healthy control dogs (HCD), 10 dogs with inflammatory bowel disease (IBD), and 15 dogs with food‐responsive diarrhea (FRD).
Methods
Prospective study. mRNA expression of COX‐2, 5‐LO, IL‐1b, IL‐4, IL‐6, TNF, IL‐10 and TFG‐β was evaluated by quantitative real‐time RT‐PCR in duodenal and colonic biopsies before and after treatment.
Results
COX‐2 expression in the colon was significantly higher in IBD and FRD before and after treatment (all P < .01). IL‐1b was higher in FRD in the duodenum after treatment (P = .021). TGF‐β expression was significantly higher in the duodenum of HCD compared to FRD/IBD before treatment (both P < .001) and IBD after treatment (P = .012). There were no significant differences among groups and within groups before and after treatment for IL‐4, IL‐6, TNF, and IL‐10. There was a significant correlation between COX‐2 and IL‐1b in duodenum and colon before treatment in FRD and IBD, whereas 5‐LO correlated better with IL‐6 and TNF. IL‐10 and TGF‐β usually were correlated.
Conclusions and Clinical Importance
COX‐2 is upregulated in IBD and FRD, whereas IL‐1b and TGF‐β seem to be important pro‐ and anti‐inflammatory cytokines, respectively. The use of dual COX/5‐LO inhibitors could be an interesting alternative in the treatment of CCE.
Keywords: Food‐responsive diarrhea, Inflammatory bowel disease, Interleukin, Leukotriene
Abbreviations
- 5‐LO
5‐lipoxygenase
- AA
arachidonic acid
- CBC
complete blood count
- CCE
canine chronic enteropathies
- CIBDAI
canine IBD activity index
- COX‐2
cyclooxygenase‐2
- CysLTs
cysteinyl‐leukotrienes
- FRD
food‐responsive diarrhea
- HCD
healthy control dogs
- IBD
inflammatory bowel disease
- IL
interleukin
- LTB4, LTC4, LTD4, LTE4
leukotriene B4, C4, D4, E4
- LTs
leukotrienes
- NSAID
nonsteroidal anti‐inflammatory drug
- PGs
prostaglandins
- TGF‐β
transforming growth factor beta
- TNF
tumor necrosis factor
Inflammatory bowel disease (IBD) and food‐responsive diarrhea (FRD) are common chronic enteropathies (CCE) in dogs that can only be differentiated by their response to treatment after exclusion of other diseases.1, 2 The etiopathogenesis of IBD is still ill‐defined, but likely involves a breakdown of the mucosal tolerance to luminal microbial and dietary antigens, possibly because of genetic susceptibility, disruption of the mucosal barrier, dysfunction of the mucosal immune system, or disturbances in the endogenous microflora, resulting in chronic inflammation.3, 4, 5, 6
Cyclooxygenase 2 (COX‐2) and 5‐lipoxygenase (5‐LO) are key enzymes in the arachidonic acid (AA) cascade, leading to the production of pro‐inflammatory mediators.7, 8 The prostaglandins (PGs) produced by COX‐2 play a major role in inflammatory reactions by inducing vasodilatation, changes in vascular permeability and chemotaxis. Leukotrienes (LTs) are lipid messengers that play a central role in the immune response and tissue homeostasis.7 They participate in the margination and migration of leukocytes, in alterations of vascular permeability and in the formation of edema.9 5‐LO is the major source of LTs and their synthesis can be divided into 2 pathways, 1 creating LTB4, and the other creating the cysteinyl‐leukotrienes (CysLTs; LTC4, LTD4, and LTE4).10
Arachidonic acid is the common substrate of the 5‐LO and COX, and there are functional interactions between both pathways. Thus, a blockade of the COX pathway by nonsteroidal anti‐inflammatory drugs (NSAID) can shunt the conversion of AA to 5‐LO, resulting in an increase in the LT production.9, 11, 12 Similarly, inhibition of 5‐LO leads to a shift of AA to the COX pathway.13 With regard to their potent biological activities, LTs and PGs have been recognized to be important inflammatory mediators in IBD in humans.7
The important role played by cytokines in the development of chronic enteropathies has been demonstrated in several laboratory animal models3, 14 and in humans.15, 16 During the last few years, several studies have been published on the mRNA expression of various cytokines in the intestinal mucosa of dogs with CCE.17, 18, 19, 20, 21,1 These studies, using either semiquantitative RT‐PCR17, 18, 21 or real‐time RT‐PCR,19, 20,1 yielded such variable cytokine profiles that it is currently impossible to establish a consistent pattern. For example, tumor necrosis factor (TNF) expression in dogs with CCE was significantly higher than in healthy dogs in 3 studies,17, 18, 20 significantly lower in 1 study,21 and the other 2 studies19,1 failed to detect any differences.
Recently, an increase in urinary LTE4 excretion has been reported in humans22 and in dogs23 suffering from IBD and CCE. Thus, the aim of this study was to compare the mRNA expression of the key enzymes 5‐LO and COX‐2 as well as pro‐ and anti‐inflammatory cytokines in healthy control dogs (HCD) and dogs suffering from IBD and FRD before and after treatment.
Materials and Methods
Animals and Study Protocol
The 25 dogs included in this prospective study were referred to the Small Animal Teaching Hospital of the University of Bern between November 2006 and November 2008 with signs of chronic gastrointestinal (GI) disease. Information concerning the standard study protocol has already been published elsewhere.24 The selection criteria consisted of a history of chronic diarrhea with or without vomiting that lasted at least 6 weeks, exclusion of identifiable underlying disorders, and histopathologic evidence of intestinal inflammatory cellular infiltration. Other potential causes of diarrhea were eliminated with a CBC, serum biochemical profile, urinalysis, bacterial and parasitic analysis of fecal samples, assessment of serum trypsin‐like immunoreactivity, vitamin B12, and folate measurements, ACTH stimulation test, abdominal ultrasonography, and endoscopy of the GI tract. All dogs were given a clinical score using the canine IBD activity index (CIBDAI)25 before and after standard treatment. Owners of dogs signed a letter of consent in which they agreed to participate in initial and follow‐up diagnostic evaluation. All experimental procedures were approved by the Cantonal Committee for Animal Experimentation, Bern, Switzerland.
Duodenoscopy and colonoscopy were performed in all dogs except those with severe hypoalbuminemia (n = 2), where the routine 36‐hour fasting period used in preparation for colonoscopy in our hospital and the very low albumin concentration with regard to anesthesia were considered an increased risk. Four dogs did not have a second endoscopy after treatment because the owners declined or the dogs had been euthanized.
After endoscopy, all dogs were treated with an elimination diet based on fish and rice2 for at least 10 weeks. Dogs that responded to the elimination diet in the first 14 days (clinical signs improved markedly or resolved) were assigned to the FRD group. Although recommendations usually state that dogs should be fed an appropriate formulation for at least 4–6 weeks,26 we limited the elimination diet trial to 14 days for owner compliance reasons. However, it is still possible that these dogs still had IBD despite their prompt response to dietary treatment alone. The dogs that did not respond during the first 14 days (clinical signs persisted while on the diet) were assigned to the IBD group and were given prednisolone (1 mg/kg PO q12h) for 2 weeks followed by tapering dosages. If the response to prednisolone was insufficient, dogs were given cyclosporine (5 mg/kg PO q24h). The FRD group was reevaluated, including CIBDAI and endoscopy, 4 weeks after starting treatment with the elimination diet. The IBD group was reexamined, including CIBDAI and endoscopy, at the end of the 10‐week treatment period, which was 2 weeks after discontinuation of the prednisolone treatment. All dogs were fed the elimination diet exclusively.
Biopsies from HCD were taken at necropsy from 15 Beagle dogs, which served either in pharmacological studies as placebo control dogs or were euthanized because of age or nongastrointestinal problems (eg, rupture of cruciate ligament, behavioral problems). The group included 10 females and 5 males (all intact), 6–14 years old (mean, 10 years), with body weight from 8.3 to 15.5 kg (mean, 12.04 kg). These dogs did not receive any drugs, were clinically healthy with no signs of diarrhea or vomiting, and had no abnormalities on CBC, serum biochemical profile, and urinalysis. Their parasitic and bacterial analyses of fecal samples were free from Giardia sp., Salmonella sp., and Campylobacter sp. Within 5 minutes after euthanasia, 6 duodenal (approximately 10 cm below the caudal duodenal flexure) and 6 colonic samples (middle portion of the descending colon) were taken from all dogs with an endoscopic biopsy forceps and stored for subsequent histopathologic evaluation in 4% neutral‐buffered formalin or in RNAlater3 at −80°C until RNA isolation. As an inclusion criterion, all HCD had to be free of histologic abnormalities.
Endoscopy
Details about the endoscopy protocol have already been published elsewhere.24 Six mucosal biopsy specimens were obtained from duodenum (approximately 10 cm below the caudal duodenal flexure), and 6 from the middle portion of the descending colon, or from where lesions were visible. Samples for subsequent histopathologic evaluation were placed in 4% neutral‐buffered formalin for 48 hours before embedding in paraffin. In addition, 6 endoscopic biopsies from duodenum and colon were immediately put into RNAlater3 and stored at −80°C until RNA isolation.
Histopathology
Blinded qualitative evaluation of the degree of inflammation and overall cellular infiltrate was performed by an ACVP board‐certified pathologist, who assigned a grade (normal = 0, mild = 1, moderate = 2, severe = 3) based on previously published guidelines.27 The pathologist was blinded with regard to 1st or 2nd endoscopy, clinical diagnosis, and treatments used and did the analysis of all samples in 1 sitting.
RNA Extraction, Reverse Transcription and Primer Design
Tissue samples stored at −80°C were thawed and used for RNA extraction. Total RNA was isolated by use of SV Total RNA isolation kit4 after the manufacturer's instructions. Throughout the procedure of RNA extraction, biopsies from the same dog (ie, before and after treatment) were always included in the same experimental run, in addition to a sample from HCD.
Total RNA (3 μg) was reversed transcribed to cDNA using High Capacity cDNA reverse Transcription kit5 following the manufacturer's instructions. Primers for the reference genes ubiquitin28 (accession number NCBI AB032025, forward primer [5′–3′] cag cta gaa gat ggc cga ac, reverse primer [5′–3′] act tct tct tgc ggc agt tg, product length 199 bp), cyclophylline29 (accession number NCBI XM_847296.1, forward primer [5′–3′] ggt cat cgg tct ctt tgg aa, reverse primer [5′–3′] gat gct ctt tcc tcc agt gc, product length 175 bp), target genes COX‐2 (forward primer [5′–3′)] cca ccc atg tca aaa cca ag, reverse primer [5′–3′] gtg acc ggg atg tca aca ca, product length 254 bp), 5‐LO (forward primer [5′–3′] gtg gac acg tgc aga tgg tg, reverse primer [5′–3′] gtg aac gtc ttg atg gcc tc, product length 166 bp), Interleukin‐1b (IL‐1b; accession number NM_001037971.1, forward primer [5′–3′] ccc tgg aaa tgt gaa gtg ct, reverse primer [5′–3′] tat ccg cat ctg ttt tgc ag, product length 242 bp), IL‐430 (accession number AF187322, forward primer [5′–3′] gct cca aag aac aca agc ga, reverse primer [5′–3′] cat gct gct gag gtt cct gt, product length 123 bp), IL‐6 (accession number NM_001003301.1, forward primer [5′–3′] cac cag gaa cga aag aga gc, reverse primer [5′–3′] ttg ttt gca gag gtg agt gg, product length 186 bp), TNF (accession number NM_001003244.4, forward primer [5′–3′] tca tct tct cga acc cca ag, reverse primer [5′–3′] acc cat ctg acg gca cta tc, product length 157 bp), IL‐1031 (accession number U33843, forward primer [5′–3′] ctc cct ggg aga gaa gct caa, reverse primer [5′–3′] aca ggg aag aaa tcg gtg aca, product length 72 bp) and transforming growth factor beta (TGF‐β;31 accession number L34956, forward primer [5′–3′] caa gta gac att aac ggg ttc agt tc, reverse primer [5′–3′] ggt cgg ttc atg cca tga at, product length 70 bp) were synthesized6 using previously published or newly designed primer sequences. The primers were tested for self priming and single PCR product amplification during PCR temperature gradients on a gradient cycler. The presence of a single amplicon of the expected size was identified by use of 1.8% agarose gel electrophoresis stained with ethidium bromide.
Real‐Time Polymerase Chain Reaction (qRT‐PCR)
The defined optimal essay conditions were tested for PCR efficiency in serially diluted pooled cDNA on the Rotor‐Gene 60007 using the Rotor Gene software version 1.7.40 and then were routinely applied to the qRT‐PCR of targets and reference genes in the intestinal samples. Each of the 10‐μL 1‐step real‐time RT‐PCR reactions contained 10 ng reverse transcribed RNA, 5 μmol/L gene‐specific forward and reverse primers, and 2× SensiMix Plus SYBR PCR Kit.8 The thermocycling conditions for fluorogenic 1‐step RT‐PCR were denaturation at 95°C for 10 minutes, followed by 40 cycles of 15 seconds at 95°C, primer annealing at 60°C for 30 seconds, and elongation at 72°C for 20 seconds. Negative controls were performed in each PCR run by the addition of nuclease‐free water instead of cDNA. A dissociation curve was run to confirm the presence of a single product. During target and reference gene amplification, the take off values (C t values) were recorded. The threshold values were placed manually so that the C t values for pooled cDNA (intercontrol) included in each PCR run remained unchanged. The relative mRNA abundance of target gene (ΔC t) was expressed by relating their respective C t values to the mean C t value of ubiquitin and cyclophylline, as published elsewhere.32 A lower ΔC t indicates higher mRNA expression. Therefore, to simplify the interpretation, the results were expressed as [(−ΔC t) + 20], so that a higher result indicates a higher mRNA expression.
Statistical Analysis
All statistical analysis were performed using NCSS© 2007.9 PCR results ([−ΔC t] + 20) were used as the basis for comparisons. Normal distribution of all measured parameters per group was tested by the Kologomorov‐mirnov and the Shapiro‐Wilk normality tests using NCSS© 2007. The data, being not normally distributed, was reported as median with the 10th and 90th percentiles. Separate analyses were run for each region (duodenum, colon) and before and after treatment. Comparison among IBD, FRD, and HCD was made using Kruskal‐Wallis one‐way ANOVA with Dunn's test for multiple comparisons (Bonferroni correction). IBD and FRD before and after treatment were compared with a Wilcoxon signed‐rank test. In addition, correlations between CIBDAI, COX‐2, 5‐LO, IL‐1b, IL‐4, IL‐6, TNF, IL‐10, TGF‐β in the IBD and FRD groups before and after treatment were evaluated with the Spearman rank correlation test. The level of statistical significance was set at P < .05.
Results
Dogs with CCE
The FRD group (n = 15) included 9 females and 6 males, of which 5 and 3 were neutered, respectively. The dogs were 9–132 months old (median, 44 months). Breeds in this group included mixed breed (4), Labrador (2), and Dachshund, Yorkshire Terrier, French Bulldog, Weimaraner, Golden Retriever, West Highland White Terrier, White Swiss Shepherd, Pomeranian, and Newfoundland (1 of each breed). Body weights ranged from 1.8 to 49 kg (median, 23.9 kg).
The IBD group (n = 10) contained 5 females and 5 males, of which 3 and 4 were neutered, respectively. The dogs were 36–154 months old (median, 81 months). Breeds include mixed breed (3), and 1 of each Chinese Shar‐Pei, Papillon, Golden Retriever, Rottweiler, Beauceron, Bernese mountain dog, and Cocker Spaniel. Body weights in the IBD group ranged from 3 to 60 kg (median, 26.8 kg).
CIBDAI
In the FRD group before treatment (n = 15), 2 dogs were classified as insignificant, 6 as mild, 4 as moderate, and 3 as severe. After treatment, 14 dogs were classified as insignificant and 1 as mild. None of the FRD dogs relapsed during the study period. In the IBD group before treatment (n = 10), 1 dog was classified as insignificant, 3 as moderate and 6 as severe. After treatment, 7 dogs were classified as insignificant and 1 dog as mild. Two dogs did not receive a second evaluation because of low owner compliance (1) and euthanasia after poor response to treatment (1). Despite initial improvement with prednisolone, the second dog relapsed and did not respond to cyclosporine. Another dog received cyclosporine as well (5 mg/kg PO q24h) and improved from severe to insignificant disease.
Comparisons between FRD, IBD, and HCD
With regard to the key enzymes, COX‐2 in the colon was significantly higher in both FRD and IBD compared to HCD before and after treatment (all P < .01; Fig 1). The differences in 5‐LO expression among HCD, FRD, and IBD did not reach significance (results not shown).
Figure 1.
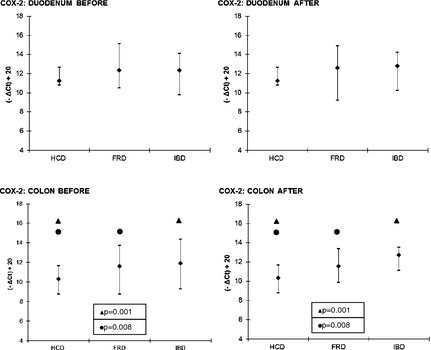
COX‐2—comparisons between FRD, IBD and HCD. Symbols (if P ≤ .05): ● between HCD and FRD; ▲ between HCD and IBD. The results are presented as median and 10th/90th percentile. COX‐2, cyclooxygenase‐2; FRD, food‐responsive diarrhea; HCD, healthy control dogs; IBD, inflammatory bowel disease.
With regard to the cytokines, significant differences were only found in the expression of IL‐1b and TGF‐β, whereas there were no significant differences among FRD, IBD, and HCD for IL‐4, IL‐6, TNF, and IL‐10. After treatment, IL‐1b was significantly higher in the duodenum in FRD than in HCD (P = .021; Fig 2). The expression of TGF‐β was significantly higher in the duodenum of HCD compared to FRD and IBD before treatment (P < .001) and IBD after treatment (P = .012; Fig 3).
Figure 2.
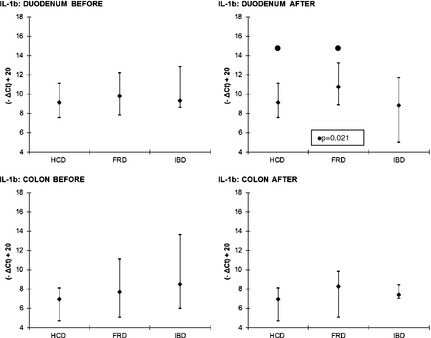
IL‐1b—comparisons between FRD, IBD and HCD. Symbols (if P ≤ .05): ● between HCD and FRD. The results are presented as median and 10th/90th percentile. FRD, food‐responsive diarrhea; HCD, healthy control dogs; IBD, inflammatory bowel disease; IL‐1b, interleukin 1b.
Figure 3.
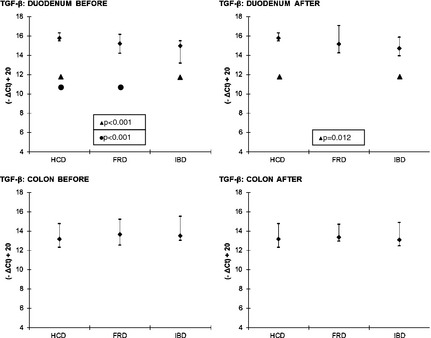
TGF‐β—comparisons between FRD, IBD and HCD. Symbols (if P ≤ .05): ● between HCD and FRD; ▲ between HCD and IBD. The results are presented as median and 10th/90th percentile. FRD, food‐responsive diarrhea; HCD, healthy control dogs; IBD, inflammatory bowel disease; TGF‐β, Transforming growth factor beta.
Correlations between CIBDAI, Key Enzymes and Cytokines in the FRD Group Before and After Treatment
In the duodenum before treatment, there was a significant correlation between COX‐2 and IL‐1b, IL‐4, IL‐6 and TNF (P = .003, r = 0.707; P = .017, r = 0.604; P = .05, r = 0.679; P = .016, r = 0.607); IL‐1b and TNF as well as IL‐10 (P = .008, r = 0.657; P = .005, r = 0.679); IL‐6 and TNF (P = .013, r = 0.621); TNF and IL‐10 (P = .018, r = 0.600); and between IL‐10 and TGF‐β (P = .002; r = 0.732). In the duodenum after treatment, there was a significant correlation between COX‐2 and IL‐1b as well as IL‐10 (P = .008, r = 0.679; P = .012, r = 0.648); 5‐LO and IL‐1b, TNF, IL‐10 and TGF‐β (P = .016, r = 0.631; P = .001, r = 0.798; P = .009, r = 0.670; P = .014, r = 0.640); IL‐1b and TNF, IL‐10 and TGF‐β (P = .006, r = 0.697; P = .027, r = 0.587; P = .035, r = 0.565); IL‐6 and TNF (P = .004, r = 0.719); TNF and IL‐10 as well as TGF‐β (P = .015, r = 0.635; P = .012, r = 0.648); and between IL‐10 and TGF‐β (P < .001, r = 0.859).
In the colon before treatment, there was a significant correlation between COX‐2 and IL‐1b as well as IL‐10 (P = .010, r = 0.686; P = .039, r = 0.576); 5‐LO and IL‐6 as well as TNF (P < .001, r = 0.890; P = .007, r = 0.703); IL‐4 and TNF (P = .024, r = 0.621); IL‐6 and TNF (P < .001, r = 0.830); and between IL‐10 and TGF‐β (P = .006, r = 0.720). In the colon after treatment, there was a significant correlation between COX‐2 and IL‐10 (P = .010, r = 0.706); 5‐LO and IL‐6 as well as TNF (P < .001, r = 0.930; P = .003, r = 0.769); IL‐1b and IL‐10 (P = .003, r = 0.776); IL‐6 and TNF (P = .007, r = 0.734); and between IL‐10 and TGF‐β (P = .039; r = 0.601).
There were no significant correlations between CIBDAI and any other parameter in duodenum and colon before and after treatment.
Correlations between CIBDAI, Key Enzymes and Cytokines in the IBD Group Before and After Treatment
In the duodenum before treatment, there was a significant correlation between COX‐2 and IL‐1b (P = .022, r = 0.709); 5‐LO and IL‐4, TNF and TGF‐β (P = .038, r = 0.661; P = .008, r = 0.782; P = .043, r = 0.648); and between TNF and TGF‐β (P = .038, r = 0.661). In the duodenum after treatment, there was a significant correlation between COX‐2 and TNF, IL‐10 and TGF‐β (P = .019, r = 0.886; P = .005, r = 0.943; P = .019, r = 0.886); 5‐LO and IL‐6 as well as IL‐10 (P = .019, r = 0.886; P = .042, r = 0.829); IL‐6 and TNF (P = .005, r = 0.943); and between TNF and TGF‐β (P = .019, r = 0.886).
In the colon before treatment, there was a significant correlation between COX‐2 and IL‐1b (P = .005, r = 0.833); 5‐LO and IL‐6 (P = .007, r = 0.817); IL‐6 and TNF (P = .030, r = 0.717); TNF and IL‐10 (P = .030, r = 0.717); and between IL‐10 and TGF‐β (P = .010, r = 0.800). In the colon after treatment, there was a significant correlation between 5‐LO and IL‐4 as well as TNF (P < .001, r = 0.901; P < .001, r = 0.898); IL‐4 and TNF (P < .001, r = 0.911); and between IL‐10 and TGF‐β (P = .037; r = 0.900).
There were no significant correlations between CIBDAI and any other parameter in duodenum and colon before and after treatment.
Discussion
In this study, COX‐2 was upregulated in dogs with IBD and FRD in the colon before and after treatment. There were no significant differences among HCD, IBD, and FRD for 5‐LO, but a significant difference was found when comparing CCE (=IBD + FRD) with HCD in the colon before treatment (results not shown). There was a significant correlation between COX‐2 and IL‐1b in duodenum and colon before treatment in FRD and IBD, whereas 5‐LO correlated better with IL‐6 and TNF. IL‐10 and TGF‐β were almost always correlated. Together with an increase in IL‐1b and significant correlations between COX‐2 and 5‐LO with most pro‐inflammatory cytokines, it seems that COX‐2, and to a much lesser extent 5‐LO, contribute in the development of intestinal inflammation in dogs with CCE.
Human patients suffering from IBD have abnormal AA metabolism.12 An overexpression of COX‐2 has been demonstrated12, 33 and the LO pathway also is upregulated with an overexpression of LTB4 12, 34 and LTE4 22 in humans, and an overexpression of LTC4 in rabbit35 and rat36 models of IBD. There are several studies evaluating the possible use of dual COX/5‐LO inhibitors in the treatment of inflammatory diseases showing promising results.37, 38, 39, 40 Particularly, 1 of these studies was performed in a dog model of local inflammation and showed that RWJ 63556, a dual COX/5‐LO inhibitor, produced significant anti‐inflammatory activity.37 Sulfasalazine (sulfonamide and salicylate), an NSAID with antibacterial properties, has been used for years in the treatment of IBD in dogs and cats, especially in the treatment of colitis, but can induce adverse GI effects. Thus, dual COX/5‐LO inhibitors could provide an interesting alternative for the effective treatment of CCE in dogs, without causing deleterious adverse GI effects, and require additional study.
Interleukin‐1b was only upregulated in the duodenum of dogs with FRD after treatment, but when comparing CCE (=IBD + FRD) with HCD, IL‐1b was significantly upregulated in the colon of dogs before and after treatment (results not shown). Together with significant correlations with COX‐2, 5‐LO and other different pro‐inflammatory cytokines, these results demonstrate that, in our study, IL‐1b plays an important role in inflammation. Similar results were found in 1 real‐time RT‐PCR study,1 where IL‐1b showed a tendency to be upregulated in IBD. On the other hand, a semiquantitative RT‐PCR study21 found a decrease in IL‐1b in IBD in duodenum compared to HCD.
Our study identified significantly decreased TGF‐β in the duodenum of dogs with FRD and IBD before treatment and in those with IBD after treatment compared to healthy dogs. The failure to upregulate this immunosuppressive cytokine could be because of dysfunction of the mucosal immune system, which results in the establishment of a chronic inflammatory reaction. This finding supports the hypothesis that TGF‐β is an important anti‐inflammatory contributor in CCE. Two other studies found similar results, 1 with decreased TGF‐β in the duodenum,1 the other in the colon.21 On the other hand, 1 semiquantitative study found increased expression in duodenum17 that suggested an attempt of TGF‐β to downregulate an ongoing inflammatory response. Finally, 3 other studies failed to find any differences in the expression of TGF‐β.18, 19, 20
In contrast to IL‐1b and TGF‐β, IL‐4, IL‐6, IL‐10, and TNF failed to reach significant differences among the different groups. The expression of IL‐4 generally was low, at the limit of detection, and was not significantly different between sick and healthy dogs. This finding is consistent with those of most of the studies published.17, 18, 19,1 There is only 1 semiquantitative study that found increased expression of IL‐4 in IBD in the colon compared to HCD.21 With regard to IL‐6, this study is consistent with 2 other studies,18, 19 but stands in contrast with a real‐time RT‐PCR study that found increased expression of IL‐6 in the duodenum in IBD.1 For IL‐10, almost all studies published did not show differences between sick and healthy animals17, 18, 19, 20,1 except 1 that found decreased expression in the duodenum of IBD.21 TNF results vary in the literature. No significant differences in TNF were found in 2 other studies reviewed,19,1 whereas 3 studies showed increased expression of TNF in the duodenum17, 20 and colon18 of sick dogs, 3 studies showed no significant differences and 1 study showed decreased expression in the duodenum in IBD.21 Thus, it seems that the cytokine pattern in CCE is very variable. This variation could be partly explained by the fact that some studies used semiquantitative RT‐PCR and others used real‐time RT‐PCR, which is considered more accurate and sensitive.41 Furthermore, different disease durations could be another explanation for the differences because the cytokine profiles in the mucosa of patients with Crohn's disease have been found to vary with different stages of the disease.15
With regard to the correlation between key enzymes and cytokines, COX‐2 showed strong correlation with IL‐1b in duodenum and colon in FRD and IBD. Because COX‐2 is induced by pro‐inflammatory cytokines among other stimuli to promote an inflammatory reaction,8 it seems that in this study, IL‐1b is an important component of this mechanism. On the other hand, 5‐LO is strongly correlated with TNF, IL‐4 and IL‐6, some of the most important inflammatory cytokines. This can be explained by the fact that 1 of the roles of 5‐LO is to enhance the release of these pro‐inflammatory cytokines by macrophages and lymphocytes.10 Furthermore, IL‐4 down‐regulates the production of 5‐LO.42 Therefore, the correlation between 5‐LO and IL‐4 might represent an internal mechanism in the intestine to keep the production of 5‐LO from escalating. Interestingly, both COX‐2 and 5‐LO correlated with IL‐10 and TGF‐β in the duodenum after treatment, but not before. Because IL‐10 and TGF‐β are anti‐inflammatory cytokines, this may represent the regeneration process that takes place after treatment.
The pro‐inflammatory cytokines (IL‐1b, IL‐6 and TNF) mostly correlated with 1 another, which might confirm the presence of an inflammatory process in the intestine. At the same time, these cytokines often correlated with the anti‐inflammatory cytokines (IL‐10 and TGF‐β) as well. Similar to IL‐4 and 5‐LO, this could represent an internal regulatory mechanism in the intestine that keeps the inflammation from escalating by keeping a balance between pro‐inflammatory and anti‐inflammatory cytokines.
A major limitation of this study was the low number of diseased dogs enrolled which made it especially difficult to find significant differences among IBD, FRD, and HCD. Another limitation of the study was that the control group consisted only of beagles. Batt and coworkers found bacterial overgrowth in 14 of 21 healthy beagles with only minimal morphologic changes in jejunal mucosa.43 Furthermore, activities of lysosomal and endoplasmic reticulum marker enzymes were higher in the beagles compared with other healthy controls. Owing to limited access to other HCD, healthy Beagle dogs serving as placebo controls in pharmacologic studies were used. These dogs did not receive any drugs, were clinically healthy with no signs of diarrhea or vomiting, and had no abnormalities on CBC, serum biochemical profile, and urinalysis. Furthermore, parasitic and bacterial analyses of fecal samples were performed, and the samples were free of Giardia sp., Salmonella sp., Campylobacter sp., and other potential causes of diarrhea. Full thickness biopsies of mesenteric lymph nodes and endoscopic biopsies as well as full thickness biopsies from duodenum and colon were free of histological abnormalities. Therefore, we suggest that these beagles accurately represented HCD.
In conclusion, COX‐2 and, to a much lesser extent 5‐LO, are upregulated in CCE and participate in intestinal inflammation. There was a significant correlation between COX‐2 and IL‐1b in duodenum and colon before treatment in FRD and IBD, whereas 5‐LO correlated better with IL‐6 and TNF. IL‐10 and TGF‐β almost always were correlated. IL‐1b seems to be an important pro‐inflammatory cytokine and TGF‐β an important anti‐inflammatory cytokine in CCE, but the cytokine profiles were very variable. The use of dual COX/5‐LO inhibitors could be an interesting alternative in the treatment of CCE, but additional studies are needed.
Acknowledgments
The authors thank the Swiss Armed Forces Veterinary Services for their financial support of S. Dumusc. E.C. Ontsouka and C. Albrecht were supported by the Swiss National Science Foundation through the National Centre of Competence in Research TransCure. The expert laboratory work of Mrs. Claudine Morel, Veterinary Physiology, Vetsuisse Faculty University of Bern, is gratefully acknowledged.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
The work was performed at the Small Animal Hospital and the Division of Veterinary Physiology of the Vetsuisse Faculty of the University of Bern, Switzerland.
This study was presented at the ECVIM‐CA Congress in Porto, Portugal, in September 2009.
Footnotes
Fujiwara S, Yasunaga H, Nakayama H, et al. Quantitative analysis of cytokine mRNAs in the duodenal mucosa in dogs with small‐intestinal inflammatory bowel disease. Abstract ACVIM 2002, J Vet Intern Med 2002;16:327
Biomill SA, Granges‐Marnand, Switzerland
Qiagen AG, Basel, Switzerland
Promega, Dübendorf, Switzerland
#0806057; Applied Biosystems, Madison, WI
Microsynth, Balgach, Switzerland
Corbett Research, Sydney, Australia
Quantace; Biolabo SA, Châtel‐St‐Denis, Switzerland
Number Cruncher Statistical Systems (NCSS), Kaysville, UT
References
- 1. Hall EJ, German AJ. Diseases of the small intestine In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 6th ed St. Louis, MO: Elsevier Saunders; 2005:1332–1378. [Google Scholar]
- 2. Allensprach K, Wieland B, Grone A, Gaschen F. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J Vet Intern Med 2007;21:700–708. [DOI] [PubMed] [Google Scholar]
- 3. Elson CO. Experimental models of intestinal inflammation: New insights into mechanisms of mucosal homeostasis In: Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, eds. Mucosal Immunology, 2nd ed San Diego, CA: Academic Press; 1999:1007–1033. [Google Scholar]
- 4. German AJ, Hall EJ, Day MJ. Chronic intestinal inflammation and intestinal disease in dogs. J Vet Intern Med 2003;17:8–20. [DOI] [PubMed] [Google Scholar]
- 5. Mizoguchi A, Mizoguchi E, Bhan AK. Immune networks in animal models of inflammatory bowel disease. Inflamm Bowel Dis 2003;9:246–59. [DOI] [PubMed] [Google Scholar]
- 6. Kathrani A, House A, Catchpole B, et al. Polymorphisms in the TLR4 and TLR5 gene are significantly associated with inflammatory bowel disease in German shepherd dogs. PLoS ONE 2010;5:e15740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Funk CD. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001;294:1871–1875. [DOI] [PubMed] [Google Scholar]
- 8. Charlier C, Michaux C. Dual inhibition of cyclooxygenase‐2 (COX‐2) and 5‐lipoxygenase (5‐LOX) as a new strategy to provide safer non steroidal anti‐inflammatory drugs. Eur J Med Chem 2003;38:645–659. [DOI] [PubMed] [Google Scholar]
- 9. Celotti F, Durand T. The metabolic effects of inhibitors of 5‐lipoxygenase and of cyclooxygenase 1 and 2 are an advancement in the efficacy and safety of anti‐inflammatory treatment. Prostag Other Lipid Mediat 2003;71:147–162. [DOI] [PubMed] [Google Scholar]
- 10. Hedi H, Norbert G. 5‐lipoxygenase pathway, dentritic cells, and adaptative immunity. J Biomed Biotechnol 2004;2:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gilroy DW, Tomlinson A, Willoughby DA. Differential effects of inhibitors of cyclooxygenase (cyclooxygenase 1 and cyclooxygenase 2) in acute inflammation. Eur J Pharmacol 1998;355:211–217. [DOI] [PubMed] [Google Scholar]
- 12. Jupp J, Hillier K, Elliot DH, et al. Colonic expression of leukotriene‐pathway enzymes in inflammatory bowel diseases. Inflamm Bowel Dis 2007;13:537–546. [DOI] [PubMed] [Google Scholar]
- 13. Bertrán X, Mañé J, Fernández‐Bañares F, et al. Intracolonic administration of zileuton, a selective 5‐lipoxygenase inhibitor, accelerates healing in a rat model of chronic colitis. Gut 1996;38:899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pizarro TT, Arseneau KO, Bamias G, Cominelli F. Mouse models for the study of Crohn's disease. Trends Mol Med 2003;9:218–222. [DOI] [PubMed] [Google Scholar]
- 15. Desreumaux P, Brandt E, Gambiez L, et al. Distinct cytokine patterns in early and chronic ileal lesions of Crohn's disease. Gastroenterology 1997;113:118–126. [DOI] [PubMed] [Google Scholar]
- 16. Melgar S, Yeung MMW, Bas A, et al. Over‐expression of interleukin‐10 in mucosal T cells of patients with active ulcerative colitis. Clin Exp Immunol 2003;134:127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. German AJ, Helps CR, Hall EJ, Day MJ. Cytokine mRNA expression in mucosal biopsies from German shepherd dogs with small intestinal enteropathies. Dig Dis Sci 2000;45:7–17. [DOI] [PubMed] [Google Scholar]
- 18. Ridyard AE, Nuttall TJ, Else RW, et al. Evaluation of Th1, Th2 and immunosuppressive cytokine mRNA expression within the colonic mucosa of dogs with idiopathic lymphocytic‐plasmacytic colitis. Vet Immunol Immunopathol 2002;86:205–214. [DOI] [PubMed] [Google Scholar]
- 19. Peters IR, Helps CR, Calvert EL, et al. Cytokine mRNA quantification in duodenal mucosa from dogs with chronic enteropathies by real‐time reverse transcriptase polymerase chain reaction. J Vet Intern Med 2005;19:644–653. [DOI] [PubMed] [Google Scholar]
- 20. Sauter SN, Allensprach K, Blum JW. Cytokine mRNA abundance in intestinal biopsies from dogs with chronic diarrhea. Vet Med 2007;52:353–364. [Google Scholar]
- 21. Jergens AE, Sonea IM, O'Connor AM, et al. Intestinal Cytokine mRNA expression in canine inflammatory bowel disease: A meta‐analysis with critical appraisal. Comp Med 2009;59:153–62. [PMC free article] [PubMed] [Google Scholar]
- 22. Stanke‐Labesque F, Pofelski J, Moreau‐Gaudry A, et al. Urinary leukotriene E4 excretion: A biomarker of inflammatory bowel disease activity. Inflamm Bowel Dis 2008;14:769–774. [DOI] [PubMed] [Google Scholar]
- 23. Im Hof M, Schnyder M, Hartnack S, et al. Urinary leukotriene E4 concentrations as a potential marker of inflammation in dogs with inflammatory bowel disease. J Vet Intern Med 2012;26:269–274. [DOI] [PubMed] [Google Scholar]
- 24. Burgener IA, Konig A, Allensprach K, et al. Upregulation of toll‐like receptors in chronic enteropathies in dogs. J Vet Intern Med 2008;22:553–560. [DOI] [PubMed] [Google Scholar]
- 25. Jergens AE, Schreiner CA, Dagmar EF, et al. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med 2003;17:291–297. [DOI] [PubMed] [Google Scholar]
- 26. Roudebush P. Adverse reaction to foods: Allergies versus intolerance In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 6th ed St. Louis, MO: Elsevier Saunders; 2005:566–570. [Google Scholar]
- 27. Jergens AE, Moore FM, Haynes JS, Miles KG. Idiopathic inflammatory bowel disease in dogs and cats: 84 cases (1987‐1990). J Am Vet Med Assoc 1992;201:1603–1608. [PubMed] [Google Scholar]
- 28. Greger DL, Gropp F, Morel C, et al. Nuclear receptor and target gene mRNA abundance in duodenum and colon of dogs with chronic enteropathies. Domest Anim Endocrinol 2006;31:327–339. [DOI] [PubMed] [Google Scholar]
- 29. Ontsouka CE, Burgener IA, Mani O, Albrecht C. Polyunsaturated fatty acid‐enriched diets used for the treatment of canine chronic enteropathies decrease the abundance of selected genes of cholesterol homeostasis. Domest Anim Endocrinol 2010;38:32–7. [DOI] [PubMed] [Google Scholar]
- 30. Peters IR, Helps CR, Calvert EL, et al. Cytokine mRNA quantification in histologically normal canine duodenal mucosa by real‐time RT‐PCR. Vet Immunol Immunopathol 2005;103:101–111. [DOI] [PubMed] [Google Scholar]
- 31. Sauter SN, Allensprach K, Gaschen F, et al. Cytokine expression in an ex vivo culture system of duodenal samples from dogs with chronic enteropathies: Modulation by probiotic bacteria. Domest Anim Endocrinol 2005;29:605–622. [DOI] [PubMed] [Google Scholar]
- 32. Ontsouka EC, Bruckmaier RM, Steiner A, et al. Messenger RNA levels and binding sites of muscarinic acetylcholine receptors in gastrointestinal muscle layers from healthy dairy cows. J Recept Signal Transduct Res 2007;27:147–166. [DOI] [PubMed] [Google Scholar]
- 33. Singer II, Kawka DW, Schloemann S, et al. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology 1998;115:297–306. [DOI] [PubMed] [Google Scholar]
- 34. Schmidt C, Baumeister B, Kipnowski J, et al. Alteration of prostaglandin E2 and leukotriene B4 synthesis in chronic inflammatory bowel disease. Hepatogastroenterology 1996;43:1508–1512. [PubMed] [Google Scholar]
- 35. Zipser RD, Nast CC, Lee M, et al. In vivo production of leukotriene B4 and leukotriene C4 in rabbit colitis. Relationship to inflammation. Gastroenterology 1987;92:33–39. [DOI] [PubMed] [Google Scholar]
- 36. Yuceyar H, Ozutemiz O, Huseyinov A, et al. Is administration of n‐3 fatty acids by mucosal enema protective against trinitrobenzene‐induced colitis in rats? Prostaglandins Leukot Essent Fatty Acids 1999;61:339–345. [DOI] [PubMed] [Google Scholar]
- 37. Kirchner T, Argentieri DC, Barbone AG, et al. Evaluation of the antiinflammatory activity of a dual cyclooxygenase‐2 selective/5‐lipoxygenase inhibitor, RWJ 63556, in a canine model of inflammation. J Pharmacol Exp Ther 1997;282:1094–1101. [PubMed] [Google Scholar]
- 38. Janusz JM, Young PA, Ridgeway JM, et al. New cyclooxygenase‐2/5‐lipoxygenase inhibitors. 1. 7‐tert‐butyl‐2,3‐dihydro‐3,3‐dimethylbenzofuran derivatives as gastrointestinal safe antiinflammatory and analgesic agents: Discovery and variation of the 5‐Keto substituent. J Med Chem 1998;41:1112–1123. [DOI] [PubMed] [Google Scholar]
- 39. Inagaki M, Tsuri T, Jyoyama H, et al. Novel antiarthritic agents with 1,2‐isothiazolidine‐1,1‐dioxide (γ‐Sultam) skeleton: Cytokine suppressive dual inhibitors of cyclooxygenase‐2 and 5‐lipoxygenase. J Med Chem 2000;43:2040–2048. [DOI] [PubMed] [Google Scholar]
- 40. Rotondo S, Dell'Elba G, Krauze‐Brzósko K, et al. Licofelone, a dual lipoxygenase–cyclooxygenase inhibitor, downregulates polymorphonuclear leukocyte and platelet function. Eur J Pharamcol 2002;453:131–139. [DOI] [PubMed] [Google Scholar]
- 41. Bustin SA. Quantification of mRNA using real‐time reverse transcription PCR (RT‐PCR): Trends and problems. J Mol Endocrinol 2002;29:23–39. [DOI] [PubMed] [Google Scholar]
- 42. Nassar GM, Montero A, Fukunaga M, Badr KF. Contrasting effects of pro‐inflammatory and T‐helper lymphocyte subset‐2 cytokines on the 5‐lipoxygenase pathway in monocytes. Kidney Int 1997;51:1520–1528. [DOI] [PubMed] [Google Scholar]
- 43. Batt RM, Hall EJ, McLean L, et al. Small intestinal bacterial overgrowth and enhanced intestinal permeability in healthy beagles. Am J Vet Res 1992;53:1935–40. [PubMed] [Google Scholar]


