Abstract
Background
Pituitary dwarfism in German Shepherd Dogs is associated with autosomal recessive inheritance and a mutation in LHX3, resulting in combined pituitary hormone deficiency. Congenital dwarfism also is encountered in breeds related to German Shepherd Dogs, such as Saarloos and Czechoslovakian wolfdogs.
Objectives
To investigate whether Saarloos and Czechoslovakian wolfdog dwarfs have the same LHX3 mutation as do Germans Shepherd Dog dwarfs. A specific aim was to determine the carrier frequency among Saarloos and Czechoslovakian wolfdogs used for breeding.
Animals
Two client‐owned Saarloos wolfdogs and 4 client‐owned Czechoslovakian wolfdogs with pituitary dwarfism, 239 clinically healthy client‐owned Saarloos wolfdogs, and 200 client‐owned clinically healthy Czechoslovakian wolfdogs.
Methods
Genomic DNA was amplified using polymerase chain reaction (PCR). In the Saarloos and Czechoslovakian wolfdog dwarfs, PCR products were analyzed by sequencing. DNA fragment length analysis was performed on the samples from the clinically healthy dogs.
Results
Saarloos and Czechoslovakian wolfdog dwarfs have the same 7 bp deletion in intron 5 of LHX3 as do German Shepherd Dog dwarfs. The frequency of carriers of this mutation among clinically healthy Saarloos and Czechoslovakian wolfdogs used for breeding was 31% and 21%, respectively.
Conclusions and Clinical Importance
An LHX3 mutation is associated with pituitary dwarfism in Saarloos and Czechoslovakian wolfdogs. The rather high frequency of carriers of the mutated gene in the 2 breeds emphasizes the need for screening before breeding. If all breeding animals were genetically tested for the presence of the LHX3 mutation and a correct breeding policy would be implemented, this disease could be eradicated completely.
Keywords: Canine, Genetic testing, Growth hormone, Mutation
Abbreviations
- 3D
3‐dimensional
- BSA
bovine serum albumin
- CPHD
combined pituitary hormone deficiency
- CV
coefficient of variation
- FAM
fluorescein amidite
- gDNA
genomic DNA
- GH
growth hormone
- GHRH
GH‐releasing hormone
- IGF‐1
insulin‐like growth factor‐1
- MR
magnetic resonance
- PCR
polymerase chain reaction
- RIA
radioimmunoassay
- TE
time of echo
- TR
time of repetition
- TSH
thyroid‐stimulating hormone
- TT4
total thyroxine
- WI
weighted images
Development of the adenohypophysis is a very complicated process and any defect in the development of this gland may result in a form of isolated or combined pituitary hormone deficiency (CPHD). In dogs, pituitary dwarfism is the most common manifestation of CPHD and this disorder is encountered most often in German Shepherd Dogs.1 Pituitary dwarfism in this breed is inherited in an autosomal recessive fashion2 and is characterized by a combined deficiency of growth hormone (GH), thyroid‐stimulating hormone (TSH), prolactin, and the gonadotropins. In contrast, adrenocorticotropic hormone secretion is preserved in these animals.3 The hormone deficiencies can lead to a wide range of clinical manifestations, but the most common ones are marked growth retardation, retention of lanugo or secondary hairs (so‐called puppy hair coat) with concurrent lack of primary or guard hairs, and bilateral symmetrical alopecia.1
Recently, we have reported that molecular defects of the LHX3 gene are associated with CPHD.4 LHX3, a member of the LIM homeodomain protein family of DNA‐binding transcription factors, is an essential regulator of pituitary development.5, 6 All but 1 analyzed German Shepherd dwarfs were homozygous for a deletion of a 7 bp sequence in intron 5 of the LHX3 gene, decreasing the intron size to 68 bp. Because of this mutation, the intron becomes too small to be spliced efficiently.4 The 1 exception was compound heterozygous for the 7 bp deletion and an insertion of an asparagine codon in the fragment that codes for the DNA‐binding homeodomain of LHX3.4
Congenital dwarfism also is known in Saarloos wolfdogs and Czechoslovakian wolfdogs. Because these breeds are both cross‐breeds of German Shepherd Dogs and wolfs, we hypothesized that the dwarfism in these breeds is associated with the same molecular defects found in German Shepherd Dog dwarfs. The aim of the present study was to investigate whether Saarloos wolfdog and Czechoslovakian wolfdog dwarfs have the same genetic basis as do German Shepherd Dog dwarfs. An additional aim was to determine the frequency of carriers of the mutated LHX3 gene among Saarloos and Czechoslovakian wolfdogs used for breeding.
Materials and Methods
Dogs and DNA Samples
Four Czechoslovakian wolfdogs, 1 male and 3 females, 3–4 months of age, and 2 Saarloos wolfdogs, both female and 1 and 5 months of age, with proportionate dwarfism were presented to the Department of Clinical Sciences of Companion Animals of Utrecht University.
Two hundred and thirty‐nine clinically healthy Saarloos wolfdogs and 200 clinically healthy Czechoslovakian wolfdogs, intended to be used for breeding, were screened for the mutations of the LHX3 gene associated with pituitary dwarfism in German Shepherd Dogs.
Blood samples or buccal swabs were collected and genomic DNA (gDNA) was obtained from the samples using magnetic beads technology and a MSM1 robot1 according to procedures prescribed by the manufacturer.
Hormone Measurements
Plasma GH concentration was measured by a commercially available radioimmunoassay (RIA) for porcine and canine GH.2 The intra‐assay coefficient of variation (CV) was 7.6% at a plasma concentration of 4.4 μg/L. The sensitivity of the assay was 1 μg/L.
Total plasma insulin‐like growth factor‐1 (IGF‐1) concentration was measured with a heterologous RIA validated for the dog,7 after acid‐ethanol extraction to remove interfering IGF binding proteins. Plasma IGF was extracted using a mixture of 87.5% (v/v) ethanol and 12.5% 2 M formic acid. Tubes containing 100 μL plasma and 400 μL of the ethanol‐formic acid mixture were mixed thoroughly and incubated for 30 minutes at room temperature. After centrifugation for 30 minutes at 5,500 × g at 4°C, a 50‐μL aliquot of the supernatant was diluted 1 : 50 with assay buffer containing 63 mM Na2HPO4 (pH 7.4), 13 mM Na2EDTA, and 0.25% (w/v) bovine serum albumin (BSA). The extraction efficiency was 92.5 ± 5.7%. The intra‐assay CV was 8.6% at a plasma concentration of 100 μg/L. The sensitivity of the assay was 10 μg/L. IGF‐I antiserum AFP4892898 and human IGF‐I for iodination were obtained from the National Hormone and Peptide Program.3
Plasma total thyroxine (TT4) concentration was measured using a homologous solid‐phase, chemiluminescent enzyme immunoassay (Immulite canine total T44 ) according to manufacturer's instructions and validated for the dog.8 The sensitivity of the assay was 0.16 μg/dL (2 nmol/L). The intra‐assay CVs were 13.8% and 8.2% at plasma TT4 concentrations of 0.62 and 1.94 μg/dL (8 and 25 nmol/L), respectively. The interassay CV was 8.5%.
Plasma TSH concentration was measured using a homologous solid‐phase, 2‐site chemiluminescent enzyme immunometric assay (Immulite canine TSH4), according to manufacturer's instructions.9 The sensitivity of the assay was 0.03 μg/L. The intra‐assay CVs were 5.0%, 4.0%, and 3.8% at plasma TSH concentrations of 0.20, 0.50, and 2.6 μg/L, respectively. The interassay CVs were 6.3% and 8.2% at plasma TSH concentrations of 0.16 and 2.8 μg/L, respectively. The upper limit of the reference range for the plasma TSH concentration in euthyroid dogs in our laboratory is 0.6 μg/L.
Hormone Function Test
A GH‐releasing hormone (GHRH)‐stimulation test was performed by IV administration of 1 μg hGHRH5 per kg body weight.10, 11 Blood samples for determination of plasma GH concentrations were collected from the jugular vein in chilled EDTA‐coated tubes immediately before and 20 minutes after the administration of GHRH.
Diagnostic Imaging
Magnetic resonance (MR) images were obtained with a 0.2T open magnet using a 16S multipurpose coil.6 Sagittal T2‐weighted images (WI) (time of repetition [TR]: 4,455 milliseconds, time of echo [TE]: 117 milliseconds) of the caudal skull were obtained in 3 of the Czechoslovakian wolfdogs and 1 of the Saarloos wolfdogs with proportionate growth retardation. Sequences consisted of 3‐mm‐thick slices. Flash 3‐dimensional (3D)‐WI (TR: 34 milliseconds, TE: 12 milliseconds) before and after contrast administration were obtained. All flash 3D series consisted of 1‐mm‐thick T1‐weighted slices. Patients were positioned in sternal recumbency.
DNA Analysis
Genomic DNA served as a template for PCR amplification. PCR was performed using a primer pair that covered part of exon 5, intron 5, and part of exon 6 of canine LHX3 sequences. The DNA sequence of the forward primer was CCAAGCAGCTGGAGACCTTGAAGAG and the reverse primer had the sequence CTGGACGCTGTCCTTGTCCGAC. The forward primer was labeled with 6‐fluorescein amidite (FAM).7 The PCR reactions were performed in a 25‐μL volume containing 25 ng of gDNA, 1 mM MgSO4, 0.2 mM dNTPs, 0.3 μM of both primers, and 0.5 U Platinum Pfx polymerase in Pfx amplification buffer and PCR enhancer.8 The thermo‐cycling program consisted of a denaturation step of 2 minutes at 94°C, followed by 35 cycles each of 15 seconds at 94°C, 30 seconds at 61.4°C, and 30 seconds at 68°C.
The labeled PCR products were diluted 10‐fold in water. One microliter of the dilution was mixed with 15 μL Hi‐Di formamide and 0.2 μL of size standard 600‐LIZ.9 The products were analyzed with the ABI 3130 XL Genetic Analyzer using POP79 and filterset G59 and Genemapper 4.0.9
In the case of DNA sequence analysis, the PCR products were treated with Shrimp Alkaline Phosphatase10 and Exonuclease I11 and used as a template in a DNA sequencing reaction containing BigDye Terminator v3.1,9 according to manufacturer's instructions. The reaction products were purified with multiscreen 96‐well Sephadex 50‐gel filtration plates, and analyzed on an ABI 3130XL Genetic Analyzer.9 The DNA sequences were aligned using the Seqman program from the Lasergene package of DNASTAR inc.12 and compared to the reference canine genome with BLAST software.13
Results
All 6 dogs with proportionate dwarfism had retention of their puppy hair coat and a variable degree of alopecia (Fig 1). History, physical examination, and routine blood examinations were otherwise unremarkable. The basal plasma GH concentration was either immeasurable or very low (median, <1.0 μg/L; range, <1.0–1.5 μg/L), as was the plasma IGF‐1 concentration (median, 72.5 μg/L; range, <10–79 μg/L). Administration of GHRH did not result in an appropriate increase in plasma GH concentration (maximal increase <1.0 μg/L). The plasma TT4 concentrations were in the lower part of or below the reference range (median, 1.5 μg/dL [19.5 nmol/L]; range, 0.6–1.79 μg/dL [8–23 nmol/L]). The plasma TSH concentrations all were in the lower quarter of the reference range (median, 0.07 μg/L; range, 0.04–0.15 μg/L).
Figure 1.
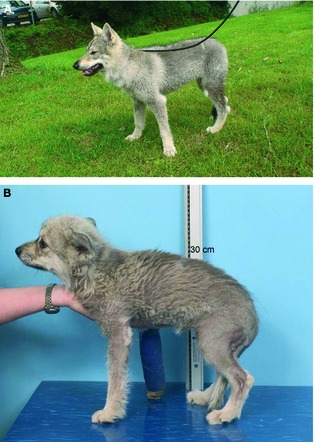
(A) An 8‐month‐old female intact Czechoslovakian wolfdog with dwarfism. The dog is proportionate and has retention of its puppy hair coat with isolated patches of adult hair. (B) A 1‐year‐old female intact Saarloos wolfdog with dwarfism. Note the proportionate growth retardation, retention of the puppy hair coat, and alopecia on the hind legs.
Sagittal T2‐WI showed a hyperintense, rounded structure in the region of the pituitary gland (Fig 2). This region was hypointense to the surrounding brain tissue on flash 3D‐WI and some peripheral enhancement was noted on postcontrast flash 3D‐WI. These changes were compatible with a cyst‐like structure.
Figure 2.
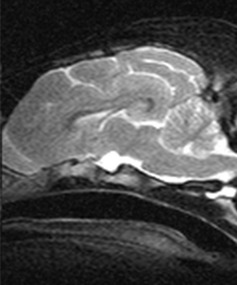
Sagittal T2‐WI of the head of a Czechoslovakian wolfdog dwarf. Note the cyst‐like structure in the region of the pituitary gland.
DNA analyses determined that intron 5 of LHX3 was 7 bp shorter in the dwarfs than in the control dogs. DNA sequence analysis showed that the size difference was the result of a deletion of 1 of 6 imperfect 7 bp repeats in intron 5 (Fig 3). This deletion is identical to the 1 found in German Shepherd dwarfs. All dwarfs displayed homozygosity for the 7 bp deletion and none of the Saarloos or Czechoslovakian wolfdog dwarfs was affected by the triplet insertion reported in a single compound heterozygous German Shepherd dwarf.
Figure 3.
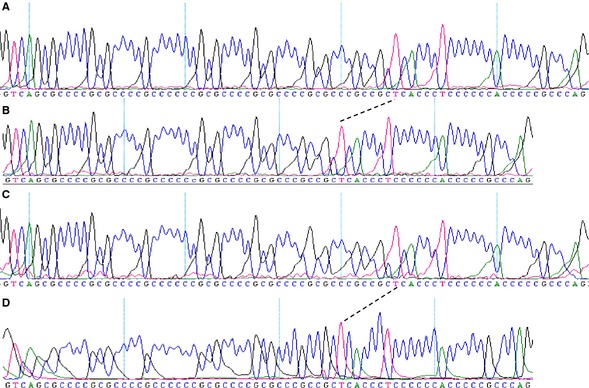
Comparison of the DNA sequence of intron 5 in normal Saarloos (A) and Czechoslovakian wolfdogs (C) and Saarloos (B) and Czechoslovakian (D) wolfdog dwarfs. The intron in the normal dogs consists of 75 bp and contains 6 imperfect repeats of the 7 bp consensus sequence GCGCCCC. Seven consecutive nucleotides in the region of repeats 4–6 are deleted from the intron of the dwarfs, indicated by dashes. The mutation is the same as found in German Shepherd dwarfs and is associated with deficient splicing of intron 5.4
Of the 239 Saarloos wolfdogs, 75 animals were carriers of the 7 bp deletion in intron 5 of LHX3 and 164 were clear of a mutation. Of the 200 Czechoslovakian wolfdogs, 42 animals were carriers of the 7 bp deletion and 158 animals did not have a mutation. Therefore, the percentage of carriers of this mutation among clinically healthy Saarloos and Czechoslovakian wolfdogs was 31% and 21%, respectively.
Discussion
This study demonstrates that pituitary dwarfism in Czechoslovakian and Saarloos wolfdogs is associated with GH deficiency and that these dwarfs have the same molecular defect of LHX3 as do German Shepherd Dog dwarfs. In addition, the high carrier frequency of the mutated allele in these 2 breeds underlines the importance of screening for this mutation before breeding.
Canine pituitary dwarfism is encountered most often in German Shepherd Dogs as a recessively inherited disorder.1, 4 The clinical signs displayed by the Czechoslovakian and Saarloos wolfdog dwarfs reported here (eg, proportionate dwarfism, retention of secondary hairs, and alopecia) strongly resembled those seen in German Shepherd dwarfs. Therefore, we tested whether the dwarfism in the Saarloos and Czechoslovakian wolfdogs also was because of GH deficiency. The basal plasma GH and IGF‐1 concentrations in the Saarloos and Czechoslovakian wolfdog dwarfs were low and the GHRH‐stimulation test failed to result in an appropriate increase in plasma GH concentration, justifying the diagnosis of hyposomatotropism.
In addition, the dwarfs had low plasma TSH concentrations and low or low‐normal plasma TT4 concentrations. German Shepherd Dog dwarfs not only have a deficiency of GH but the mutated LHX3 gene also results in TSH deficiency (ie, secondary hypothyroidism). The plasma concentrations of TT4 and TSH in the Saarloos and Czechoslovakian wolfdog dwarfs are comparable to those reported in German Shepherd dwarfs.3 Provocative testing of the thyrotropes with TSH‐releasing hormone will be required to prove impaired TSH release in Saarloos and Czechoslovakian wolfdog dwarfs.
Diagnostic imaging of the pituitary gland of the Saarloos and Czechoslovakian wolfdog dwarfs identified cystic changes. Also, in most German Shepherd dwarfs, comparable intrapituitary cysts can be identified.12 Originally, pituitary dwarfism in German Shepherd Dogs was ascribed to pressure atrophy of the cranial lobe of the pituitary gland by cystic enlargement of the residual craniopharyngeal duct or Rathke's cleft.13 However, a more recent study indicated that this is an implausible theory because German Shepherd dwarfs have been found with only very small pituitary cysts, unlikely to be responsible for pressure atrophy.12 Also, the finding that ACTH secretion is preserved in German Shepherd Dogs with dwarfism argues against cyst formation in Rathke's pouch as the primary cause of pituitary dwarfism in this breed.3 Therefore, it is more likely that cyst formation is a consequence of an underlying genetic defect.
Saarloos and Czechoslovakian wolfdogs are both German Shepherd Dog—wolf cross‐breeds. Pituitary dwarfism in German Shepherd Dogs is associated with molecular defects in LHX3. To evaluate if pituitary dwarfism in the Saarloos and Czechoslovakian wolf dwarfs is associated with the same mutations found in German Shepherd Dogs, the animals were subjected to genetic testing. All dwarfs were found to be homozygous for the same 7 bp deletion in intron 5 of LHX3. This mutation is identical to the 1 that is associated with deficient splicing and pituitary dwarfism in German Shepherd dwarfs.4 This finding strongly suggests that the dwarfs of the different dog breeds share a common ancestor.
Genealogic investigations indicate that the origin of the recessive gene is a mutation that occurred in 1940 or sometime before that year.2 In addition, these investigations suggest various champion dogs as being carriers of the trait, and thus reflect the relative importance of pituitary dwarfism. The Czechoslovakian wolfdog originates from 1955 and results from crosses of German Shepherd Dogs and Carpathian wolves. The objective was to create a breed that would have the trainability, pack mentality, and temperament of the German Shepherd Dog and the strength, physical build, and resilience of the Carpathian wolf. Because the breed was developed after the mutation that causes pituitary dwarfism in German Shepherd Dogs arose, it is likely that 1 or more of the German Shepherd Dogs used for breeding were carriers of the mutated LHX3 gene, and introduced the disease into the Czechoslovakian wolfdog breed.
In 1921, the Dutch Mr Leendert Saarloos created the Saarloos wolfdog by breeding a European wolf to a German Shepherd Dog to combine the willfulness to work of the German Shepherd Dog with the endurance and strength of the European wolf. According to their documented pedigrees, the German Shepherd Dog that was originally used to create the breed was the only German Shepherd Dog that was ever used in this breed. Because this dog was born long before 1940, either additional German Shepherd Dogs were used at a later stage in the breeding of the Saarloos wolfdogs or the mutation arose before the year 1921.
Pituitary dwarfism is a serious illness and clinical signs are not limited to physical appearance. Instead, the dwarfs suffer from a wide range of clinical manifestations. Without proper treatment, the long‐term prognosis is poor. Many dwarfs will not live more than 4–5 years and although the prognosis improves considerably when dwarfs are properly treated with porcine GH and synthetic l‐thyroxine, their prognosis still remains guarded.1 Consequently, it is important to include screening for carriers in the breeding programs of the affected breeds. Screening of a large group of clinically healthy Saarloos and Czechoslovakian wolfdogs showed that the percentage of carriers of the mutated allele (31% and 21%, respectively) is quite high, emphasizing the need for screening. Nevertheless, dwarfs are seen only occasionally. Most likely, CPHD and the associated DNA defects render affected individuals so weak that they either die in utero or shortly after birth.
Possibly, owners of dogs with dwarfs in their pedigree may be more likely to test their dogs, making the high frequency of carriers of the mutated allele in the tested population not representative of the whole breed. However, because it is mandatory for breeders of 1 of the 2 large breeder's associations of Saarloos wolfdogs to have their dogs tested for CPHD before breeding, this carrier percentage is likely a good indication of the carrier percentage of this breed as a whole. If all breeding animals were genetically tested for the presence of the LHX3 mutations associated with CPHD and a correct breeding policy would be implemented, this disease could be eradicated completely.
Acknowledgments
The authors are grateful for the technical support of M. Vos‐Loohuis and E.E.C.P. Martens and to L.W.L. van Bruggen for interpretation of the MRI scans and providing the images for Figure 2. The authors are also grateful to the owners of the dogs that were part of this study and to the breeders who supplied blood samples and buccal swabs.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
The work was performed at Utrecht University.
Footnotes
PE‐Chemagen, Baesweiler, Germany
PGH‐46HK; Linco Research, St. Charles, MS
Harbor‐UCLA Medical Center, Torrance, CA
Diagnostic Products Corporation, Los Angeles, CA
Peninsula Laboratories Inc, San Carlos, CA
Siemens Medical Systems, Hoffman Estates, IL
Eurogentec, Maastricht, The Netherlands
Invitrogen, Breda, The Netherlands
Applied Biosystems, Foster City, CA
Promega, Leiden, The Netherlands
New England BioLabs, Leiden, The Netherlands
Madison, WI
References
- 1. Voorbij AMWY, Kooistra HS. Pituitary dwarfism in German shepherd dogs. J Vet Clin Sci 2009;2:4–11. [Google Scholar]
- 2. Andresen E, Willeberg P. Pituitary dwarfism in German shepherd dogs: Additional evidence of simple autosomal recessive inheritance. Nord Vet Med 1976;28:481–486. [PubMed] [Google Scholar]
- 3. Kooistra HS, Voorhout G, Mol JA, Rijnberk A. Combined pituitary hormone deficiency in German shepherd dogs with dwarfism. Domest Anim Endocrinol 2000;19:177–190. [DOI] [PubMed] [Google Scholar]
- 4. Voorbij AMWY, van Steenbeek FG, Vos‐Loohuis M, et al. A contracted DNA repeat in LHX3 intron 5 is associated with aberrant splicing and pituitary dwarfism in German shepherd dogs. PLoS One 2011;6:e27940. doi: 10.1371/journal.pone.0027940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sheng HZ, Zhadanov AB, Mosinger B Jr, et al. Specification of pituitary cell lineages by the LIM homeobox gene LHX3. Science 1996;272:1004–1007. [DOI] [PubMed] [Google Scholar]
- 6. Sheng HZ, Moriyama K, Yamashita T, et al. Multistep control of pituitary organogenesis. Science 1997;278:1809–1812. [DOI] [PubMed] [Google Scholar]
- 7. Favier RP, Mol JA, Kooistra HS, Rijnberk A. Large body size in the dog is associated with transient GH excess at a young age. J Endocrinol 2001;170:479–484. [DOI] [PubMed] [Google Scholar]
- 8. Bruner JM, Scott‐Moncrieff JCR, Williams DA. Effect of time on sample collection on serum thyroid‐stimulating hormone concentrations in euthyroid and hypothyroid dogs. J Am Vet Med Assoc 1998;212:1572–1575. [PubMed] [Google Scholar]
- 9. Kooistra HS, Diaz‐Espineira M, Mol JA, et al. Secretion pattern of thyroid‐stimulating hormone in dogs during euthyroidism and hypothyroidism. Domest Anim Endocrinol 2000;18:19–29. [DOI] [PubMed] [Google Scholar]
- 10. Meij BP, Mol JA, Hazewinkel HAW, et al. Assessment of a combined anterior pituitary function test in beagle dogs: Rapid sequential intravenous administration of four hypothalamic releasing hormones. Domest Anim Endocrinol 1996;13:161–170. [DOI] [PubMed] [Google Scholar]
- 11. Meij BP, Mol JA, Rijnberk A. Thyroid‐stimulating hormone responses after single administration of thyrotropin‐releasing hormone and combined administration of four hypothalamic releasing hormones in beagle dogs. Domest Anim Endocrinol 1996;13:465–468. [DOI] [PubMed] [Google Scholar]
- 12. Kooistra HS, Voorhout G, Selman PJ, Rijnberk A. Progestin‐induced growth hormone (GH) production in the treatment of dogs with congenital GH deficiency. Domest Anim Endocrinol 1998;15:93–102. [DOI] [PubMed] [Google Scholar]
- 13. Müller‐Peddinghaus R, El Etreby MF, Siefert J, Ranke M. Hypophysärer Zwergwuchs beim Deutschen Schäferhund. Vet Pathol 1980;17:406–421. [DOI] [PubMed] [Google Scholar]


