Abstract
Background
People with renal disease develop a dyslipidemia that contributes to progression of renal injury and development of cardiovascular disease. Lipoproteins in dogs with renal disease have not been investigated.
Hypothesis
Dogs with chronic kidney disease (CKD) have dyslipidemia characterized by increased lower density lipoproteins and decreased high‐density lipoproteins (HDLs). The degree of dyslipidemia is positively correlated with severity of disease, as reflected by serum creatinine concentration.
Animals
Prospective study of client‐owned dogs presented to the Cornell University Hospital for Animals: 29 dogs with confirmed CKD, 5 dogs with nephrotic syndrome (NS), and 12 healthy control dogs presented for routine vaccinations, dental cleaning, or owned by students.
Methods
Lipoprotein electrophoresis was used to quantify relative proportions of the 3 main classes of lipoproteins in canine serum: low‐density lipoproteins (LDL), very low‐density lipoproteins (VLDL), and HDL. Serum cholesterol and creatinine concentrations; urinalysis and urine protein‐to‐creatinine ratio were measured by standard methods.
Results
Dyslipidemia was consistently found in dogs with CKD and NS and was characterized by a decrease in HDL and variable increases in LDL and VLDL. Dogs with NS had a proportionately greater increase in the VLDL fraction, as compared with dogs with CKD.
Conclusion and Clinical Importance
Dyslipidemia similar to that documented in people with renal disease occurs in dogs with CKD, despite serum cholesterol concentrations often being within the reference interval. The contribution of altered lipoproteins to the pathogenesis of renal disease in dogs warrants additional study.
Keywords: Cholesterol, Chronic kidney disease, Protein‐losing nephropathy
Abbreviations
- CKD
chronic kidney disease
- HDL
high‐density lipoproteins
- LDL
low‐density lipoprotein
- NS
nephrotic syndrome
- RI
reference interval(s)
- VLDL
very low‐density lipoprotein
Alterations in lipid metabolism in people with chronic kidney disease (CKD) are characterized by increases in low‐density lipoproteins (LDL), very low‐density lipoproteins (VLDL), and their oxidized derivatives, as well as by decreased high‐density lipoproteins (HDL).1, 2 These changes are reported in 35–85% of patients with CKD and are considered largely responsible for associated cardiovascular disease.3 Lipoprotein changes also are thought to be key contributors to the vicious cycle of oxidative injury and inflammation in the kidney.2 Studies of the impact of lipid‐lowering therapies on the progression of renal disease have yielded mixed results. Some of the complexity in interpreting these data stems from the impact of dialysis on serum lipoprotein changes and other confounding variables.4, 5 Consequently, the role of lipoproteins in the pathogenesis of renal disease remains controversial. The incidence, severity, and consequences of altered lipid metabolism in dogs with CKD are unknown.
In dogs, hypercholesterolemia is a component of nephrotic syndrome (NS). However, unlike in people, dyslipidemia based on measurement of serum concentrations of cholesterol and triglycerides is not a recognized component of CKD in dogs. This may be because of species differences in lipid metabolism or, alternatively, to limited analysis of lipoprotein changes in these dogs. Subfractionation of classes of lipoproteins is not routinely available in the clinical setting; therefore, without overt hyperlipidemia, dyslipidemia is likely to go unrecognized. To the best of the author's knowledge, reports characterizing lipoprotein changes in dogs with CKD have not been published.
In this study, the hypothesis tested was that dyslipidemia characterized by decreased HDL, increased LDL/VLDL, or both would be present in dogs with CKD and would be positively correlated with biomarkers of disease severity. Extrapolation from the well‐recognized clinical association of marked proteinuria and hypercholesterolemia led to testing of an additional hypothesis that proteinuria, as quantified by the urine protein‐to‐creatinine ratio, would be positively correlated with serum cholesterol concentration.
Materials and Methods
Study Design and Patient Data
This prospective observational study was conducted from October 2012 to July 2013 in accordance with all institution guidelines and with an approved institutional animal care and use protocol. Patient study groups included healthy control dogs, dogs with CKD, and dogs with NS. Healthy dogs had CBC and serum biochemical results within reference intervals (RI) established at the Cornell University Hospital for Animals (CUHA), normal physical examination findings, and urinalyses within normal limits. Inclusion criteria for the CKD group were based on guidelines developed by the International Renal Interest Society (IRIS http://www.iris-kidney.com) and included serum creatinine concentration ≥1.4 mg/dL with a concurrent urine specific gravity ≤1.025. These findings had to be present on at least 2 occasions separated by a minimum of 3 months for dogs to be included in the study. Dogs were further stratified into IRIS stages 2 through 4 based on the serum creatinine concentration reported for the visit corresponding to the day the serum lipoprotein electrophoresis was performed. Dogs that had a serum creatinine concentration <1.4 mg/dL at the time of sample collection for lipoprotein electrophoresis were excluded from the study. Additional exclusion criteria included a positive Osp F titer on the Lyme Mulitplex assay or a positive titer for leptospirosis (both tests were performed by the Animal Health Diagnostic Center, Ithaca, NY). These tests were performed at the discretion of the attending clinician. Dogs selected for the NS group had urine protein‐to‐creatinine ratios >5.0 with an inactive urine sediment, serum albumin concentrations <2.9 g/dL (RI, 3.1–4.2 g/dL), serum cholesterol concentrations >332 mg/dL (RI, 136–332 g/dL), and notation of concurrent or recent (documented within 3 days of the day the blood sample used for lipoprotein analysis was drawn) fluid extravasation (cavitary or within interstitial tissues) in the medical record. Biochemical evidence of any additional disease process (eg, endocrinopathies, organ injury) was used as exclusionary criteria for both the CKD and NS groups. Miniature schnauzers were excluded to ensure that dogs with hereditary hypertriglyceridemia were not included. Dogs were fasted overnight according to the owners.
Serum Lipoprotein Agarose Electrophoresis
In this study, lipoproteins were semiquantified using agarose electrophoresis. This method was selected because the overlap in hydrated density of canine HDL1 and LDL and large sample size required make ultracentrifugation less useful in analysis of canine lipoproteins in clinical samples.6 Additionally, previous studies have documented that electrophoresis can be used to semiquantify canine lipoproteins and is particularly useful in identifying beta‐migrating VLDL.7, 8 Serum samples were prepared for electrophoresis by addition of 5 μL of loading buffer (60% sucrose, 0.1% bromophenol blue in double‐distilled [dd] H2O) to 15 μL serum. Diluted serum samples were loaded by volume (15 μL) onto 1% agarose gel1 in 60 mM sodium barbital buffer.2 Lipoproteins were separated by horizontal electrophoresis at 80 V for 55 minutes in 60 mM sodium barbital buffer.2 Gels were stained overnight at room temperature (20–22°C) in 0.18% Sudan black B1 in 70% ethanol. Gels were destained in a 15% acetic acid/20% acetone solution in dH2O for 2–3 hours, until the background was light gray to clear and the bands were clearly visible. Gels were scanned3 as negative images, and lipoprotein bands were quantified by converting the pixel intensity of the scanned lane to a linear peak plot followed by calculation of the area under the curve (AUC) for each defined peak using NIH Image J software (http://rsbweb.nih.gov/ij/). The HDL : non‐HDL ratio was calculated using the following equation: HDL AUC/(LDL AUC + VLDL AUC). All samples were analyzed within 24 hours of collection.
Statistical Analysis
The mean AUC for lipoprotein fractions and the HDL : non‐HDL ratios for control animals and patient groups were compared using a one‐way ANOVA followed by a Tukey pairwise comparison. Correlation analyses were performed using a Spearman regression for nonparametric data. For all analyses, P < .05 was considered significant. All analysis was performed using Prism GraphPad software, v. 5.4
Results
Patient Data
Forty‐six dogs were included in the study: 12 healthy controls and 34 diseased dogs. Diseased dogs included 29 dogs with CKD and 5 dogs with NS. For each patient group, the breeds represented, ages (median and range), and sex distribution are listed in Table 1. In the CKD group, 6 dogs were classified as IRIS stage 2, 6 as IRIS stage 3, and 17 as IRIS stage 4. Three of the dogs in IRIS stage 4 had acute increases in their serum creatinine concentrations, and may have been suffering an acute renal injury overlying their CKD. Two of these dogs were euthanized within 2 months of the appointment at which the sample for this study was collected, and 1 was lost to follow‐up. Seven of the 29 CKD dogs had positive results on a SNAP 4DX5 test performed at the referring veterinarian, but lacked any clinical signs of active borelliosis. Three dogs had leptospirosis titers performed at the discretion of the clinician, and all were negative. Three of the dogs with CKD were being provided a renal diet and 6 of them were eating homemade diets. Body condition scores, using a 9‐point scale, were recorded in the medical record for 7 of the 29 dogs with CKD and included values from 2 to 6. Two dogs were noted to be losing weight, but there was no notation of their current body condition in the written record. One of the control dogs had a body condition score of 6 by 1 clinician and was noted to be obese by a second clinician. Seventeen of the dogs with CKD were being treated with enalapril, 5 were being treated with both enalapril and amlodipine, 5 were receiving supplemental fish oil, and 1 was receiving niacin. Blood pressure was measured the same day the blood sample used for electrophoresis was drawn and recorded in the medical record for only 11 of the CKD dogs. The reported systolic blood pressures ranged from 150 to 240 mmHg. A systolic blood pressure of 150–159 mmHg is considered low risk for end‐organ damage and complications, 160–179 mmHg places the animal in the moderate risk group, and a systolic blood pressure ≥180 mmHg is considered high risk.
Table 1.
Patient signalments
| Control | CKD | NS | |
|---|---|---|---|
| Breeds |
Mixed breed (3) Labrador Retriever (2) Miniature Poodle Golden Retriever Cocker Spaniel Pug Pomeranian Rat Terrier Pekingese |
Mixed breed (18) Labrador Retriever (5) Golden Retriever (3) Beagle (2) Yorkshire Terrier (2) Boston Terrier Bichon Frise Border Collie Bullmastiff Chihuahua Corgi Coonhound Duck Tolling Retriever English Mastiff German Shorthair Pointer Irish Setter Miniature Poodle Pug Shetland Sheepdog Springer Spaniel Standard Poodle West Highland White Terrier Whippet |
Mixed breed (3) Beagle Labrador Retriever |
| Agea | 5 years (10 months–16 years) | 9 years (6 months–14 years) | 10 years (4–11 years) |
| Sexa | 5 FS, 4 MC, 3 M | 27 FS, 2 F, 17 MC, 11 M | 2 FS, 2 MC, 1 M |
CKD, chronic kidney disease; NS, nephrotic syndrome; F, female; M, male; FS, female‐spayed; MC, male‐castrated.
Median (range).
Serum and Urine Biomarkers
Median serum concentrations of creatinine and cholesterol, urine specific gravity, and urine protein‐to‐creatinine ratios (UPCs) for the control and diseased dogs are presented in Table 2. The serum cholesterol concentration was above the RI for all dogs in the NS group, because this was a selection criterion. The median serum cholesterol concentration also was above the RI in the CKD group. When individual patients were investigated, 16/29 (55%) of dogs with CKD had serum cholesterol concentrations above the RI.
Table 2.
Renal biomarkers and serum cholesterol concentrations for healthy control dogs and 3 patient groups
| Controls | CKD | NS | Reference Interval | |
|---|---|---|---|---|
| Number of dogs | 12 | 29 | 5 | |
| Creatinine (mg/dL) | 0.9 (0.5–1.2) | 5.0 (1.4–11.5) | 2.9 (1.7–10.2) | 0.6–1.4 |
| UPC | NA | 3.0 (0.4–4.7)a | 13.2 (7–21.3) | <0.2 |
| Urine specific gravity | 1.031 (1.017–1.051) | 1.018 (1.008–1.032) | 1.022 (1.016–1.025)b | |
| Cholesterol (mg/dL) | 226 (180–277) | 311 (104–512)c | 408.5 (350–735) | 136–332 |
| Albumin (g/dL) | 3.7 (3.4–4.3) | 3.2 (2.5–4) | 2.1 (1.5–2.2) | 3.1–4.2 |
Values are medians (range) for the groups.
CKD, chronic kidney disease; NS, nephrotic syndrome; UPC, urine protein‐to‐creatinine ratio.
Indicates 2 missing data points.
Indicates a missing data point. One dog in the nephrotic syndrome group did not have urine specific gravity recorded on the day lipoprotein electrophoresis was performed.
16/29 of the dogs with CKD had cholesterol values above the upper end of the RI.
Lipoprotein Fractions
Initial validation studies of the electrophoresis method employed were performed. A single sample was run in 30 replicates. The average HDL to non‐HDL ratio of the 30 replicate runs was 2.15 with a SD of 0.15 and a CV of 0.07 (data not shown). Lipoprotein fractions in healthy dogs consisted primarily of the alpha lipoprotein HDL, migrating farthest from the anode. Non‐HDL lipoproteins, which include LDL, VLDL, and any chylomicrons, were low in healthy dogs (Fig 1A,B, upper panel). Dogs with CKD had a markedly different lipoprotein profile, characterized by decreased HDL and increased LDL and VLDL fractions (Fig 1A,B, lower panel). The relative amount of each lipoprotein class is represented numerically in the HDL : non‐HDL ratio, where the non‐HDL portion includes LDL, VLDL, and any retained chylomicrons. The HDL : non‐HDL ratio was significantly higher in control dogs (median, 4.85; 95% CI, 3.3–6.8) than in the CKD group (median, 1.1; 95% CI, 0.9–1.5) or the NS group (median, 1.02; 95% CI 0.48–1.3; Fig 2).
Figure 1.
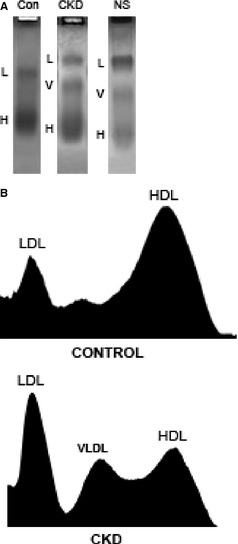
Serum lipoprotein fractions in control and diseased dogs. (A) Serum lipoprotein electrophoresis of a representative sample from each group of dogs: Control dogs (Con), dogs with chronic kidney disease (CKD), and dogs with nephrotic syndrome (NS). (B) Densitometry scans of the agarose gels from a control dog (upper) and a single dog with CKD (lower). LDL, low‐density lipoproteins; VLDL, very low‐density lipoproteins; HDL, high‐density lipoproteins. Note the decrease in the proportion of HDL in the dogs with CKD.
Figure 2.
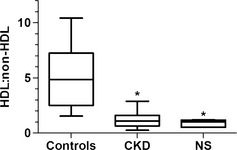
Box and whisker plot of mean HDL : non‐HDL ratio [HDL area under the curve (AUC)/(LDL AUC + VLDL AUC)] for control dogs and dogs with chronic kidney disease (CKD), and dogs with nephrotic syndrome (NS). Boxes represent the interquartile intervals from the 25 to 75th percentiles, solid horizontal lines through the boxes represent the medians, and the minimum and maximum values are represented by the capped vertical bars. *Different from control, P < .05 (ANOVA, Tukey pairwise comparison). HDL, high‐density lipoproteins; LDL, low‐density lipoproteins; VLDL, very low‐density lipoprotein.
HDL:non‐HDL Ratio, IRIS Stage, and UPC
To determine if changes in lipoprotein fractions were more pronounced in the more severe cases of renal disease, HDL : non‐HDL ratios were compared among the 3 IRIS stages included in the study. Dogs in IRIS stages 2–4 all had significantly lower HDL : non‐HDL ratios than did healthy controls. There was no statistically significant difference among the evaluated IRIS stages (Fig 3A). The HDL : non‐HDL ratio was decreased compared with ratio in controls when the CKD patients were grouped by their UPC value, but there was no difference among the ratios across the varying degrees of proteinuria (Fig 3B).
Figure 3.
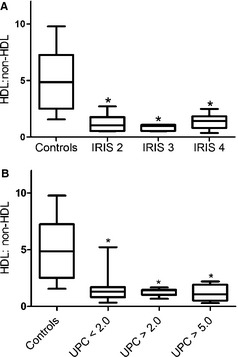
Box and whisker plot of mean HDL : non‐HDL ratio [HDL area under the curve (AUC)/(LDL AUC + VLDL AUC)] for dogs with CKD (n = 34) (A) Dogs with CKD were segregated into groups by IRIS stages divided into IRIS stages based on serum creatinine concentration at the time of blood collection for lipoprotein electrophoresis. Control n = 12; IRIS 2, n = 6; IRIS 3, n = 6; IRIS 4, n = 17. (B) Dogs with CKD were segregated into groups based on their UPC value. Control n = 12, UPC < 2.0 n = 17, UPC > 2.0 n = 9, UPC > 5.0 n = 8. Boxes represent the interquartile intervals from the 25 to 75th percentiles, solid horizontal lines through the boxes represent the medians, and the minimum and maximum values are represented by the capped vertical bars. *Different from control, P < .05 (ANOVA, Tukey pairwise comparison). HDL, high‐density lipoproteins; LDL, low‐density lipoproteins; VLDL, very low‐density lipoprotein; IRIS, International Renal Interest Society.
Lipoprotein Fractions and Disease Group
Relative proportions of individual lipoprotein classes were different among patient groups (Fig 4). HDL was consistently lower in dogs with CKD or NS, as compared with controls. Dogs with NS had HDL results that were statistically lower than dogs with CKD. The proportion of lipoproteins that were HDL had a relatively wide range in dogs with CKD and NS and a narrower range in control dogs (Fig 4A). The proportion of lipoproteins that were LDL in dogs with CKD was highly variable. Despite some visually striking increases in LDL in individual dogs (Fig 1A), the trend toward higher LDL in dogs with CKD and NS did not reach statistical significance (Fig 4B). The proportion of VLDL was significantly higher in dogs with CKD and NS with the range being wider in dogs with CKD (Fig 4C).
Figure 4.
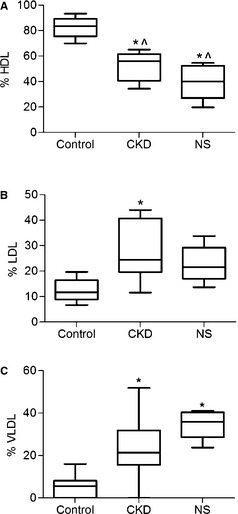
Box and whisker plot of individual lipoprotein classes among groups of dogs: control dogs (n = 12), dogs with chronic kidney disease (CKD, n = 34), and dogs with nephrotic syndrome (NS, n = 5) (A) HDL fraction (%); (B) LDL fraction (%); (C) VLDL fraction (%). Boxes represent the interquartile intervals from the 25 to 75th percentiles, solid horizontal lines through the boxes represent the medians, and the minimum and maximum values are represented by the capped vertical bars. *Different from control, ^Different from each other, P < .05 (ANOVA, Tukey pairwise comparison). HDL, high‐density lipoproteins; LDL, low‐density lipoproteins.
Correlations between Selected Biomarkers of Renal Disease and Serum Lipoproteins
Correlations between HDL : non‐HDL ratio and serum creatinine concentration, UPC, and urine specific gravity were not found (data not shown). There was a weak correlation between serum cholesterol concentration and UPC (Fig 5A). When the 5 dogs with NS were removed and the analysis was repeated, there was no significant correlation between UPC and total serum cholesterol concentration (Fig 5B). Analysis was not performed on the NS group because of small sample size.
Figure 5.
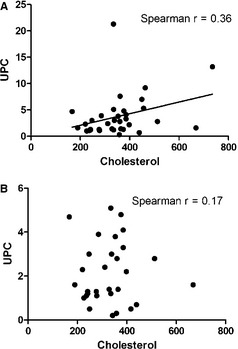
Urine protein‐to‐creatinine ratio is weakly correlated with total serum cholesterol concentration in dogs with renal disease. (A) UPC values plotted against total serum cholesterol (mg/dL) in all dogs with available data N = 34 dogs: 5 with nephrotic syndrome (NS) and 29 dogs with chronic kidney disease (CKD). Spearman r = 0.36, P < .016. (B) UPC values plotted against total serum cholesterol for only the 29 dogs with CKD.
Discussion
This study demonstrates that dyslipidemia, characterized by a decrease in HDL and an increase in LDL, VLDL, or both, is a consistent finding in dogs with CKD and NS. Similar changes have not only been documented in people with renal disease but also have an impact on disease progression and clinical outcome.1, 2, 9 The magnitude of change in lipoprotein profile was not different across the IRIS stages evaluated. Although this finding suggests that changes in lipoprotein metabolism occur early in the progression of renal disease, the cross‐sectional nature of this study does not allow for inferences regarding the evolution of these changes during the onset or progression of renal disease. Cardiovascular disease, largely attributed to dyslipidemia, is the leading cause of death in people with CKD.1, 2, 3 Dogs with renal failure have higher serum concentrations of cardiac troponin I compared with healthy dogs, and it has been proposed that these dogs may indeed have subclinical cardiovascular disease.10 Additional studies may be warranted to investigate the impact of the lipid changes detected in this study on cardiovascular disease in dogs with renal disease.
In people, the dyslipidemia of renal disease also is thought to contribute to progression of renal injury.1, 2, 3 HDL are known to have potent anti‐inflammatory and antioxidant effects.11 Conversely, the lower density lipoproteins and their metabolic by‐products have been shown to contribute to inflammation, oxidative damage, and fibrosis that lead to progression of renal disease.12, 13, 14 Animal models indicate that oxidized LDLs are deposited in renal tissue of remnant kidneys after surgical removal of 5/6 of renal mass, and accumulation of oxidized LDLs correlates with areas of increased inflammatory cells.15 The literature and the decrease in HDLs and increase in LDLs detected in dogs with CKD in this study, taken together, suggest that alterations in lipid metabolism may have an unrecognized clinical impact in dogs with renal disease.
Hypercholesterolemia is one of the clinical criteria in the diagnosis of NS in dogs, and is thought to reflect a potential increase in lipoprotein synthesis as a compensatory means to increase oncotic pressure.16 Lipoprotein profiles of affected dogs have not been reported previously. Dogs with NS had lower HDL, increased LDL, and an increase in VLDL that was higher than the increase observed in dogs with CKD. These findings are similar to what has been reported in people with NS.17 These changes may contribute to the prothrombotic tendency in these dogs, as high LDL and low HDL both are recognized to promote platelet activation in people.17, 18 Studies are currently underway to investigate this hypothesis.
The mechanistic links between declining renal function and lipid changes are complex and multifactorial. Studies in people have shown that patients with renal disease have decreased expression of a number of key lipoprotein uptake receptors and decreased activity of many enzymes responsible for clearing lipoproteins from circulation.9 Similar defects may be present in dogs with renal disease. However, key enzymes in lipid metabolism have been shown to have differential to no activity in dogs, as compared with enzymes in humans. Salient examples including lecithin:cholesterol acyltransferase and cholesteryl ester transfer protein.19, 20 Therefore, alternative or additional mechanisms probably are involved.
This study has several limitations. The sample size of the groups is relatively small. This is particularly true of comparisons made between IRIS stages and the NS group. However, changes reflected in the average of the 5 dogs with NS were consistently present in all individuals in this group. Body composition, age, diet, and drug treatment may have an impact on lipoprotein profiles. Obesity has been shown to correlate with higher total and HDL cholesterol concentrations in dogs <8 years of age, and a mild increase in the proportion of beta lipoproteins (VLDL and LDL) in male dogs >8 years of age.21 Other studies have shown obese dogs to maintain an HDL‐rich status, despite an increase in total cholesterol concentration.22 In this study, only 1 of the control dogs was noted to be obese by 1 clinician, and classified as overweight by another. None of the dogs with CKD or NS were documented to be obese, minimizing the expected contribution of body condition to the lipoprotein status. Age has been shown to correlate with an increase in LDL in a 1 study. However, 4 of the 12 aged dogs in that study were miniature schnauzers, a breed known to have a predisposition toward developing hyperlipidemia that worsens with age.7, 23 In another study, male dogs >8 years of age had an approximate 6% increase in the combined LDL/VLDL fraction as compared to male dogs <8 years of age.21 In the current study, the median age of the CKD dogs was higher than the median age of the control dogs. However, given the magnitude of the difference detected, the potential contribution of age is probably minor. The impact of the homemade diets administered to some of the dogs with CKD in the current study on their lipoprotein profile is unknown. Diets high in fish oil and omega 3 fatty acids, as many renal diets are, have been shown to lower total cholesterol concentration.24 The main drugs the dogs with CKD received (enalapril and amlodipine) have not been reported to impact lipoprotein profiles in people or dogs. Niacin has been shown to decrease LDL in dogs.25 This could have altered the profile of the single dog with CKD that was being treated with this supplement. Agarose gel electrophoresis provides accurate separation of canine lipoproteins.7, 26, 27, 28 This method, however, depends in part on Sudan black B staining of the lipid bands. Sudan black B preferentially stains neutral lipids, and may lead to a slight overestimation of the relative proportion of LDL and VLDL because of their higher triglyceride content. Comparing the degree of oxidation of lipoprotein fractions across groups of dogs would have been of interest, but was not performed in this study because of limitations in sample volume and currently available methodology.
This study is the first to document dyslipidemia in dogs with CKD and the composition of serum lipoproteins in dogs with NS. Marked dyslipidemia occurred in just over half the dogs with CKD despite serum cholesterol concentrations within the RI. Based on current clinical standards, which do not include lipoprotein profiling, many of these dogs would have been considered to have normal lipid metabolism. Dyslipidemias also have been reported in dogs with serum cholesterol and triglyceride concentrations within the RI in a recent study comparing healthy dogs and miniature schnauzers with hyperlipidemia.7 The multitude of interactions between serum lipids and the axis of renal inflammation and fibrosis have made modulation of lipid metabolism and reduction of non‐HDL lipoproteins a primary therapeutic target in the management of CKD in people.1 Lipid‐lowering statins decrease the incidence of cardiovascular disease, myocardial infarctions, and strokes in people with renal disease.29 Statins are also under investigation in human patients as agents to decrease renal injury.30 These drugs, or other means of lipid modulation, also may prove beneficial in dogs. Additional studies are warranted to determine if the lipid changes reported in this study evolve during the progression of renal disease and if they contribute to renal or cardiovascular injury or both in dogs. Lipoprotein modulation may represent an unrecognized target for therapeutic intervention in CKD and NS in dogs.
Acknowledgments
The author thanks Rebekah Collins‐Cronkright, Cornell University, for technical assistance and Dr Karen Young, University of Wisconsin‐Madison, for assistance in preparing the manuscript. This study was supported by start‐up funds provided to Dr Behling‐Kelly.
Conflict of Interest Declaration: The author discloses no conflict of interest.
Off‐label Antimicrobial Declaration: The author declares no off‐label use of antimicrobials.
This study was performed at Cornell University, College of Veterinary Medicine, Ithaca, NY.
Footnotes
Agarose Unlimited, Gainseville, FL
BioRad gel systems, Hercules, CA
Epson Perfection V500, Long beach, CA
GraphPad Software, Inc, La Jolla, CA
IDEXX laboratories, Westbrook, ME
References
- 1. Nitta K. Clinical assessment and management of dyslipidemia in patients with chronic kidney disease. Clin Exp Nephrol 2012;16:522–529. [DOI] [PubMed] [Google Scholar]
- 2. Kasiske BL. Hyperlipidemia in patients with chronic renal disease. Am J Kidney Dis 1998;32:S142–S156. [DOI] [PubMed] [Google Scholar]
- 3. Foley RN, Parfrey PS, Sranak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kideny Dis 1998;32:S112–S119. [DOI] [PubMed] [Google Scholar]
- 4. Vaziri ND, Norris KC. Reasons for the lack of salutary effects of cholesterol‐lowering interventions in end‐stage renal disease populations. Blood Purif 2013;35:313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi HD, Shin WG. Safety and efficacy of statin treatment alone and in combination with fibrates in patients with dyslipidemia: A meta‐analysis. Curr Med Res Opin 2014;30:1–10. [DOI] [PubMed] [Google Scholar]
- 6. Mahley RW, Weisgraber KH, Innerarity T. Canine lipoproteins and atherosclerosis. II. Characterization of the plasma lipoproteins associated with atherogenic and nonatherogenic hyperlipidemia. Circ Res 1974;35:722–733. [DOI] [PubMed] [Google Scholar]
- 7. Whitney MS1, Boon GD, Rebar AH, et al. Ultracentrifugal and electrophoretic characteristics of the plasma lipoproteins of miniature schnauzer dogs with idiopathic hyperlipoproteinemia. J Vet Intern Med 1993;7:253–260. [DOI] [PubMed] [Google Scholar]
- 8. Wright AS1, Bauer JE, Bigley KE, et al. Maternal dietary fatty acids modify canine puppy plasma lipoprotein distributions during the suckling period. J Nutr 2004;134:2106S–2109S. [DOI] [PubMed] [Google Scholar]
- 9. Laquaniti A, Bolignano D, Donato V, et al. Alterations in lipid metabolism in chronic nephropathies: Mechanisms, diagnosis and treatment. Kidney Blood Press Res 2010;33:100–110. [DOI] [PubMed] [Google Scholar]
- 10. Sharkey LC1, Berzina I, Ferasin L, et al. Evaluation of serum cardiac troponin I concentration in dogs with renal failure. J Am Vet Med Assoc 2009;234:767–770. [DOI] [PubMed] [Google Scholar]
- 11. Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of HDL. Circ Res 2006;98:1352–1364. (Review) [DOI] [PubMed] [Google Scholar]
- 12. Gyebi L, Soltani Z, Reisin E. Lipid nephrotoxicity: New concept for an old disease. Curr Hypertens Rep 2012;14:177–181. [DOI] [PubMed] [Google Scholar]
- 13. Vaziri ND, Norris K. Lipid disorders and their relevance to outcomes in chronic kidney disease. Blood Purif 2011;31:189–196. [DOI] [PubMed] [Google Scholar]
- 14. Ng KF, Aung HH, Rutledge JC. Role of triglyceride‐rich lipoproteins in renal injury. Contrib Nephrol 2011;170:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Satirapoj B, Bruhn KW, Nast CC, et al. Oxidized low density lipoprotein antigen transport induces autoimmunity in the renal tubulointerstitium. Am J Nephrol 2012;35:520–530. [DOI] [PubMed] [Google Scholar]
- 16. Klosterman ES, Pressler BM. Nephrotic syndrome in dogs: Clinical features and evidence‐based treatment considerations. Top Companion Anim Med 2011;26:135–142. [DOI] [PubMed] [Google Scholar]
- 17. Kronenberg F. Dyslipidemia and nephrotic syndrome: Recent advances. J Ren Nutr 2005;15:195–203. [DOI] [PubMed] [Google Scholar]
- 18. Akkerman JW. From low‐density lipoprotein to platelet activation. Int J Biochem Cell Biol 2008;40:2374–2378. [DOI] [PubMed] [Google Scholar]
- 19. Liu M1, Bagdade JD, Subbaiah PV. Specificity of lecithin:cholesterol acyltransferase and atherogenic risk: Comparative studies on the plasma composition and in vitro synthesis of cholesteryl esters in 14 vertebrate species. J Lipid Res 1995;36:1813–1824. [PubMed] [Google Scholar]
- 20. Ouguerram K1, Nguyen P, Krempf M, et al. Selective uptake of high density lipoproteins cholesteryl ester in the dog, a species lacking in cholesteryl ester transfer protein activity: An in vivo approach using stable isotopes. Comp Biochem Physiol B Biochem Mol Biol 2004;138:339–345. [DOI] [PubMed] [Google Scholar]
- 21. Mori N, Lee P, Kondo K, et al. Potential use of cholesterol lipoprotein profile to confirm obesity status in dogs. Vet Res Commun 2011;35:223–235. [DOI] [PubMed] [Google Scholar]
- 22. Jeusette IC, Lhoest ET, Istasse LP, Diez MO. Influence of obesity on plasma lipid and lipoprotein concentrations in dogs. Am J Vet Res 2005;66:81–86. [DOI] [PubMed] [Google Scholar]
- 23. Kawasumi K, Kashiwado N, Okada Y, et al. Age effects on plasma cholesterol and triglyceride profiles and metabolite concentrations in dogs. BMC Vet Res 2010;10:57. (Open Access) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pasquini A, Luchetti E, Cardini G. Plasma lipoprotein concentrations in the dog: The effects of gender, age, breed and diet. J Anim Physiol Anim Nutr (Berl) 2008;92:718–722. [DOI] [PubMed] [Google Scholar]
- 25. Le Bloc'h J, Leray V, Chetiveaux M, et al. Nicotinic acid decreases apolipoprotein B100‐containing lipoprotein levels by reducing hepatic very low density lipoprotein secretion through a possible diacylglycerol acyltransferase 2 inhibition in obese dogs. J Pharmacol Exp Ther 2010;334:583–589. [DOI] [PubMed] [Google Scholar]
- 26. Xenoulis PG, Cammarata PJ, Walzem RL, et al. Novel lipoprotein density profiling in healthy dogs of various breeds, healthy Miniature Schnauzers, and Miniature Schnauzers with hyperlipidemia. BMC Vet Res 2013;8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rogers WA, Donovan EF, Kociba GJ. Lipids and lipoproteins in normal dogs and in dogs with secondary hyperlipoproteinemia. J Am Vet Med Assoc 1975;166:1092–1100. [PubMed] [Google Scholar]
- 28. Abate O, Zanatta R, Malisano T, Dotta U. Canine serum protein patterns using high‐resolution electrophoresis (HRE). Vet J 2000;159:154–160. [DOI] [PubMed] [Google Scholar]
- 29. Galvao TF, Aaraujo ME, Penha AP, Silva MT. Statins for early stage chronic kidney disease: An overview of reviews. Cardiovasc Hematol Disord Drug Targets 2014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30. Kasahara M1, Nakagawa T, Yokoi H, et al. Do statins play a role in renoprotection? Clin Exp Nephrol 2014;18:282–285. [DOI] [PubMed] [Google Scholar]


