Abstract
Background
Remission occurs in 10–50% of cats with diabetes mellitus (DM). It is assumed that intensive treatment improves β‐cell function and increases remission rates.
Hypothesis
Initial intravenous infusion of insulin that achieves tight glycemic control decreases subsequent insulin requirements and increases remission rate in diabetic cats.
Animals
Thirty cats with newly diagnosed DM.
Methods
Prospective study. Cats were randomly assigned to one of 2 groups. Cats in group 1 (n = 15) received intravenous infusion of insulin with the goal of maintaining blood glucose concentrations at 90–180 mg/dL, for 6 days. Cats in group 2 (n = 15) received subcutaneous injections of insulin glargine (cats ≤4 kg: 0.5–1.0 IU, q12h; >4 kg 1.5–2.0 IU, q12h), for 6 days. Thereafter, all cats were treated with subcutaneous injections of insulin glargine and followed up for 6 months. Cats were considered in remission when euglycemia occurred for ≥4 weeks without the administration of insulin. Nonparametric tests were used for statistical analysis.
Results
In groups 1 and 2, remission was achieved in 10/15 and in 7/14 cats (P = .46), and good metabolic control was achieved in 3/5 and in 1/7 cats (P = .22), respectively. Overall, good metabolic control or remission occurred in 13/15 cats of group 1 and in 8/14 cats of group 2. In group 1, the median insulin dosage given during the 6‐month follow‐up was significantly lower than in group 2 (group 1: 0.32 IU/kg/day, group 2: 0.51 IU/kg/day; P = .013).
Conclusions and Clinical Importance
Initial intravenous infusion of insulin for tight glycemic control in cats with DM decreases insulin requirements during the subsequent 6 months.
Keywords: Endocrinology, Feline, Hyperglycemia, Pancreas, Treatment
Abbreviations
- CGMS
continuous glucose monitoring system
- DM
diabetes mellitus
- PBGM
portable blood glucose meter
Diabetes mellitus (DM) is a frequent endocrine disease in cats. Approximately 80% of diabetic cats are believed to have a form similar to type 2 diabetes in humans, which is characterized by inadequate insulin secretion and impaired insulin action.1 Current cornerstones of diabetes management in cats are twice daily injections of insulin and feeding a high‐protein and low‐carbohydrate diet. Ten to 50% of diabetic cats experience remission and no longer require exogenous insulin because of resolution of clinical signs and normalization of blood glucose and fructosamine concentrations.2, 3, 4, 5, 6, 7
A number of trials in human medicine evaluated short‐term intensive administration of insulin in the management of newly diagnosed type 2 diabetic patients.8, 9, 10, 11, 12 Treatment included either multiple daily injections or continuous subcutaneous infusion of insulin for 2–3 weeks. Early intensive insulin treatment resulted in improvement of β‐cell function and prolongation of remission.10 In addition, remission rates after 1 year were significantly higher in humans treated with intensive insulin treatment compared with those treated with oral hypoglycemic agents.10, 11, 12 The effect of intensive insulin treatment in cats needs to be explored; to date, only two studies have investigated this treatment modality using the long‐acting insulin analogs glargine and detemir.13, 14 Owners were required to follow a strict treatment protocol that included measurement of blood glucose several times per day. In both studies, remission rates of up to 64 and 67% were achieved, which might suggest that intensive insulin treatment is beneficial in cats.13, 14
Because intensive insulin treatment is associated with favorable results in human patients with type 2 diabetes and appears to be advantageous in diabetic cats, the aim of this investigation was to determine the effect of early and tight glycemic control using short‐term intravenous infusion of insulin in cats with newly diagnosed diabetes.
Materials and Methods
Animals
Cats with newly diagnosed diabetes were enrolled in the study from July 2008 to January 2011. Cats were excluded from the study if they had received insulin treatment for longer than 1 week before admission and if glucocorticoids or progestagens had been administered during the previous 4 months. All cats underwent a thorough evaluation including physical examination, complete blood cell count, serum biochemical profile, measurement of fructosamine and total T4 concentrations, urinalysis, including bacterial culture and urinary protein‐to‐creatinine ratio, blood pressure measurement, abdominal and thoracic radiographs and abdominal ultrasonography. Diabetic cats with ketoacidosis were included in the study, if acidemia resolved and their general condition improved within 48 hours of insulin treatment. Cats with concurrent diseases at diagnosis (eg, renal failure, gastrointestinal disorders, heart disease, other endocrinopathies, neoplasia) were not enrolled.
The study was approved by the Cantonal Veterinary Office of Zurich and conducted in accordance with guidelines established by the Animal Welfare Act of Switzerland (permission no.: 83/2008). Informed consent to participate in the study was provided by the owners.
Randomization and Treatments
Enrolled cases were hospitalized for 7 consecutive days. The cats were allocated to one of two treatment groups using a software1 providing a partial minimization procedure to adjust the randomization probabilities between groups and to balance for covariates collected at baseline. The covariates sex, age, body weight, treatment with insulin before admission (ie, for <1 week), presence of ketoacidosis, blood glucose, and serum fructosamine concentrations were selected.
After the initial work‐up, all cats were sedated with midazolam/butorphanol2 , 3 and anesthetized with propofol4 to place a central venous catheter5 in the jugular vein and to insert the sensor of the continuous glucose monitoring system (CGMS)6 in the subcutaneous tissue of the lateral chest wall, as described previously.15, 16, 17 The CGMS measures glucose in the subcutaneous interstitial fluid every 10 seconds and displays the 5‐minute average on the monitor. After a 2‐hour period of initialization, the first calibration was carried out; thereafter the CGMS was calibrated after 6 hours and then every 10 hours. To calibrate the CGMS, capillary blood glucose was measured with a portable blood glucose meter (PBGM),7 validated for the cat.18 In addition to CGMS measurements, capillary blood glucose concentrations were also determined with the PBGM7 every 4–6 hours to ensure reliability of the system.
Cats of group 1 received intravenous infusion of insulin for 6 consecutive days with the goal of achieving tight glycemic control by adjusting the insulin dosage as required. Insulin was infused via the central venous catheter. The insulin solution consisted of 12.5 IU rapid‐acting insulin aspart8 dissolved in 250 mL 0.9% NaCl. The solution was renewed daily, and the tube was flushed with the same solution to prevent insulin adsorption to the solid surfaces of the infusion sets.19 The initial insulin dosage used in the infusion was 0.05 IU/kg/h (equal to 1 mL/kg/h). In addition, 0.9% NaCl infusion was given concurrently through the same catheter, and the infusion rate was adjusted to maintain the total amount of intravenous fluids at 2 mL/kg/h. The cats were monitored 24 hours a day. The infusion rate of insulin was adjusted to achieve a target glucose concentration range of 90–180 mg/dL. For this purpose, the infusion rate of insulin was adjusted in steps of 0.025–0.05 IU/kg/h, every 15–30 minutes, if necessary. The infusion of insulin was discontinued at 6:00 am on day 7 of hospitalization, and at 8:00 am, the cats were started on subcutaneous injections of insulin glargine.9 To ensure that the same dosage conversion was used for each cat, an arbitrary calculation was used. The total amount of insulin infused IV on day 6 was divided by 4. This calculated dosage was administered twice daily on day 7, while the cats were still being monitored by the CGMS. The cats were discharged on day 8 with insulin injections prescribed twice daily. If glucose concentrations dropped below 72 mg/dL on day 7 or 8, the insulin dosage was reduced by 50%.
Cats of group 2 were treated during the same 7‐day period of hospitalization as cats of group 1 and received subcutaneous injections of insulin glargine9; tight glycemic control was not a goal in this group. The insulin dosage was 0.5–1.0 IU, q12h, in cats weighing <4 kg and 1.5–2.0 IU, q12h, in cats >4 kg. In addition, all cats received an infusion of 0.9% NaCl via the central venous catheter at a rate of 2 mL/kg/h. The subcutaneous dosage of insulin was not adjusted during the 7‐day period unless the blood glucose concentration dropped below 72 mg/dL, in which case the insulin dosage was reduced by 50%. The insulin dosage was increased by 0.5 IU per cat, q12h, if hyperglycemia persisted after the 7 days of hospitalization.
All cats of both groups were fed a high‐protein, low‐carbohydrate diet10 during hospitalization and after discharge from the clinic. The study was carried out by two internal medicine residents under the supervision of two board‐certified specialists in internal medicine. Cats were continuously monitored during the 7 days.
Analyses During Hospitalization
To calculate the duration of time that glucose was within and outside the target range in cats of group 1, the glucose concentrations recorded by the CGMS and calibrated with the PBGM were divided into the following ranges: hypoglycemia (<90 mg/dL), target range (90–180 mg/dL) and hyperglycemia (mild: 181–270 mg/dL; and moderate to severe: >270 mg/dL). This calculation included all glucose values recorded with the CGMS starting 12 hours after initiation of the insulin infusion (group 1) or after the first insulin injection (group 2), until day 7 at 6:00 am. The percentage of glucose measurements within each glycemic range was calculated for each cat in both groups. On day 8, a blood sample was collected for determination of a complete blood cell count, serum biochemical profile, and fructosamine concentration in all cats. The central venous catheter was then removed, and bacterial culture of the catheter tip was carried out.
Serum potassium and phosphorus concentrations were measured at admission and discharge in all cats. At admission, if hypokalemia or hypophosphatemia was documented, supplementation was provided with potassium chloride or phosphate diluted in the 0.9% NaCl infusion and electrolytes were measured every 2–6 hours until the concentration normalized. During hospitalization, serum potassium, and phosphorus concentrations were measured only if clinical signs compatible with electrolyte abnormalities were documented.
Follow‐up
Re‐evaluations were scheduled 1, 2–3, 6–8, 12–16, and 24 weeks after discharge from the clinic and were carried out by the same internal medicine residents who cared for the cats during the first week of hospitalization. The insulin dosage was adjusted based on clinical signs and the results of physical examination, blood glucose curves and fructosamine levels1; the goal was to resolve clinical signs of DM and to maintain blood glucose curves between 90 and 270 mg/dL and fructosamine concentrations <400 μmol/L. To exclude the development of concurrent diseases, diagnostic tests including a complete blood cell count, serum biochemical profile, fructosamine concentration, urinalysis, urinary protein‐to‐creatinine ratio, and blood pressure measurement were done at each re‐evaluation. Abdominal ultrasonography and total T4 measurement were carried out 6–8 weeks and 24 weeks after hospitalization. A dexamethasone suppression test was done 6–8 weeks after discharge from the clinic to rule out hyperadrenocorticism. Remission of diabetes was defined as absence of signs of DM (eg, polyuria/polydipsia, polyphagia) with normal blood glucose (72–162 mg/dL) and fructosamine concentrations (<340 μmol/L) for at least 4 weeks after discontinuation of the insulin injections.1, 4, 5 In cats in which remission occurred before insulin administration had been discontinued, the dosage was decreased in increments of 0.5 IU per dosage, once weekly. The last dosage before insulin was discontinued was 0.5 UI once daily, for at least 1 week. The onset and duration of remission were recorded. Good metabolic control was defined as absence of clinical signs of DM, fructosamine concentrations <400 μmol/L and blood glucose curve measurements ranging from 90 to 270 mg/dL.1 In both groups, the median insulin dosage per kg per day was calculated over the 6‐month study period; phases of remission were excluded from the calculation.
Statistical Analysis
Data are presented as median and ranges. Differences in rate of remission and rate of good metabolic control between groups were analyzed using Fisher's exact test. Differences in laboratory results, blood pressure measurements, glucose measurements within each glycemic range during hospitalization, onset of remission and daily insulin dosage given over the 6‐month study period between groups were analyzed using the Mann–Whitney U‐test. A commercial software11 was used for all analyses. The level of significance was set at P < .05.
Results
Animals
A total of 51 cats with newly diagnosed DM were admitted to our clinic during the study period. Thirty cats fulfilled the inclusion criteria and were enrolled in the study; each of the 2 groups consisted of 15 cats. In group 1, the median age was 11.0 years (range: 7.0–15.0) and median body weight was 5.8 kg (range: 3.4–9.0). Eleven were domestic shorthair or longhair cats, and 4 were purebred cats (Abyssinian, Burmese, Siamese and Norwegian forest cat). Nine cats were neutered males and six were spayed females. In group 2, the median age was 11.0 years (range: 8.0–17.0) and median body weight was 4.7 kg (range: 2.5–9.6). Fourteen were domestic shorthair or longhair cats and one was a Ragdoll cat. Eight cats were neutered males and seven were spayed females. There was no significant difference in sex, age, or bodyweight between the two groups. At the time of initial presentation, 2 of the cats enrolled in the study had ketoacidosis, which resolved within 1 day of intramuscular injections of insulin; one was allocated to group 1 and the other to group 2. In group 2, 1 cat died of pyelonephritis (3 months after admission) and another died of alimentary lymphoma (3 days before the end of the study). Only cats that were alive for the duration of the study were included in the analysis; the cat that died 3 months after admission was excluded.
Laboratory Results and Blood Pressure Measurement on Admission
In group 1, the median serum glucose concentration was 439 mg/dL (range: 203–581) and the median fructosamine concentration was 615 μmol/L (range: 528–783). In group 2, the median serum glucose concentration was 425 mg/dL (range: 248–560) and the median fructosamine concentration was 575 μmol/L (range: 449–778). The laboratory results and blood pressure measurements at the time of admission are shown in Table 1. There were no significant differences between groups.
Table 1.
Laboratory results and blood pressure measurements in cats on initial intensive intravenous infusion of insulin with tight glycemic control achieved by insulin dosage adjustment (group 1) and in cats started on subcutaneous injections of insulin without the goal of initial tight glycemic control (group 2)
| Parameter | Unit | Group 1 | Group 2 | Reference Range | ||
|---|---|---|---|---|---|---|
| Median | Range | Median | Range | |||
| Hematocrit | % | 39 | 31–49 | 38 | 23–42 | 33–45 |
| Leukocytes | 103/μL | 13.3 | 5.8–27.0 | 8.7 | 5.0–24.0 | 4.6–12.8 |
| Platelets | 103/μL | 306 | 192–594 | 322 | 194–649 | 180–680 |
| Glucose | mg/dL | 439.6 | 203.6–582.0 | 425.2 | 248.6–560.4 | 72–162 |
| Fructosamine | μmol/L | 615 | 528–783 | 575 | 449–778 | 202–340 |
| Cholesterol | mg/dL | 220.1 | 158.3–359.1 | 251.0 | 169.9–393.8 | 101–263 |
| Triglyceride | mg/dL | 141.6 | 35.4–557.5 | 119.5 | 44.2–2,247.8 | 26.5–115.0 |
| Total proteins | g/dL | 7.7 | 7.0–9.1 | 7.4 | 5.8–8.5 | 6.4–8.0 |
| Albumin | g/dL | 3.4 | 2.9–4.1 | 3.5 | 2.6–3.9 | 3.0–4.0 |
| Urea | mg/dL | 27.7 | 16.0–35.3 | 10.3 | 12.3–15.6 | 20.7–35.3 |
| Creatinine | mg/dL | 1.2 | 0.8–2.0 | 1.1 | 0.7–1.6 | 1.1–1.8 |
| Sodium | mEq/L | 159 | 151–163 | 160 | 155–166 | 158–165 |
| Chloride | mEq/L | 113 | 103–121 | 115 | 109–119 | 121–131 |
| Potassium | mEq/L | 4.9 | 3.3–5.7 | 4.7 | 3.7–6.5 | 3.8–5.4 |
| Phosphorus | mg/dL | 3.8 | 1.8–6.2 | 3.7 | 2.0–5.6 | 2.8–5.6 |
| Calcium | mg/dL | 10.5 | 9.2–12.4 | 9.9 | 7.9–11.2 | 9.6–11.2 |
| Bilirubin | mg/dL | 0.08 | 0.02–0.22 | 0.08 | 0.02–0.16 | 0.1–0.4 |
| Lipase | U/L | 24 | 14–75 | 26 | 15–75 | 8–26 |
| ALP | U/L | 65 | 37–92 | 61 | 14–111 | 16–43 |
| ASAT | U/L | 34 | 21–91 | 36 | 15–137 | 19–44 |
| ALAT | U/L | 92 | 45–326 | 61 | 32–235 | 34–98 |
| Urine ketone bodies | mg/dL | 0 | 0–40 | 0 | 0–15 | 0 |
| UPC | 0.41 | 0.02–1.64 | 0.24 | 0.03–2.82 | ≤0.40 | |
| Basal thyroxin | μg/dL | 1.5 | 0.5–2.5 | 1.0 | 0.5–2.5 | <3.5 |
| Systolic blood pressure | mmHg | 129 | 90–162 | 133 | 110–175 | <160 |
ALAT, alanine aminotransferase; ALP, alkaline phosphatase; ASAT, aspartate aminotransferase; UPC, urine protein‐to‐creatinine ratio.
Intensive Intravenous Infusion of Insulin
In group 1, blood glucose concentrations decreased to the target range within 12 hours of initiation of treatment in 13 of 15 cats. In those cats, it was necessary to reduce the insulin infusion rate to 0.02–0.03 IU/kg/h to avoid hypoglycemia. In 2 obese cats with a body condition score12 of 9 of 9, the initial insulin infusion rate was not sufficient to achieve the target glucose concentration within 12 hours and it was therefore increased to 0.07 IU/kg/h. Within 24 hours, all 15 cats had blood glucose concentrations within the target range. In 2/15 cats of group 1, the glucose concentration decreased below 72 mg/dL during the 6‐day infusion, and the insulin infusion was stopped temporarily. On day 7, 2 of the cats received 0.5 IU, 8 received 1 IU, 4 received 2 IU and 1 received 3 IU of insulin injected SC, q12h. Bacterial culture of the tip of the jugular catheter after removal revealed bacterial growth in 2 cats; one culture yielded Pseudomonas aeruginosa and the other Enterococcus spp.
Subcutaneous Injections of Insulin
In group 2, nine of the 15 cats weighed <4 kg; the initial dosage of insulin injected SC was 0.5 IU, q12h, in 1 cat and 1.0 IU, q12h, in the other 8. Of the 6 cats that weighed >4 kg, 4 received an initial dosage of insulin injected SC of 1.5 IU, q12h, and 2 received 2.0 IU, q12h. In 4 of the 15 cats of group 2, glucose concentrations decreased to the target range within 12 hours. During hospitalization, 4 of the 15 cats of group 2 had glucose concentrations <72 mg/dL, which necessitated a 50% decrease in the insulin dosage for the remainder of the study; the dosage was reduced after a median of 2.6 days (range: 0–4). One of those 4 cats had normoglycemia at the time of discharge (day 8), and insulin was therefore not prescribed. In 6 of the 15 cats, the insulin dosage was increased at the time of discharge because the amount given during hospitalization was considered insufficient for adequate glycemic control; the insulin dosage was increased to 1 IU in one cat, to 1.5 IU in two cats and to 2 IU in the remaining three cats, administered SC, q12h. Bacterial culture of the tip of the jugular catheter after removal revealed bacterial growth of Pantoea agglomerans in one cat and Acinetobacter spp. in one other.
Analyses During Hospitalization and Costs
To determine whether short‐term intravenous infusion of insulin maintained blood glucose levels within the target range during hospitalization, the percentage of glucose measurements that fell into different concentration ranges was compared with those of cats that were started on subcutaneous injections of insulin without aiming at tight glycemic control. The percentage of glucose measurements for cats within the target range of 90–180 mg/dL was significantly higher for cats in group 1 than in group 2 (group 1: median 59% [range: 15–96]; group 2: median 16% [range: 0–65]; P = .001). The percentage of glucose measurements with moderate to severe hyperglycemia (>270 mg/dL) was significantly lower for cats in group 1 than in group 2 (group 1: median 5% [range: 0–40]; group 2: median 42% [range: 0–100]; P = .004). The percentage of glucose measurements for each cat within the range of mild hyperglycemia (181–270 mg/dL) (group 1: median 27% [range: 0–45]; group 2: median 20% [range: 0–83]) and of hypoglycemia (<90 mg/dL) (group 1: median 0% [range: 0–30]; group 2: median 0% [range: 0–40]) did not differ significantly between groups.
In both groups, median fructosamine concentrations decreased significantly after 1 week of hospitalization (admission versus discharge: group 1, 615 μmol/L [range: 528–783] versus 402 μmol/L [range: 322–528], P < .0001; group 2, 575 μmol/L [range: 449–778] versus 441 μmol/L [range: 351–527], P < .001). Fructosamine concentrations at discharge did not differ significantly between groups.
On admission, 3 cats presented with mild hypokalemia (range: 3.3–3.7 mEq/L; reference range: 3.8–5.4) and 5 with mild hyperkalemia (range: 5.5–6.5 mEq/L), 2 cats presented with mild hypophosphatemia (1.8 and 2.0 mg/dL, respectively; reference range: 2.8–5.6), and one with mild hyperphosphatemia (6.2 mg/dL). Abnormal serum potassium or phosphorus concentrations normalized within 24 hours from admission; potassium chloride or phosphate supplementation was provided in cats with hypokalemia or hypophosphatemia, respectively. None developed clinical signs suggestive of electrolyte imbalance during the period of hospitalization. At discharge, all cats had normal serum potassium concentrations and all but one had normal phosphorus. The cat with abnormal serum phosphorus had very mild hyperphosphatemia (5.8 mg/dL). There were no differences between groups at admission and discharge for either electrolyte. The cost of the 6‐day intensive intravenous infusion of insulin was approximately US$1,700 per cat.
Diabetic Remission and Metabolic Control During Follow‐up
The remission occurred in 10/15 in group 1 and in 7/14 in group 2, P = .462. Of the cats that went into remission, relapse occurred 4 weeks after insulin had been discontinued in one cat of group 1 and 7 weeks after discontinuation of insulin in one cat of group 2. The remaining cats were in remission until the end of the study. In the majority of all cats, remission occurred within 16 weeks of discharge (Table 2). The onset of remission did not differ between groups.
Table 2.
Onset of remission in cats started on initial intensive intravenous infusion of insulin with tight glycemic control achieved by insulin dosage adjustment (group 1) and in cats started on subcutaneous injections of insulin without the goal of initial tight glycemic control (group 2)
| Onset of Remission | Group 1 (Number of Cats) | Group 2 (Number of Cats) |
|---|---|---|
| At discharge | 0 | 1 |
| ≤4 weeks after discharge | 2 | 3 |
| 5–6 weeks after discharge | 2 | 0 |
| 7–16 weeks after discharge | 5 | 2 |
| >16 weeks after discharge | 1 | 1 |
Good metabolic control was obtained in 3/5 cats that did not achieve remission in group 1, and in 1/7 cats that did not achieve remission in group 2 (P = .222). Overall, good metabolic control or remission was achieved in 13/15 cats in group 1 and in 8/14 cats in group 2. During the 6‐month follow‐up period, the median insulin dosage was significantly lower in group 1 cats compared with group 2 (group 1: 0.32 IU/kg/day [range: 0.13–0.53]; group 2: 0.51 IU/kg/day [range: 0.05–1.52]; P = .013) (Fig 1).
Figure 1.
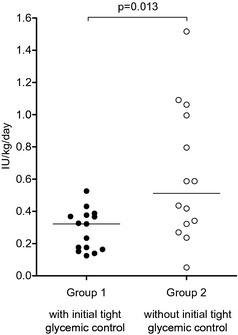
Dot plots of median insulin dosage administered per kg body weight per day for each cat during the 6‐month study period in cats on initial intensive intravenous infusion of insulin with tight glycemic control achieved by insulin dosage adjustment (group 1) and in cats started on subcutaneous injections of insulin without the goal of initial tight glycemic control (group 2). The horizontal lines mark the medians for each group.
During the 6‐month follow‐up period, 9 cats developed concurrent diseases, including chronic kidney disease (n = 4, 3 in group 1, 1 in group 2), hyperthyroidism (n = 2, 1 in each group), acute pancreatitis and hyperadrenocorticism (1 in group 2), pyelonephritis (1 in group 2), and alimentary lymphoma (1 in group 2).
Discussion
Intravenous infusion of insulin was effective in quickly reducing glucose concentrations to within the target range, and compared with subcutaneous injections of insulin, prevented long periods of moderate to severe hyperglycemia (ie, >270 mg/dL). Glucose concentrations were rarely below the reference range, and episodes of clinical hypoglycemia did not occur. These findings support the conclusion that our method of intensive intravenous infusion of insulin can be safely used to rapidly decrease hyperglycemia without adverse effects. In previous studies, we demonstrated that CGMS is clinically accurate for generating 12‐ and 24‐hour glucose curves in diabetic cats.15, 16 The results of the present study showed that a CGMS is a valuable tool for long‐term monitoring of glucose curves (ie, 1 week) in hospitalized cats and allows fine‐tuning of the insulin dosage.
Remission occurred in 10/15 cats initially treated with intensive intravenous infusion of insulin compared to 7/14 cats treated with subcutaneous injections of insulin. When cats that went into remission were excluded, good glycemic control was achieved in 3/5 cats that had been initially treated with intensive intravenous infusion of insulin and in 1/7 cats treated with subcutaneous injections of insulin. However, differences between the 2 groups were not significant, possibly because of the relatively low number of cats in the study. It was interesting to note that cats started on intensive intravenous infusion of insulin required significantly less insulin than cats started with subcutaneous injections of insulin (approximately 40% less, based on differences between median values). Roomp and Rand carried out two intensive glucose monitoring studies in cats, one with insulin glargine and the other with insulin detemir.13, 14 In both investigations, the aim was to achieve tight glycemic control by frequent monitoring of blood glucose concentration and adjustment of insulin dosage. Owners were asked to measure blood glucose concentrations of their diabetic cats at least three times daily at home and to adjust the insulin dosage with the help of a web‐based protocol. The rate of remission was 64% in diabetic cats treated with insulin glargine and 67% in diabetic cats treated with insulin detemir.13, 14 Their rates of remission were similar to our results in cats started on intensive intravenous infusion of insulin. These results suggest that strict blood glucose monitoring and frequent insulin dosage adjustments are as effective as intensive intravenous infusion of insulin. However, the study design of those previous investigations differed substantially from that of the present study making direct comparison difficult. In the study by Roomp and Rand, owners of cats with DM were responsible for maintaining tight glycemic control,13 whereas in our study, veterinarians were in charge of insulin dosage adjustments. One would expect that treatment decisions made by veterinarians in a hospital setting are more consistent than those made by owners at home. In addition, it should be noted that in contrast to our study, a substantial percentage of the cats included in the study by Roomp and Rand had been treated with glucocorticoids before being diagnosed with DM.13 In our study, cats with prior steroid treatment were excluded. The chance of remission in cats with glucocorticoid‐induced DM is good,13 which may explain the slightly higher remission rate found in the glargine‐treated cats in their study compared with the cats of group 2, in which initial tight glycemic control was not a goal. Of note, by excluding glucocorticoid‐treated cats from that study,13 the remission rate would have decreased from 64% to 51%, yielding a remission rate similar to our group 2 cats as well as to other cats started on subcutaneous injections of insulin without initial tight glycemic control.5
Clinical studies in human medicine demonstrated that initial intensive infusion of insulin has positive effects on metabolic control in patients with newly diagnosed type 2 diabetes compared with oral antidiabetic drug treatment.10, 11, 12 This beneficial effect was attributable to improved β‐cell function, evidence of which was seen as augmented glucose stimulated first‐phase insulin secretion after intensive infusion of insulin.12 The mechanisms involved in improved β‐cell function after intensive insulin treatment in humans include reversal of glucotoxicity and lipotoxicity, reduction in islet inflammation, or both, all of which contribute to loss of β‐cell function and mass.20, 21, 22 Glucotoxicity is considered to be the major cause of loss of β‐cell function and mass.22 Similar to findings in humans, hyperglycemia has been shown to induce early and severe dysfunction and loss of β cells via apoptosis in healthy cats.23, 24 Thus, eliminating the detrimental effects of hyperglycemia using initial intensive infusion of insulin may improve β‐cell viability in diabetic cats, ultimately decreasing insulin requirements for the maintenance of glycemic control. Evidence of this in the present study was the lower median insulin dosages required by cats in group 1.
This study had some limitations including the relatively low number of cats; studying a larger number of cats may or may not have shown significant differences in rates of remission or good metabolic control. The follow‐up period was set at 6 months and it is possible that this period was too short for remission to occur in some of the cats. However, based on an earlier study from our clinic, more than 90% of cats with newly diagnosed diabetes achieved remission within 6 months,5 and therefore the duration of the follow‐up period presumably had little effect on the remission rate. The central venous catheters from 4 cats yielded positive bacteriological cultures, but because none of these cats had related clinical or laboratory abnormalities, it was assumed that the adverse effect of the recovered microorganisms was negligible. Finally, fine‐tuning of the insulin dosage to maintain blood glucose levels within the target range was difficult in some cats; concentrations above the target range occurred despite strict 24‐hour monitoring. It is possible that in some cats this interfered with the beneficial effects of intensive intravenous infusion on β cells.
In summary, cats that initially received intensive intravenous infusion of insulin with tight glycemic control had significantly decreased insulin requirements in the 6‐month follow‐up period. Remission rate and metabolic control did not differ significantly between the two groups, possibly because of the small number of cases. Intensive intravenous infusion of insulin is a demanding treatment, and owner compliance may not be satisfactory because of the high cost and the relatively long hospitalization period.
Acknowledgment
The study is partly supported by a grant from the Policlinico di Monza, Italy.
Conflict of Interest Disclosure: The authors disclose no conflict of interest.
Footnotes
Minim, Altman DG, Bland JM, http://www.sghms.ac.uk/depts/phs/guide/randser.htm
Dormicum; Roche Pharma, Reinach, Switzerland
Morphasol; Dr E. Gräub, Bern, Switzerland
Propofol 1% MCT; Fresenius Kabi, Stans, Switzerland
Certofix Mono Paed S, B. Braun, Melsungen, Switzerland
Guardian REAL‐Time continuous glucose monitoring system; Medtronic, Munchenbuchsee, Switzerland
AlphaTRAK portable blood glucose meter; Abbott Animal Health, Maidenhead, UK
NovoRapid; Novo Nordisk Pharma, Kusnacht, Switzerland
Lantus; Sanofi Aventis, Meyrin, Switzerland
DM Purina Veterinary Diets, Medical solution, Steinhausen, Switzerland
GraphPad Prism version 4.0; GraphPad Software, La Jolla, CA
Body condition chart for the cat; Nestlé Purina PetCare Research, St. Louis, MO
References
- 1. Reusch CE. Feline diabetes mellitus In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 7th ed St. Louis, MO: Saunders; 2010:1796–1816. [Google Scholar]
- 2. Nelson RW, Griffey SM, Feldman EC, et al. Transient clinical diabetes mellitus in cats: 10 cases (1989–1991). J Vet Intern Med 1999;13:28–35. [PubMed] [Google Scholar]
- 3. Bennett N, Greco DS, Peterson ME, et al. Comparison of a low carbohydrate‐low fiber diet and moderate carbohydrate‐high fiber diet in the management of feline diabetes mellitus. J Feline Med Surg 2006;8:73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sieber‐Ruckstuhl NS, Kley S, Tschuor F, et al. Remission of diabetes mellitus in cats with diabetic ketoacidosis. J Vet Intern Med 2008;22:1326–1332. [DOI] [PubMed] [Google Scholar]
- 5. Zini E, Hafner M, Osto M, et al. Predictors of clinical remission in cats with diabetes mellitus. J Vet Intern Med 2010;24:1314–1321. [DOI] [PubMed] [Google Scholar]
- 6. Smith JR, Vrono Z, Rapoport GS, et al. A survey of southeastern United States veterinarians' preferences for managing cats with diabetes mellitus. J Feline Med Surg 2012;14:716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Callegari C, Mercuriali E, Hafner M, et al. Survival time and prognostic factors in cats with newly diagnosed diabetes mellitus: 114 cases (2000–2009). J Am Vet Med Assoc 2013;243:91–95. [DOI] [PubMed] [Google Scholar]
- 8. Ilkova H, Glaser B, Tunckale A, et al. Induction of long‐term glycemic control in newly diagnosed type 2 diabetic patients by transient intensive insulin treatment. Diabetes Care 1997;20:1353–1356. [DOI] [PubMed] [Google Scholar]
- 9. Ryan EA, Imes S, Wallace C. Short‐term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care 2004;27:1028–1032. [DOI] [PubMed] [Google Scholar]
- 10. Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on β‐cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: A multicenter randomised parallel‐group trial. Lancet 2008;371:1753–1760. [DOI] [PubMed] [Google Scholar]
- 11. Alvarsson M, Sundkvist G, Lager I, et al. Beneficial effects of insulin versus sulphonylurea on insulin secretion and metabolic control in recently diagnosed type 2 diabetic patients. Diabetes Care 2003;26:2231–2237. [DOI] [PubMed] [Google Scholar]
- 12. Chen HS, Wu TE, Jap TS, et al. Beneficial effects of insulin on glycemic control and β‐cell function in newly diagnosed type 2 diabetes with severe hyperglycemia after short‐term intensive insulin therapy. Diabetes Care 2008;31:1927–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roomp K, Rand J. Intensive blood glucose control is safe and effective in diabetic cats using home monitoring and treatment with glargine. J Feline Med Surg 2009;11:668–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roomp K, Rand J. Evaluation of detemir in diabetic cats managed with a protocol for intensive blood glucose control. J Feline Med Surg 2012;14:566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moretti S, Tschuor F, Osto M, et al. Evaluation of a novel real‐time continuous glucose‐monitoring system for use in cats. J Vet Intern Med 2010;24:120–126. [DOI] [PubMed] [Google Scholar]
- 16. Dietiker‐Moretti S, Müller C, Sieber‐Ruckstuhl N, et al. Comparison of a continuous glucose monitoring system with a portable blood glucose meter to determine insulin doses in cats with diabetes mellitus. J Vet Intern Med 2011;25:1084–1088. [DOI] [PubMed] [Google Scholar]
- 17. Hafner M, Lutz TA, Reusch CE, et al. Evaluation of sensor sites for continuous glucose monitoring in cats with diabetes mellitus. J Feline Med Surg 2013;15:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zini E, Moretti S, Tschuor F, et al. Evaluation of new portable glucose meter designed for the use in cats. Schweiz Arch Tierheilk 2009;151:448–451. [DOI] [PubMed] [Google Scholar]
- 19. Peterson L, Caldwell J, Hoffman J. Insulin adsorbance to polyvinylchloride surfaces with implications for constant‐infusion therapy. Diabetes 1976;25:72–74. [DOI] [PubMed] [Google Scholar]
- 20. Maedler K, Schulthess FT, Bielman C, et al. Glucose and leptin induce apoptosis in human beta‐cells and impair glucose‐stimulated insulin secretion through activation of c‐Jun N‐terminal kinases. FASEB J 2008;22:1905–1913. [DOI] [PubMed] [Google Scholar]
- 21. Maedler K, Spinas GA, Dyntar D, et al. Distinct effects of saturated and monounsaturated fatty acids on beta‐cell turnover and function. Diabetes 2001;50:69–76. [DOI] [PubMed] [Google Scholar]
- 22. Tomas E, Lin YS, Dagher Z, et al. Hyperglycemia and insulin resistance: Possible mechanisms. Ann N Y Acad Sci 2002;967:43–51. [DOI] [PubMed] [Google Scholar]
- 23. Zini E, Osto M, Franchini M, et al. Hyperglycaemia but not hyperlipidaemia causes beta cell dysfunction and beta cell loss in the domestic cat. Diabetologia 2009;52:336–346. [DOI] [PubMed] [Google Scholar]
- 24. Link KRJ, Allio I, Rand JS, et al. The effect of experimentally induced chronic hyperglycaemia on serum and pancreatic insulin, pancreatic islet IGF‐I and plasma and urinary ketones in the domestic cat (Felis felis). Gen Comp Endocrinol 2013;188:269–281. [DOI] [PubMed] [Google Scholar]


