Abstract
Background
The use of cardiac biomarkers to assist in the diagnosis of occult and symptomatic hypertrophic cardiomyopathy (HCM) in cats has been established. There is limited data describing their prognostic utility in cats with HCM.
Hypothesis
Circulating concentrations of N‐terminal B‐type natriuretic peptide (NTproBNP) and cardiac troponin I (cTnI) predict cardiac death in cats with HCM.
Animals
Forty‐one cats diagnosed with HCM at a veterinary teaching hospital, between February 2010 and May 2011.
Methods
Prospective investigational study. Plasma samples were collected from cats diagnosed with HCM and concentrations of NTproBNP and cTnI were analyzed at a commercial laboratory. Echocardiographic measurements from the day of blood sampling were recorded. Long‐term outcome data were obtained. Associations with time to cardiac death were analyzed using Cox proportional hazards models.
Results
When controlling for the presence/absence of heart failure and echocardiographic measures of left atrial size and function, cTnI > 0.7 ng/mL was independently associated with time to cardiac death. In univariable analysis, NTproBNP > 250 pmol/L was associated with cardiac death (P = .023), but this did not remain significant (P = .951) when controlling for the effect of clinical signs or left atrial size/function.
Conclusions and Clinical Importance
Plasma concentration of cTnI (cutoff >0.7 ng/mL) is a predictor of cardiac death in cats with HCM that is independent of the presence of heart failure or left atrial dilatation.
Keywords: Biomarkers, Cardiology, Cardiovascular, Monitoring, Outcome
Abbreviations
- ATE
arterial thromboembolism
- CHF
congestive heart failure
- cTnI
cardiac troponin I
- HCM
hypertrophic cardiomyopathy
- HR
heart rate
- LA : Ao
ratio of left atrium to aortic root diameter (short‐axis)
- LAD
maximum left atrial diameter (long‐axis)
- LAEF
left atrial ejection fraction
- LAFS
left atrial fractional shortening
- LA
left atrial/atrium
- LVIDd
left ventricular internal diameter in diastole
- LV
left ventricular
- MST
median survival time
- NTproBNP
N‐terminal pro‐B type natriuretic peptide
- RR
respiratory rate
- SAM
systolic anterior motion of the mitral valve
- SBP
systolic blood pressure (Doppler)
- T4
total thyroxine
Hypertrophic cardiomyopathy (HCM) is the most common cardiac disease in cats.1, 2, 3, 4, 5, 6 It also affects 1 in 500 people and is the most frequent cause of cardiac death in young adults.7 Prognostic indicators have been identified in both humans and cats.4, 8, 9, 10 In humans, independent predictors of death because of cardiac disease include family history of cardiac death, nonsustained ventricular tachycardia, severe hypertrophy (≥30 mm) of the left ventricular (LV) wall, LV outflow tract obstruction, late gadolinium enhancement on magnetic resonance imaging and younger age at diagnosis.11, 12, 13 Poor prognostic indicators for cats with HCM include arterial thromboembolism (ATE), congestive heart failure (CHF), increased left atrial (LA) size, decreased LA and LV systolic function, and LV hypertrophy ≥9 mm.4, 10, 14, 15
The use of circulating cardiac troponin I (cTnI) and N‐terminal pro‐B‐type natriuretic peptide (NTproBNP) concentrations to assist diagnosis, guide treatment, and screen individuals for HCM has recently received much attention in human and veterinary cardiology research.16, 17, 18, 19, 20, 21, 22, 23, 24, 25 However, little information is available on the prognostic value of circulating biomarker concentrations in HCM. A single measurement of NTproBNP, cardiac troponin, or a combination of both, act as independent predictors of adverse cardiovascular events, including death because of cardiac disease, in human patients with HCM.26, 27, 28, 29 One study in cats with heart failure suggested that increased NTproBNP concentrations predicted survival to 1 month after diagnosis of CHF,1 while another recent study suggested that troponins I and T are associated with cardiac death in pedigree cats with HCM.30
The primary aim of this study was to determine if a single measurement of circulating NTproBNP, cTnI, or both, in cats of any breed diagnosed with HCM could provide useful prognostic information. We hypothesized that increases in both NTproBNP and cTnI would be significantly associated with reduced time to cardiac death.
Materials and Methods
Animals
Cats presented to a veterinary teaching hospital2 for investigation of heart disease from February 2010 to May 2011 were eligible for prospective enrollment. The inclusion criteria were a diagnosis of HCM on 2‐dimensional (2D) echocardiography and a blood sample taken within 24 hours of presentation, from which residual EDTA plasma was available. Echocardiography was performed by a Diplomate in Cardiology or Resident under supervision. Exclusion criteria were any diagnosis other than HCM; chronic kidney disease (IRIS Stage >II) or other significant systemic disease; cats ≥8 years old without a recorded blood pressure or where total thyroxine (T4) concentration was unavailable. In addition, cats with other possible causes of LV hypertrophy such as hyperthyroidism, hypertension, or clinical evidence of hypovolemia (“pseudohypertrophy”) were excluded.
Clinical Data
At presentation age, sex, breed, heart rate (HR), respiratory rate (RR), weight, and systolic blood pressure (SBP) were recorded. Echocardiographic measurements were performed at the time of presentation to the clinic and later reviewed by a single cardiologist (DJC). A maximum diastolic LV free wall or interventricular septum measurement of ≥6 mm was considered diagnostic for HCM.1, 6 The ratio of LA diameter to aortic root (LA : Ao) was measured from a 2D short axis view at the heart base, optimized for the LA and aortic valve, in the frame before aortic valve opening (end‐ventricular diastole) using an inner edge to inner edge technique.31 Maximum LA diameter (LAD) was measured from the 2D right parasternal long‐axis view (RPLA), in the frame before mitral valve opening (end‐ventricular systole).32 LV internal diameter in diastole (LVIDd) was measured in 2D from the RPLA, in the first frame in which the mitral valve was closed. M‐mode measurements LV fractional shortening (LVFS) and LA fractional shortening (LAFS) were made using a leading edge to leading edge method.10, 33, 34 LA ejection fraction (LAEF) was measured in the 2D RPLA, optimized for the LA, using a modified Simpson's method.10, 33, 34 Transmitral flow velocities (E and A waves) were recorded from pulsed wave Doppler interrogation of diastolic mitral inflow in the left‐apical 4‐chamber view.35 Waves were considered summated at an E‐at‐A intersection velocity >0.25 m/s. The presence of mitral systolic cranial motion, regional LV wall hypokinesis, and restrictive mitral inflow pattern (E : A ratio > 2, nonsummated36) were recorded as categorical variables (yes/no). Regional hypokinesis was defined as localized subjective lack of systolic excursion with thinning of a segment of LV wall.10
Congestive heart failure was defined as present if the cat had radiographic or ultrasonographic evidence of cardiogenic pulmonary edema or pleural effusion in the presence of LA dilatation (LA : Ao ≥ 1.5,31 LAD ≥ 16 mm,32 or both), or tachypnea responsive to furosemide in the presence of LA dilatation (LA : Ao ≥ 1.5,31 LAD ≥ 16 mm,32 or both). If another potential cause of pleural effusion (eg, intrathoracic neoplasia) was present, the cat was excluded from the study.
Cardiac Biomarkers
Residual EDTA plasma samples were obtained after jugular venipuncture for clinical purposes. Samples were processed within 20 minutes of collection. Plasma was separated by centrifugation at 800 × g for 3 minutes, immediately before storage at −80°C. After transport on dry ice to a commercial veterinary laboratory,3 batch analysis of NTproBNP and cTnI (using a high sensitivity assay4 ) was performed. The lower limit of detection was 24 pmol/L for the NTproBNP assay37 and 0.01 ng/mL for the cTnI assay. For the purposes of statistical analysis, results below these limits were recorded as 24 pmol/L and 0.01 ng/mL respectively.
Outcome
The end of the study period was September 1, 2013. Owners of cats were contacted annually, according to a protocol approved by the institutional ethics committee. Owners were sent a letter and questionnaire, followed by a telephone call if no response was received within 30 days. Primary veterinarians were contacted regarding the circumstances of death if euthanasia was performed. The end‐point of the study was death, categorized as death because of cardiac disease or noncardiac causes. Cardiac causes included death or euthanasia because of clinical signs of CHF or ATE, or sudden death unrelated to known systemic disease or trauma. Noncardiac causes were death or euthanasia after clinical signs not attributable to heart disease (eg, vomiting, diarrhea, trauma, or diagnosed neoplasia).
Statistical Analysis
Statistical analysis was performed using commercially available software.5 For continuous data, normality was assessed graphically and using a Shapiro–Wilk test. Normally distributed continuous data (represented mean ± SD) were compared using an independent samples t‐test and nonnormally distributed data (represented median and range) were compared using a Mann–Whitney U‐test. To look for linear correlation between two continuous variables, Spearman's rank correlation (ρs) was used.
The end‐point for survival analysis was death because of cardiac disease. Animals dying of noncardiac disease or alive at the end of the study period were right‐censored for survival analysis. Univariable time‐to‐event models were constructed using Cox proportional hazards analysis. Continuous variables were initially analyzed as quintiles (cTnI) or quartiles (all others) using Kaplan–Meier curves and pairwise Log‐rank tests. Variables where hazard increased ordinally were eligible for inclusion in time‐to‐event analysis as continuous variables. Where one or more groups had disproportionately different hazards to the others, these variables were presumed to exhibit a threshold effect, and were included in Cox models as categorical variables using a cutoff established by the exceptional quartile/quintile.
Multivariable analysis was performed in a forwards stepwise manner, to investigate the association of cardiac biomarkers with time to death because of cardiac disease. Variables significant at P < .2 at the univariable level were carried forward to multivariable Cox analysis. The assumptions of Cox proportional hazards were tested using log cumulative hazard plots and Schoenfeld residuals, and overall model fit was evaluated using Cox Snells. Where variables showing statistically significant correlation were included in a model, a variance inflation factor (VIF) were calculated for each pair of correlated variables. Colinearity was considered insignificant for the purposes of Cox multivariable analysis if the VIF was <10. Aikaike's information criteria were calculated for each of the final multivariable Cox models to assess ability of the overall model to explain difference in time to cardiac death. Statistical significance was set at P < .05.
Results
Population
Fifty‐nine cats were eligible for inclusion. Eighteen were excluded because of hyperthyroidism (2), non‐HCM cardiac disease (8), or no diagnosis of heart disease (8), leaving 41 cats that met the inclusion criteria. Population characteristics, clinical data and echocardiographic measurements are summarized in Table 1.
Table 1.
Population characteristics, clinical data, and echocardiographic findings of the 41 cats enrolled in the study
| Parameter | Alive or Noncardiac Death | Cardiac Death | P‐Value |
|---|---|---|---|
| Number of cats | 20 | 21 | – |
| Follow‐up period (days) | 1,050 (162–1,274) | 241 (2–946) | <.001a |
| Age (years) | 6.2 (0.96–16.4) | 6.9 (0.72–16.1) | .873 |
| Male, n (%) | 12/20 (60) | 16/21 (76) | .244 |
| Purebred, n (%) | 4/20 (20) | 8/21 (38.1) | .177 |
| Body weight (kg) | 4.2 (2.6–5.6) | 4.43 (2.77–6.34) | .589 |
| Heart rate (per minute) | 184 ± 36 | 180 ± 35 | .666 |
| Respiratory rate (per minute) | 39 ± 14 | 46 ± 16 | .259 |
| CHF confirmed, n (%) | 5/20 (25) | 19/21 (90.5) | <.001a |
| Systolic blood pressure (mmHg) | 140 ± 22 | 121 ± 20 | .013a |
| NTproBNP (pmol/L) | 266 (24–786) | 573 (24–1,451) | .008a |
| cTnI (ng/mL) | 0.11 (0.01–0.73) | 0.27 (0.05–4.56) | .527 |
| LA : Ao ratio | 1.52 ± 0.34 | 2.51 ± 0.76 | <.001a |
| LA maximum diameter (mm) | 16.2 ± 3.98 | 23.7 ± 3.61 | <.001a |
| LA fractional shortening (%) | 29 (7.69–46.2) | 8.54 (2.94–31.3) | <.001a |
| LA ejection fraction (%) | 53.5 (1.18–67.6) | 16.4 (5.13–60.1) | <.001a |
| LV fractional shortening (%) | 54.5 ± 12.9 | 36.3 ± 13.1 | <.001a |
| IVSd (mm) | 6.70 ± 1.37 | 5.84 ± 2.00 | .198 |
| LVFWd (mm) | 7.13 ± 1.60 | 6.91 ± 2.50 | .794 |
| LV maximum thickness (mm) | 7.74 ± 1.14 | 7.69 ± 1.35 | .915 |
| SAM present, n (%) | 13/20 (65) | 8/21 (38.1) | .121 |
| Restrictive mitral inflow present, n (%) | 3/17 (17.6) | 7/10 (70) | .013a |
| Regional LV hypokinesis present, n (%) | 0/20 (0) | 6/21 (28.6) | .021a |
CHF, congestive heart failure; NTproBNP, N‐terminal of pro‐B‐type natriuretic peptide; cTnI, cardiac troponin I; LA : Ao, left atrium to aortic root ratio; LA, left atrial/atrium; LV, left ventricular; IVSd, interventricular septum measured at end‐diastole; LVFWd, left ventricular free wall measured at end‐diastole; SAM, systolic anterior motion of the mitral valve.
Indicates variables significantly different between cats suffering a cardiac death and those that died a noncardiac death or alive at the end of the study period. Normally distributed data are represented as mean ± SD, and nonnormally distributed data are represented as median (range).
At the end of the study period, 24/41 cats (59%) had died, 17 (41%) were alive and none were lost to follow‐up. Death was considered to be because of cardiac disease in 21/24 cats (ie, 51% of the entire study population reached the primary end‐point). Cardiac causes of death were euthanasia because of CHF (14), euthanasia because of clinical signs of ATE (2), sudden death associated with dyspnea (3) and sudden death after clinical signs of limb ATE (2). Noncardiac causes of death were euthanasia because of advanced chronic kidney disease (2) and mediastinal lymphoma (1).
Associations between Biomarkers and Clinical/Echocardiographic Findings
There was significant but weak correlation between cTnI and LA : Ao (ρs 0.50), LAD (ρs 0.42), LAFS (ρs −0.49), LAEF (ρs −0.48), LVFS (ρs −0.47), weight (ρs 0.44), and NTproBNP (ρs 0.46). VIF for each of these significant correlations was <10 (1.10–7.58), suggesting that colinearity was not great enough to impact upon multivariable Cox proportional hazards analysis. There was no correlation between age, HR, RR, maximum LV thickness, or LVIDd. cTnI (but not NTproBNP) concentrations were significantly higher in cats with regional LV wall hypokinesis than those without (0.99 ng/mL, 0.3–4.56 ng/mL; versus 0.2 ng/mL, 0.01–3.8 ng/mL; P = .027). All 6 cats with regional hypokinesis had the inferior LV free wall affected.
NTproBNP was significantly correlated with LA : Ao (ρs 0.63), LAD (ρs 0.57), LAFS (ρs −0.77), LAEF (ρs −0.69), LVFS (ρs −0.42), and SBP (ρs −0.47). VIF for each of these significant correlations was <10 (1.64–5.73), suggesting that colinearity was not great enough to impact upon multivariable Cox proportional hazards analysis. There was no correlation between age, weight, HR, RR, maximum LV thickness, or LVIDd. Cats with CHF had significantly higher median NTproBNP concentration than those without (569 pmol/L, 126–1,451 pmol/L; versus 249 pmol/L, 24–786 pmol/L; P = .016).
Among echocardiographic variables, significant correlation was present between LA : Ao and LAD (ρs 0.87), LAEF (ρs 0.81), LAFS (ρs 0.85), and LVFS (ρs 0.60). In addition, LAD was correlated with LAEF (ρs −0.74), LAFS (ρs −0.75), and LVFS (ρs −0.58); LAFS with LAEF (ρs 0.84) and LVFS (ρs 0.54); and LAEF with LVFS (ρs 0.62, P < .05 for each pairwise correlation listed). SBP was correlated with LAFS (ρs 0.57). These significant interactions were considered when performing multivariable Cox proportional hazards analysis.
Survival Analysis
Median survival time (MST) to cardiac death for the whole population was >946 days. Median follow‐up period was 834 days (2–1,274 days). Cats with cTnI >0.7 ng/mL had a significantly shorter MST (40 days, 95% CI 0–168 days; versus >1,274 days, P = .001; Fig 1). Cats with NTproBNP > 250 pmol/L also had a significantly shorter MST than those with NTproBNP ≤ 250 pmol/L (764 days, 95% CI 159–1,369 days; versus >1,257 days, P = .005; Fig 1). A combined measurement of NTproBNP > 250 pmol/L and cTnI > 0.7 ng/mL did not yield additional information regarding survival time when compared to measurement of cTnI > 0.7 ng/mL alone.
Figure 1.
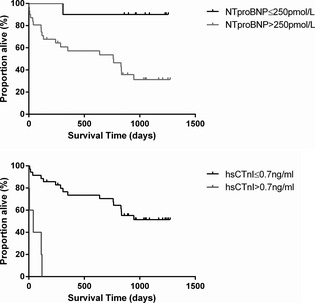
Kaplan–Meier curves to show the effect on survival of cardiac biomarker concentrations at the univariable level. NTproBNP, N‐terminal pro‐B type natriuretic peptide; cTnI, cardiac troponin I.
The results of univariable Cox proportional hazards analysis are shown in Table 2. Two models were devised to evaluate the use of NTproBNP and cTnI as predictors of time to cardiac death in multivariable analysis. First, a model employing cardiac biomarkers where the presence/absence of CHF is confirmed, but echocardiography is unavailable. Second, a multivariable model was constructed where all data were eligible for inclusion (biomarkers, CHF present/absent, and echocardiographic measurements).
Table 2.
Variables associated (P < .2) with a shorter time to cardiac death in univariable Cox proportional hazards analysis
| Parameter | HR | 95% CI | P‐Value |
|---|---|---|---|
| Age | |||
| ≥3.53 years | Ref | ||
| <3.53 years | 4.82 | 1.12–20.8 | .035 |
| LA : Ao | |||
| ≤1.76 | Ref. | ||
| >1.76 | 12.3 | 3.56–42.4 | <.001 |
| LA maximum diameter | |||
| ≤16 mm | Ref | ||
| >16 mm | 16.3 | 2.18–122.2 | .007 |
| LA fractional shortening | |||
| ≥14.2% | Ref | ||
| <14.2% | 7.8 | 2.58–23.5 | <.001 |
| LA ejection fraction | |||
| ≥33.5% | Ref | ||
| <33.5% | 12.3 | 3.56–42.4 | <.001 |
| LV fractional shortening | |||
| ≥47% | Ref | ||
| <47% | 5.49 | 1.98–15.2 | .001 |
| NTproBNP | |||
| ≤250 pmol/L | Ref | ||
| >250 pmol/L | 10.4 | 1.39–77.6 | .023 |
| cTnI | |||
| ≤0.7 ng/mL | Ref | ||
| >0.7 ng/mL | 4.77 | 1.68–13.5 | .003 |
| Systolic BP | |||
| ≥130 mmHg | Ref | ||
| <130 mmHg | 3.08 | 1.13–8.44 | .028 |
| Regional LV hypokinesis | |||
| No | Ref | ||
| Yes | 35.9 | 6.69–192.5 | <.001 |
| Nonrestrictive mitral inflow | Ref | ||
| Restrictive mitral inflow present | 6.92 | 1.76–27.2 | .006 |
| Congestive heart failure | |||
| No | Ref | ||
| Yes | 15 | 3.4–66.4 | <.001 |
HR, hazard ratio; 95% CI, 95% confidence intervals; LA : Ao, left atrium to aortic root ratio; LA, left atrial; LV, left ventricular; NTproBNP, N‐terminal of pro‐B‐type natriuretic peptide; cTnI, cardiac troponin I.
In the first model, when controlling for the confirmed presence/absence of CHF, NTproBNP did not provide additional prognostic value. However, cTnI provided additional, independent predictive value of a significantly shorter time to death because of cardiac disease (Table 3). Other measured variables were not significant.
Table 3.
Results of Cox multivariable analyzes, performed to reflect each of two clinical scenarios. In each, cTnI provided independent predictive value for cardiac death in cats with HCM. The exception was where regional LV hypokinesis was detected by 2D echocardiography
| Multivariable Model | Variable | HR | 95% CI | P‐Value | AIC value |
|---|---|---|---|---|---|
| Model 1: CHF status established, echocardiography unavailable | CHF (yes) | 17.4 | 3.84–78.6 | <.001 | 114.2 |
| cTnI (>0.7 ng/mL) | 6.78 | 1.98–23.2 | .002 | ||
| Model 2.1: CHF status established, echocardiography available | CHF (yes) | 6.35 | 1.10–36.7 | .039 | 111.0 |
| cTnI (>0.7 ng/mL) | 5.61 | 1.61–19.5 | .007 | ||
| LA : Ao (>1.76) | 4.53 | 1.04–19.7 | .044 | ||
| Model 2.2: CHF status established, echocardiography available | CHF (yes) | 11.9 | 2.60–54.4 | .001 | 104.0 |
| Regional LV hypokinesis (yes) | 16.3 | 2.71–97.6 | .002 |
HCM, hypertrophic cardiomyopathy; HR, hazard ratio; 95% CI, 95% confidence intervals; AIC, Aikaike's information criteria; NTproBNP, N‐terminal of pro‐B‐type natriuretic peptide; cTnI, cardiac troponin I; CHF, congestive heart failure; LA : Ao, left atrium to aortic root ratio; LV, left ventricular.
In the second model, NTproBNP did not remain predictive of shorter time to death because of cardiac disease in any multivariable model featuring confirmed CHF status and echocardiographic measurement of LA size or function. Conversely, cTnI did provide additional prognostic information even when accounting for the effect of LA size, LA function, or LV systolic function. However, if echocardiographic evidence of regional LV hypokinesis was detected, cTnI lost significance, suggesting some interrelationship. Detailed evaluation of the echocardiographic data resulted in the construction of 2 alternate final models (Table 3). In one, CHF status, LA : Ao > 1.76 and cTnI > 0.7 ng/mL were independent predictors of time to cardiac death. In the other, CHF status and presence of regional LV hypokinesis were independent predictors of time to death because of cardiac disease.
Discussion
This prospective study demonstrates the long‐term, independent prognostic value of a single measurement of cTnI in cats of any breed with HCM. One recent study by demonstrated that pedigree cats suffering an HCM‐related death had higher cTnI at initial diagnosis than survivors.30 In our data, not only was cTnI > 0.7 ng/mL associated with a shorter survival time to cardiac death but also it had predictive value in addition to the presence of CHF and LA dilatation. In our study, higher NTproBNP was not an independent predictor of cardiac death, but both increased NTproBNP and cTnI concentrations were significantly associated with a worse prognosis in univariable analysis (cutoff values >250 pmol/L and >0.7 ng/mL respectively). A combination of both biomarkers did not provide additional prognostic information compared to measurement of cTnI > 0.7 ng/mL alone.
The presence of CHF and LA dilatation are widely acknowledged indicators of risk of cardiac death in cats with HCM.4, 14, 15, 38 Echocardiographic measures of poor LA function, extreme LV hypertrophy, and reduced LV systolic function were independent predictors of shorter time to cardiac death in a large population of cats with HCM.10 In our univariable analysis, CHF status (present/absent), measures of LA size and function, LV systolic function, the presence of restrictive mitral inflow, and regional LV hypokinesis were significantly associated with time to death because of cardiac disease (as previously reported10).
In multivariable analysis, to investigate whether cardiac biomarker measurement could provide useful additional prognostic information on survival when the effects of CHF status and echocardiographic measurements were accounted for, we derived two alternate models to reflect different clinical situations, where the clinician either has or does not have access to echocardiographic information (for example, in many general practice clinics). In the first model, multivariable analysis evaluated the prognostic value of biomarkers where CHF was already confirmed as present or absent. In this model, NTproBNP did not remain significant, suggesting that CHF status was a more powerful prognostic predictor. High sensitivity cTnI remained significant; however, likely reflecting its ability to detect myocardial cellular damage.39 Although the ability of cardiac biomarkers to discriminate between cardiac and respiratory causes of tachypnea in cats is well‐established,17, 18, 19, 23, 40, 41 our results show the additional prognostic value of measuring cTnI in cats with HCM and CHF.
In the second model, CHF status and echocardiographic data were included in the multivariable analysis. The model that best accounted for differences in MST included the presence of CHF, LA : Ao and cTnI. If LV regional hypokinesis identified by 2D echocardiography was included in the model, cTnI lost significance. This suggests a close association between LV regional hypokinesis and increased cTnI, similar to elevations in cardiac troponins reported in humans with ischemic myocardial damage.42, 43 Regional LV hypokinesis is predictive of a shorter survival time in cats with HCM10 and our results suggest that indicators of myocardial damage (whether increased cTnI concentration or regional LV hypokinesis) provide additional prognostic information independent of the presence of CHF or LA dilatation.
The prognostic value of cTnI identified in our study is similar to reports in humans, and recently cats, with HCM.26, 27, 30 Although our study enrolled a relatively small number of cats, its prospective nature and high event rate add to the statistical power.44 Similar studies in humans with HCM, although enrolling a greater number of subjects, have reported a notably lower event rate.26, 27
Possible misclassification of cause of death (cardiac/noncardiac) or CHF status is a limitation of this study. Classification of death because of cardiac disease was likely to be relatively accurate for cats euthanized with CHF or ATE by their primary veterinarian, but may have been less accurate when based on owner reports for cats that died at home. Further limitations include a potential bias of owners or veterinarians in the decision making process surrounding euthanasia, lack of standardization in treatment, and the exclusion of cats with comorbidities that may elevate biomarker concentrations. Although this latter point may limit the applicability of these results to a more heterogeneous feline population in clinical practice, it should make these findings more accurate when considering cats with HCM.
In conclusion, the key finding from this study is that, although NTproBNP did not remain an independent predictor of death because of cardiac disease, a circulating concentration of cTnI >0.7 ng/mL was a significant predictor of cardiac death in cats with HCM that is independent of the presence of heart failure or left atrial dilatation.
Acknowledgments
The authors thank IDEXX Ltd. for their support of this project.
Conflict of Interest Declaration: This study was not supported by any grant or other source of direct funding. IDEXX Ltd. performed cardiac biomarker assay free of charge, but had no involvement in the preparation of this manuscript. Mr Borgeat and Drs Luis Fuentes and David Connolly have performed consultancy work for Boehringer Ingelheim. Drs Jessie Payne and Luis Fuentes have received research funding from IDEXX Ltd. for previous studies.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
Royal Veterinary College, Hawkshead Lane, Hatfield AL9 7TA, UK.
Footnotes
Fox PR, Ettinger SJ, Lamb KE, et al. Evaluation of NTpro‐brain natriuretic peptide (NTproBNP) as a prognostic indicators of short‐term outcome in cats with heart failure. J Vet Int Med 2013;27:637 (abstract)
Queen Mother Hospital for Animals, Royal Veterinary College, Hertfordshire, UK
IDEXX Veterinary Laboratories, Wetherby, Yorkshire, UK
AccuTnI, a paramagnetic particle chemiluminescent immunoassay from Access Immunoassay Systems (Ref. A78803), Beckman Coulter, High Wycombe, UK
IBM SPSS 20 for Windows 7, IBM (UK) Ltd, Portsmouth, UK; GraphPad Prism 6 for Windows 7, GraphPad Software Inc., San Diego, CA
References
- 1. Wagner T, Fuentes VL, Payne JR, et al. Comparison of auscultatory and echocardiographic findings in healthy adult cats. J Vet Cardiol 2010;12:171–182. [DOI] [PubMed] [Google Scholar]
- 2. Paige CF, Abbott JA, Elvinger F, Pyle RL. Prevalence of cardiomyopathy in apparently healthy cats. J Am Vet Med A 2009;234:1398–1403. [DOI] [PubMed] [Google Scholar]
- 3. Kittleson MD, Meurs KM, Munro MJ, et al. Familial hypertrophic cardiomyopathy in maine coon cats—An animal model of human disease. Circulation 1999;99:3172–3180. [DOI] [PubMed] [Google Scholar]
- 4. Payne J, Luis Fuentes V, Boswood A, et al. Population characteristics and survival in 127 referred cats with hypertrophic cardiomyopathy (1997–2005). J Small Anim Pract 2010;51:540–547. [DOI] [PubMed] [Google Scholar]
- 5. Lefbom BK, Rosenthal S, Tyrell WDJ, et al. Severe hypertrophic cardiomyopathy in 10 young ragdoll cats. J Vet Int Med 2001;15:308. [Google Scholar]
- 6. Fox PR, Liu SK, Maron BJ. Echocardiographic assessment of spontaneously occurring feline hypertrophic cardiomyopathy. An animal model of human disease. Circulation 1995;92:2645–2651. [DOI] [PubMed] [Google Scholar]
- 7. Maron BJ. Hypertrophic cardiomyopathy—A systematic review. J Am Med Assoc 2002;287:1308–1320. [DOI] [PubMed] [Google Scholar]
- 8. Gersh BJ, Maron BJ, Bonow RO, et al. 2011 accf/aha guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. J Thorac Cardiovasc Surg 2011;142:e153–e203. [DOI] [PubMed] [Google Scholar]
- 9. Christiaans I, Birnie E, Bonsel GJ, et al. Manifest disease, risk factors for sudden cardiac death, and cardiac events in a large nationwide cohort of predictively tested hypertrophic cardiomyopathy mutation carriers: Determining the best cardiological screening strategy. Eur Heart J 2011;32:1161–1170. [DOI] [PubMed] [Google Scholar]
- 10. Payne JR, Borgeat K, Connolly DJ, et al. Prognostic indicators in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2013;27:1427–1436. [DOI] [PubMed] [Google Scholar]
- 11. Maron BJ. Contemporary insights and strategies for risk stratification and prevention of sudden death in hypertrophic cardiomyopathy. Circulation 2010;121:445–456. [DOI] [PubMed] [Google Scholar]
- 12. Chan RH, Maron B, Olivotto I, et al. Prognostic utility of contrast‐enhanced cardiovascular magnetic resonance in hypertrophic cardiomyopathy: An international multicenter study. J Am Coll Cardiol 2012;59:E1570–E1570. [Google Scholar]
- 13. Maron BJ, Maron MS, Wigle ED, Braunwald E. The 50‐year history, controversy, and clinical implications of left ventricular outflow tract obstruction in hypertrophic cardiomyopathy from idiopathic hypertrophic subaortic stenosis to hypertrophic cardiomyopathy: From idiopathic hypertrophic subaortic stenosis to hypertrophic cardiomyopathy. J Am Coll Cardiol 2009;54:191–200. [DOI] [PubMed] [Google Scholar]
- 14. Rush JE, Freeman LM, Fenollosa NK, Brown DJ. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990–1999). J Am Vet Med Assoc 2002;220:202–207. [DOI] [PubMed] [Google Scholar]
- 15. Atkins CE, Gallo AM, Kurzman ID, Cowen P. Risk‐factors, clinical signs, and survival in cats with a clinical‐diagnosis of idiopathic hypertrophic cardiomyopathy: 74 cases (1985–1989). J Am Vet Med Assoc 1992;201:613–618. [PubMed] [Google Scholar]
- 16. Connolly DJ, Cannata J, Boswood A, et al. Cardiac troponin I in cats with hypertrophic cardiomyopathy. J Feline Med Surg 2003;5:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Connolly DJ, Brodbelt DC, Copeland H, et al. Assessment of the diagnostic accuracy of circulating cardiac troponin I concentration to distinguish between cats with cardiac and non‐cardiac causes of respiratory distress. J Vet Cardiol 2009;11:71–78. [DOI] [PubMed] [Google Scholar]
- 18. Connolly DJ, Soares Magalhaes RJ, Fuentes VL, et al. Assessment of the diagnostic accuracy of circulating natriuretic peptide concentrations to distinguish between cats with cardiac and non‐cardiac causes of respiratory distress. J Vet Cardiol 2009;11(Suppl 1):S41–S50. [DOI] [PubMed] [Google Scholar]
- 19. Fox PR, Oyama MA, Reynolds C, et al. Utility of plasma N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) to distinguish between congestive heart failure and non‐cardiac causes of acute dyspnea in cats. J Vet Cardiol 2009;11(Suppl 1):S51–S61. [DOI] [PubMed] [Google Scholar]
- 20. Wess G, Daisenberger P, Mahling M, et al. Utility of measuring plasma N‐terminal pro‐brain natriuretic peptide in detecting hypertrophic cardiomyopathy and differentiating grades of severity in cats. Vet Clin Pathol 2011;40:237–244. [DOI] [PubMed] [Google Scholar]
- 21. Fox PR, Rush JE, Reynolds CA, et al. Multicenter evaluation of plasma N‐terminal probrain natriuretic peptide (NT‐pro BNP) as a biochemical screening test for asymptomatic (occult) cardiomyopathy in cats. J Vet Intern Med 2011;25:1010–1016. [DOI] [PubMed] [Google Scholar]
- 22. Oyama MA, Boswood A, Connolly DJ, et al. Clinical usefulness of an assay for measurement of circulating N‐terminal pro‐B‐type natriuretic peptide concentration in dogs and cats with heart disease. J Am Vet Med Assoc 2013;243:71–82. [DOI] [PubMed] [Google Scholar]
- 23. Singletary GE, Rush JE, Fox PR, et al. Effect of NT‐pro‐BNP assay on accuracy and confidence of general practitioners in diagnosing heart failure or respiratory disease in cats with respiratory signs. J Vet Intern Med 2012;26:542–546. [DOI] [PubMed] [Google Scholar]
- 24. Fernandes F, Arteaga‐Fernandez E, Antunes Mde O, et al. Plasma pro‐B‐type natriuretic peptide testing as a screening method for hypertrophic cardiomyopathy. J Card Fail 2012;18:564–568. [DOI] [PubMed] [Google Scholar]
- 25. Moreno V, Hernandez‐Romero D, Vilchez JA, et al. Serum levels of high‐sensitivity troponin T: A novel marker for cardiac remodeling in hypertrophic cardiomyopathy. J Card Fail 2010;16:950–956. [DOI] [PubMed] [Google Scholar]
- 26. Kubo T, Kitaoka H, Yamanaka S, et al. Significance of high‐sensitivity cardiac troponin T in hypertrophic cardiomyopathy. J Am Coll Cardiol 2013;62:1252–1259. [DOI] [PubMed] [Google Scholar]
- 27. Kubo T, Kitaoka H, Okawa M, et al. Combined measurements of cardiac troponin I and brain natriuretic peptide are useful for predicting adverse outcomes in hypertrophic cardiomyopathy. Circulation 2011;75:919–926. [DOI] [PubMed] [Google Scholar]
- 28. Geske JB, McKie PM, Ommen SR, Sorajja P. B‐type natriuretic peptide and survival in hypertrophic cardiomyopathy. J Am Coll Cardiol 2013;61:2456–2460. [DOI] [PubMed] [Google Scholar]
- 29. Coats CJ, Gallagher MJ, Foley M, et al. Relation between serum N‐terminal pro‐brain natriuretic peptide and prognosis in patients with hypertrophic cardiomyopathy. Eur Heart J 2013;34:2529–2537. [DOI] [PubMed] [Google Scholar]
- 30. Langhorn R, Tarnow I, Willesen JL, et al. Cardiac troponin I and T as prognostic markers in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2014; doi: 10.1111/jvim.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abbott JA, MacLean HN. Two‐dimensional echocardiographic assessment of the feline left atrium. J Vet Int Med 2006;20:111–119. [DOI] [PubMed] [Google Scholar]
- 32. Schober KE, Maerz I, Ludewig E, Stern JA. Diagnostic accuracy of electrocardiography and thoracic radiography in the assessment of left atrial size in cats: Comparison with transthoracic 2‐dimensional echocardiography. J Vet Intern Med 2007;21:709–718. [DOI] [PubMed] [Google Scholar]
- 33. Bang CN, Dalsgaard M, Greve AM, et al. Left atrial size and function as predictors of new‐onset of atrial fibrillation in patients with asymptomatic aortic stenosis: The simvastatin and ezetimibe in aortic stenosis study. Int J Cardiol 2013;168:2322–2327. [DOI] [PubMed] [Google Scholar]
- 34. Blume GG, McLeod CJ, Barnes ME, et al. Left atrial function: Physiology, assessment, and clinical implications. Eur J Echocardiogr 2011;12:421–430. [DOI] [PubMed] [Google Scholar]
- 35. Schober KE, Fuentes VL, Bonagura JD. Comparison between invasive hemodynamic measurements and noninvasive assessment of left ventricular diastolic function by use of doppler echocardiography in healthy anesthetized cats. Am J Vet Res 2003;64:93–103. [DOI] [PubMed] [Google Scholar]
- 36. Schober KE, Maerz I. Assessment of left atrial appendage flow velocity and its relation to spontaneous echocardiographic contrast in 89 cats with myocardial disease. J Vet Int Med 2006;20:120–130. [DOI] [PubMed] [Google Scholar]
- 37. Lyons HR, Foster WM, Esty KJ, et al. Bioanalytical method validation of a second‐generation immunoassay for the quantification of N‐terminal pro‐B‐type natriuretic peptide in feline blood. J Vet Int Med 2013;27:632–632. [Google Scholar]
- 38. Trehiou‐Sechi E, Tissier R, Gouni V, et al. Comparative echocardiographic and clinical features of hypertrophic cardiomyopathy in 5 breeds of cats: A retrospective analysis of 344 cases (2001–2011). J Vet Int Med 2012;26:532–541. [DOI] [PubMed] [Google Scholar]
- 39. Peacock WF 4th, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008;358:2117–2126. [DOI] [PubMed] [Google Scholar]
- 40. Humm K, Hezzell M, Sargent J, et al. Differentiating between feline pleural effusions of cardiac and non‐cardiac origin using pleural fluid NT‐proBNP concentrations. J Small Anim Pract 2013;54:656–661. [DOI] [PubMed] [Google Scholar]
- 41. Herndon WE, Rishniw M, Schrope D, et al. Assessment of plasma cardiac troponin I concentration as a means to differentiate cardiac and noncardiac causes of dyspnea in cats. J Am Vet Med Assoc 2008;233:1261–1264. [DOI] [PubMed] [Google Scholar]
- 42. Perna ER, Macin SM, Cimbaro Canella JP, et al. Minor myocardial damage detected by troponin T is a powerful predictor of long‐term prognosis in patients with acute decompensated heart failure. Int J Cardiol 2005;99:253–261. [DOI] [PubMed] [Google Scholar]
- 43. Perna ER, Aspromonte N, Cimbaro Canella JP, et al. Minor myocardial damage is a prevalent condition in patients with acute heart failure syndromes and preserved systolic function with long‐term prognostic implications: A report from the CIAST‐HF (Collaborative Italo‐Argentinean Study on Cardiac Troponin T in Heart Failure) study. J Card Fail 2012;18:822–830. [DOI] [PubMed] [Google Scholar]
- 44. Ohno‐Machado L. Modeling medical prognosis: Survival analysis techniques. J Biomed Inform 2001;34:428–439. [DOI] [PubMed] [Google Scholar]


