Abstract
Background
Many dogs suffering from inflammatory bowel disease (IBD) are presented to veterinary clinics. These patients are diagnosed based on a history of chronic gastrointestinal signs and biopsy‐confirmed histopathologic intestinal inflammation. Intestinal intraepithelial lymphocytes (IEL) are part of the first line of defense in the gastrointestinal immune system. Alterations in IEL subsets may play a role in the pathogenesis of IBD.
Hypothesis
The aim of this study was to characterize the phenotypes of IEL in dogs with IBD compared with healthy control dogs.
Animals
Intestinal intraepithelial lymphocytes subpopulations of control dogs (n = 5) obtained from endoscopic biopsies (EB) were compared to those obtained from full thickness biopsies (FTB) on the same day. In addition, the phenotypes of IEL from FTB of control dogs (n = 10) were compared with EB of IBD dogs (n = 10). Each participant was scored clinically using the canine inflammatory bowel disease activity index (CIBDAI), and all samples were graded histopathologically. Three‐color flow cytometry of isolated IEL was performed using monoclonal antibodies against T‐ and B‐lymphocyte subpopulations.
Results
No significant differences in the composition of IEL subpopulations were found in control dogs based on method of biopsy. The IBD dogs had significantly higher CIBDAI and histopathologic scores compared with control dogs and their IEL contained a significantly higher frequency TCRγδ T‐cells.
Conclusions and Clinical Importance
Endoscopic biopsies provide suitable samples for 3‐color flow cytometry when studying canine intestinal IEL and IBD patients show significant changes of major T‐cell subsets compared to healthy control dogs.
Keywords: Dogs, Flow‐cytometry, Intestinal immune‐cells
Abbreviations
- Alexa647
Alexa Fluor 647
- WSAVA
World Small Animal Veterinary Association
- APC
allophycocyanin
- CD
cluster of differentiation
- CIBDAI
canine inflammatory bowel disease activity index
- DTT
1,4‐Dithiotreitol
- EB
endoscopic biopsies
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- FSC/SSC
forward scatter/sideward scatter
- FTB
full‐thickness biopsies
- HHB
hepes‐buffered Hanks’ balanced salt solution
- IBD
inflammatory bowel disease
- IEL
intraepithelial lymphocytes
- IgG
immunoglobulin G
- mAb
monoclonal antibody
- PBS
phosphate buffered saline
- PE
phycoerythrin
- SD
standard deviation
- TCR
T‐cell receptor
A large number of dogs suffer from chronic or recurrent gastrointestinal signs.1, 2 Inflammatory bowel disease (IBD) represents a heterogeneous group of disorders characterized by inflammation of the intestinal tract. After excluding infectious, endocrine and neoplastic causes, the diagnosis of canine IBD is established based on histopathologic evidence of intestinal inflammation. In most cases, lymphocytic‐plasmacytic cellular infiltration of the mucosa predominates.3, 4 Furthermore, dogs with IBD have been differentiated clinically with regard to response to therapeutic trials as “diet‐responsive,”5 “antibiotic‐responsive,”6 and “steroid‐responsive.”1, 3
Dogs may share a similar, multifactorial pathogenesis as do human IBD patients,7, 8 but little is known about the underlying pathologic mechanisms.9 Genetic factors,10 disruption of the mucosal barrier,11, 12 changes in the intestinal microbiome,13, 14 and dysregulation of the intestinal immune system may lead to the breakdown of immunologic tolerance and the onset of IBD. Intestinal intraepithelial lymphocytes (IEL) are an important part of adaptive immunity. Two major subsets can be defined15, 16: (1) conventional IEL characterized by T‐cell receptor (TCR) αβ+ expression with co‐receptor cluster of differentiation (CD) 4+ and CD8αβ+ and (2) non‐conventional IEL expressing TCRαβ+ or TCRγδ+ combined with co‐receptor CD8αα+.17 Pro‐ and anti‐inflammatory functions are described for both subsets.18 Human patients with Crohn's have increased numbers of CD8+ cytotoxic T‐cells19 and TCRγδ+ T‐cells in inflamed colonic mucosa.20 Based on this information, it can be concluded that IEL play an important role in the pathogenesis of canine IBD. Hence, the aim of the present study was to characterize the phenotypes of IEL by flow cytometry. The suitability of intestinal EB for flow cytometric analysis in dogs also was validated. The phenotypic characterization of IEL from control dogs and dogs with IBD was performed to identify differences in their lymphocyte subsets.
Materials and Methods
Protocols for this study were approved by the Institutional Ethics Committee, the Advisory Committee for Animal Experiments (§12 of Law for Animal Experiments, Tierversuchsgesetz [TVG]), and the Federal Ministry for Science and Research [reference number: GZ 68.205/0201‐II/3b/2010].
Study Groups
Control Dogs
Ten healthy control dogs of different breeds were included in the study. They were presented to the Clinic for Internal Medicine at the Veterinary University of Vienna for non‐gastrointestinal problems. These dogs had not received antibiotic or immunosuppressive treatment in the 10 days before biopsy acquisition and were euthanized for reasons not related to the study. Full thickness biopsies (FTB) and EB from the duodenum were obtained 15–30 minutes post‐mortem21 and were immediately placed in ice‐cold phosphate‐buffered saline (PBS)1 and 4% buffered paraformaldehyde solution2 until processing.
Inflammatory Bowel Disease Dogs
Ten dogs presented to the Clinic for Internal Medicine at the Veterinary University of Vienna with chronic gastrointestinal signs were selected for this prospective study. Inclusion criteria were vomiting, diarrhea, anorexia, weight loss, or some combination of these signs for at least 4 weeks, with no immunosuppressive drugs or antibiotics administered by the owners for at least 10 days before biopsy acquisition. Furthermore, a complete clinical evaluation was performed, including hematology, clinical biochemistry (including canine serum trypsin‐like immunoreactivity, vitamin B12, and folate concentrations), urinalysis, fecal flotation, Giardia antigen test, and abdominal ultrasound examination to exclude infectious, endocrine or neoplastic diseases as explanations for the gastrointestinal signs. Owners gave written consent for their dogs to take part in the study. Gastroduodenoscopy was performed under general anesthesia, and EB samples from the stomach and descending duodenum were taken with flexible endoscopic biopsy forceps. Endoscopic procedures and sample storage were performed the same as for control dogs. All dogs had intestinal infiltration with inflammatory cells and lesions were graded using the World Small Animal Veterinary Association (WSAVA) guidelines. Based on the chronicity of gastrointestinal signs, the exclusion of underlying infectious, endocrine or neoplastic diseases, and the intestinal histopathologic inflammatory findings, these dogs were diagnosed as suffering from IBD.
Clinical and Histopathologic Scoring
All cases were scored according to the canine inflammatory bowel disease activity index (CIBDAI).22 All tissue samples from the 3 study groups were graded by a single independent board‐certified pathologist according to the WSAVA International Gastrointestinal Standardization Group guidelines.4 Because a number of samples had suboptimal orientation of mucosal villi, the morphologic criteria of villus stunting were not taken into account. In total, 4 morphologic parameters (epithelial injury, crypt distension, lacteal dilatation, and mucosal fibrosis) and 4 inflammatory histologic parameters (IEL, lamina propria lymphocytes and plasma cells, lamina propria eosinophils, and lamina propria neutrophils) were scored as 0 = normal, 1 = mild, 2 = moderate, 3 = marked.4 The sum of the scores from single parameters were totaled and dogs were subdivided into histologic severity groups: WSAVA score of 0 = normal, 1–6 = mild (≤25% of the maximal score of 24), 7–12 = moderate (25–50% of the maximal score), 13–18 = severe (50–75% of the maximal score), and >18 = very severe (>75% of the maximal score).23
IEL Isolation
Immediately after sample collection, IEL were isolated as previously described.24, 25 In brief, all duodenal biopsies (FTB and EB) had to contain intact lamina propria mucosa. The FTB samples were cut into pieces approximately 1 cm in length. All specimens were washed 2–6 times in ice‐cold PBS1 or Hepes‐buffered Hanks’ balanced salt solution (HHB) to remove attached feces. Afterward, they were washed twice in HHB with 2% fetal calf serum (FCS), 2 mM 1,4‐dithiotreitol,3 and 0.5 mM EDTA3 for 20 minutes each time at 37°C with constant stirring. After each wash, cells were passed through a 70 μm and then a 40 μm nylon cell strainer. Cells then were centrifuged on a discontinuous density gradient with 40% and 70% Percoll4 (920 × g, 30 minutes, room temperature). The interphase was harvested and then washed twice in HHB containing 5% FCS. Cells were counted in a Neubauer counting chamber and live/dead discrimination was determined using trypan blue exclusion.5
Flow Cytometry
After IEL isolation from the biopsy samples, 3‐color flow cytometry using anti‐canine specific and anti‐human cross‐reactive monoclonal antibodies (mAb) against CD21,6 CD79αcy,7 CD3‐12,8 TCRαβ,9 TCRγδ,10 CD4,8 CD8α,8 and CD8β8 (Table 1) was performed to characterize the cells. For each analysis, 500,000 or 1,000,000 cells were incubated with the listed mAb for 15 minutes at room temperature. Cells were then washed in PBS, without Ca2+ and Mg2+, and supplemented with 3% FCS. Those samples containing mAb without directly conjugated fluorochromes were labeled with anti‐mouse secondary antibodies and incubated for an additional 15 minutes at room temperature. For intracellular staining (CD3‐12, CD79αcy), the IntraStain‐Kit7 was used according to manufacturers' instructions. After the last incubation step, cells were washed again and then analyzed using a FACSCanto II flow cytometer.6 Data analysis was performed by the FACSDiva software, version 6.1.3.6
Table 1.
List of mAb used for flow cytometry
| mAb | Clone | Isotype | Fluorescence Labeling |
|---|---|---|---|
| CD45 | YKIX716.13a | rIgG2b | APC |
| CD79αcy | HM57b | mIgG1 | PE |
| CD21 | B‐ly‐4b | mIgG1 | APC |
| CD3 | CD3‐12b | rIgG1 | FITC |
| TCRαβ | CA15.8G7a | mIgG1 | α‐mIgG1‐FITCc , d |
| TCRγδ | CA20.8H1a | mIgG2a | α‐mIgG2a‐FITCc , e |
| CD4 | YKIX302.9a | rIgG2a | APC |
| CD8α | YCATE 55.9a | rIgG1 | PE |
| CD8β | CA15.4G2a | mIgG1 | α‐mIgG1‐APCc , f |
mAb, monoclonal antibodies; CD, cluster of differentiation; IgG, immunoglobulin G; TCR, T‐cell receptor; m, mouse; α‐m, anti‐mouse; r, rat; APC, allophycocyanin; FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Anti‐canine antibody.
Anti‐human cross‐reactive antibody (CD79αcy47; CD3‐12: Serotec, technical datasheet MCA1477; CD21).48
Fluorescence labeling was achieved by use of a secondary antibody.
Goat anti‐mouse IgG1‐Alexa488; Life Technologies, Carlsbad, CA.
Goat F(ab′)2 anti‐mouse IgG2a‐FITC; SouthernBiotech, Birmingham, AL.
Goat anti‐mouse IgG1‐Alexa647; Life Technologies.
Statistical Analysis
Age and weight of the 2 dog groups were summarized by descriptive statistics. Data were tested for normal distribution using the Shapiro–Wilk‐test. Dog groups were compared by nonparametric tests when the data was not normally distributed. All analyses were performed using IBM SPSS 20.010 software. The level of statistical significance was set at P < .05.
Results
Descriptive Data—Age, Sex, Weight
The control dogs (n = 10) consisted of several different breeds (4 cross‐breeds, 2 Yorkshire Terriers, 1 Boxer, 1 Cocker Spaniel, 1 Maltese, 1 Shi Tzu) and consisted of 5 males (4 intact, 1 neutered), and 5 females (3 intact, 2 neutered). The median (range) age of the dogs was 10.3 years (2.3–15.4 years); age mean ± SD was 5.8 ± 2.8 years. The median (range) body weight was 21.4 kg (2.3–45.6 kg); body weight mean ± SD was 21.6 ± 16.6 kg.
The breeds in the IBD group (n = 10) included 2 cross‐breeds and 1 each of the following breeds: American Staffordshire Terrier, Boxer, Collie, Groenendael, Jack Russell Terrier, Maltese, Pit Bull Terrier and Shar Pei. There were 6 males (2 intact, 4 neutered) and 4 neutered female dogs. IBD dogs had a median (range) age of 6.0 years (1.8–9.6 years), with an age mean ± SD of 5.8 ± 2.8 years. The median (range) body weight of this group was 24.2 kg (7.2–37 kg), with a body weight mean ± SD of 22.3 ± 10.4 kg. Control dogs were significantly older compared with IBD dogs (P < .05). There was no significant difference between body weights.
Clinical Scoring
All dogs were clinically evaluated by CIBDAI scores.22 Control dogs had a median (range) CIBDAI score of 1 (0–5), and mean ± SD score of 1.7 ± 1.7. The IBD dogs were classified as mild, with median (range) CIBDAI score of 4.5 (2–8), and mean ± SD score of 4.6 ± 2.1. The CIBDAI scores of IBD dogs were significantly higher compared with control dogs (P < .01; Fig 1).
Figure 1.
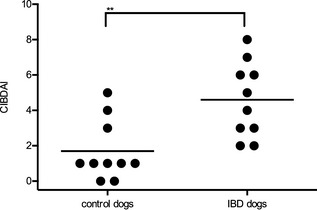
Clinical disease scores for individual dogs in each study group. The canine inflammatory bowel disease activity index (CIBDAI) for control dogs (n = 10) and dogs with inflammatory bowel disease (IBD; n = 10) was calculated for individual dogs. Each dot indicates an individual dog score. The horizontal lines show the mean score in each group (**P < .01).
Histopathologic Examination
Comparison of histopathologic results between IBD dogs and control dogs identified marked differences in inflammatory criteria, but morphologic abnormalities were less diverse. Three control dogs had 1 of the following abnormalities: mild lacteal dilatation, mild mucosal fibrosis, or moderate increase of lamina propria eosinophils. Two control dogs showed no histologic abnormalities. In all remaining control dogs, a mild increase in lamina propria lymphocytes was reported. The cellular infiltrates in IBD dogs ranged from normal to moderately increased. No abnormalities in morphologic criteria were reported. The WSAVA scores of IBD dogs were median (range) 2 (1–3) and mean ± SD scores of 1.9 ± 0.57. Scores were significantly higher compared with the control dogs that had median (range) 1.9 (0–2) and mean ± SD scores of 0.9 ± 0.57 (P < .01; Fig 2).
Figure 2.
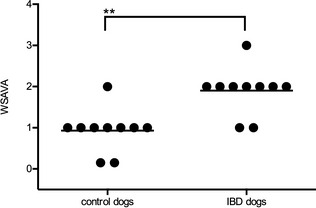
Histopathology scores for individual dogs in each study group. Histopathology grading was performed according to the World Small Animal Veterinary Association (WSAVA) guidelines for control dogs (n = 10) and dogs with inflammatory bowel disease (IBD; n = 10). Each dot indicates an individual dog score. The horizontal lines show the mean score in each group (**P < .01).
Immunophenotyping by Flow Cytometry
Gating of IEL and Analysis of T‐ and B‐cells
In forward/sideward scatter (FSC/SSC) analysis, isolated IEL were detected as a distinct population (Fig 3A), and represented on average 7.3% of all acquired cells (minimum, 2.4%; maximum, 27.5%). Less than 1% of the gated IEL were B‐cells expressing CD21, CD79αcy or both (Fig 3B,C). In contrast, the majority of all cells were CD3+ T‐cells (median ± SD in control dogs, 97.6 ± 5.1%; and in IBD dogs, 89.5 ± 6.8% (Fig 3D). Therefore, results were normalized to CD3+ T‐cells.
Figure 3.
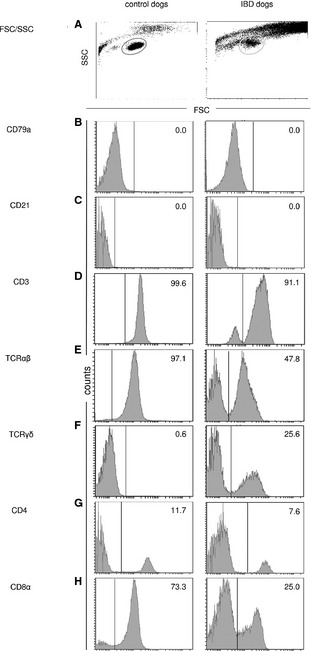
Flow cytometry histograms for intestinal intraepithelial lymphocytes (IEL) from 1 control dog and 1 inflammatory bowel disease (IBD) dog. Both are representative of their respective dog groups. Cells were gated by forward/sideward scatter (FSC/SSC) properties (A). The IEL were stained with anti‐canine‐specific or anti‐human‐cross‐reactive monoclonal antibodies (mAb) against CD79αcy (B), CD21 (C), CD3‐12 (D), TCRαβ (E), and TCRγδ (F), CD4 (G), CD8α (H) and CD8β (CD8β data not shown). Histograms show negative cells on the left and positive cells on the right side of each border. Borders were set according to the corresponding isotype controls. Numbers indicate positive cells as a percent of CD3+ T‐cells.
Immunophenotyping—FTB versus EB
The cell yield from FTB comprised a mean ± SD of 24,800,000 ± 22,620,000 cells/mL, and median (range) of 15,000,000 cells/mL (9,000,000–64,000,000 cells/mL). The cell yield from EB consisted of mean ± SD of 2,840,000 ± 1,450,000 cells/mL, and median (range) of 2,800,000 cells/mL (1,000,000–5,000,0005 cells/mL). In the lymphocyte gate, there were no significant differences in the distribution of lymphocyte subpopulations, comparing IEL from FTB and EB of the same control dog, collected at the same time from the same duodenal localization (data not shown).
Immunophenotyping—IBD Dogs versus Healthy Control Dogs
In both healthy and control dogs, the CD8α+ T‐cells were predominant (control dogs, 52.9 ± 24.0%; IBD dogs, 53.1 ± 19.3%) compared with CD4+ T‐cells (control dogs, 14.2 ± 11.1%; IBD dogs, 7.9 ± 6.3%); (Table 2). In their IEL subsets, IBD dogs had fewer TCRαβ+ T‐cells (IBD dogs, 64.4 ± 15.6%; control dogs, 79.7 ± 17%; P = .059; Table 2; Fig 3E) and significantly more TCRγδ+ T‐cells (IBD dogs, 19.9 ± 8.7%; control dogs 8.4 ± 6.1%; P < .01; Table 2; Fig 4).
Table 2.
Phenotypes of intestinal IEL from control dogs and dogs with IBD, expressed as percent of CD3+ T‐cells
| CD4a | CD8αa | TCRαβb | CD8ααTCRαβa | CD8αβTCRαβb | TCRγδb | |
|---|---|---|---|---|---|---|
| Control dogs (n = 10) | ||||||
| IEL, mean ±SD | 14.2 ± 11.1 | 52.9 ± 24 | 79.7 ± 17 | 6.9 ± 3.9 | 44.4 ± 25.4 | 8.4 ± 6.1c |
| IEL, median (range) | 10.5 (4.6–39.1) | 60.7 (15.3–74.8) | 83.4 (44.6–101.4) | 6.9 (2.1–14.8) | 46.1 (11.7–85.9) | 5.8 (0.6–17.7) |
| IBD dogs (n = 10) | ||||||
| IEL, mean ± SD | 7.9 ± 6.3 | 53.1 ± 19.3 | 64.4 ± 15.6 | 6.9 ± 2.4 | 43.7 ± 18.6 | 19.9 ± 8.7c |
| IEL, median (range) | 6.9 (1.1–24.3) | 50.0 (29.2–93.8) | 62.8 (44–96.1) | 5.7 (4.6–11.5) | 43.3 (21.0–84.6) | 21.7 (1.5–29.6) |
IEL, intraepithelial lymphocytes; IBD, inflammatory bowel disease.
Parameters that were not normally distributed were analyzed by the Mann–Whitney test.
Parameters that were normally distributed were analyzed by Student's t‐test.
Significant difference between groups (P < .05).
Figure 4.
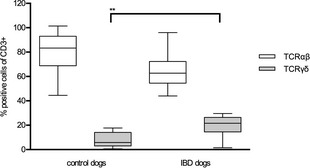
Distribution of TCR (T‐cell receptor)αβ+ and TCRγδ+ T‐cells within intestinal intraepithelial lymphocytes (IEL). Box and whisker plots show TCRαβ and yδ expression within IEL from controls dogs (n = 10) and dogs with inflammatory bowel disease (IBD; n = 10). Each box and whisker plot illustrates the median and quartiles (**P < .01).
Immunophenotyping—CD8αα Homodimer versus CD8αβ Heterodimer Expression in TCRαβ+ and TCRγδ+ IEL
Both subpopulations, TCRαβ+ and TCRγδ+ IEL, can be further described depending on their CD8αα and CD8αβ expression pattern. We analyzed these subpopulations by gating on TCRγδ+ and TCRγδ− cells in combination with staining for CD8αα and CD8αβ expression (Fig 5). Because of the extreme predominance of CD3+ T‐cells among total IEL (Fig 3d), we concluded that TCRγδ− cells represent TCRαβ+ T‐cells. In all study groups, the majority of TCRαβ+ T‐cells identified by this strategy expressed the CD8αβ heterodimer (control dogs, 44.4 ± 25.4%; IBD dogs, 43.7 ± 18.6%) and less frequently the CD8αα homodimer (control dogs, 6.9 ± 3.9%; IBD dogs, 6.9 ± 2.4%; Table 2; Fig 5). TCRγδ+ T‐cells were not analyzed statistically with respect to their CD8 expression pattern because the homodimer CD8αα and the heterodimer CD8αβ showed high variability among individuals (Table 2). No significant differences between these subpopulations comparing IBD dogs with control dogs could be detected.
Figure 5.
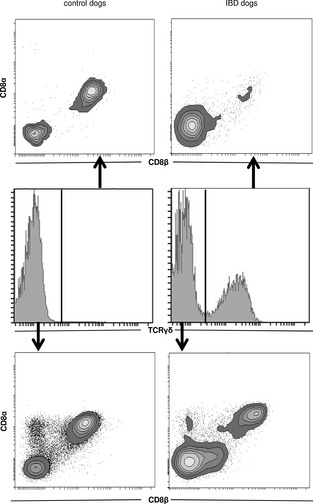
CD8αα and CD8αβ expression on TCR (T‐cell receptor)αβ+ and TCRγδ+ T‐cells within intestinal intraepithelial lymphocytes (IEL). Analysis of CD8αα homodimers and CD8αβ heterodimers expressed on TCRγδ+ T‐cells (upper contour plots) and TCRγδ− (lower contour plots, classified as TCRαβ+) T‐cells in control dogs and dogs with inflammatory bowel disease (IBD). A representative individual is shown for each dog group. The gating strategy for TCRγδ expression is shown in histograms in the middle plots, and explained in Figure 3.
Discussion
Intestinal intraepithelial lymphocytes play a crucial role in the development and maintenance of inflammation in chronic enteropathies in animal models and human IBD patients.17, 18 This study was performed to characterize canine intestinal IEL phenotypes in dogs with IBD, because different phenotypes in humans and mice seem to be associated with different disease characteristics18, 19, 20, 26 and different responses to treatment.27, 28
Dogs with IBD had significantly higher WSAVA scores compared with control dogs. These results support the WSAVA scoring system as an appropriate method to verify histopathologic changes in dogs with IBD. Although WSAVA scores in control dogs were lower than in IBD dogs, control dogs were not free of pathological findings. Abnormalities were primarily the result of increased inflammatory cell infiltrates. These findings are similar to previous reports, whereby mild histopathology changes were found in healthy dogs, and characterized predominantly as lymphoplasmacytic infiltration of the lamina propria.21, 29 Again, WSAVA scores were lower compared with IBD dogs in those studies.21 An age‐dependent increase in intestinal inflammation has been suggested in healthy dogs, but is controversial.21, 30, 31 The intestinal mucosa is continuously exposed to numerous antigenic stimuli throughout life. The control dogs in this study were older compared with IBD dogs, which makes an age‐dependent increase in intestinal inflammation a possible explanation for the mildly increased WSAVA scores in control dogs.
Another goal of the present study was to establish the usefulness of EB for flow cytometry analyses in canine IBD patients, because usually EB are obtained for the diagnosis in IBD dogs.32 However, even for EB diagnosis of IBD there is considerable inter‐observer variability in interpretation of histopathology findings,33 and in many cases inadequate sample size or quality of tissue samples is problematic.34 Therefore, we anticipated EB might not be adequate for flow cytometry studies. The IEL from EB and FTB of the same control dogs were isolated, stained and analyzed identically. We found no significant difference with respect to T‐cell subpopulations between EB and FTB, demonstrating that EB are suitable for IEL isolation and evaluation by flow cytometry. Although previous studies have compared FTB with EB in healthy dogs of different breeds,29 or compared EB to FTB from IBD dogs and healthy Beagle dogs,35 to our knowledge this is the first study comparing flow cytometry data from both biopsy methods.
The majority of isolated IEL in the current study were T‐cells. Fewer than 1% expressed a common B‐cell marker (CD21+, CD79α+). This finding mirrored results of previous studies, whereby IEL were defined predominantly as T‐cells either by immunohistochemistry21, 36, 37, 38 or flow cytometry.25, 29, 35 In both control and IBD dogs, CD8+ T‐cells were predominant over CD4+ T‐cells, which also is in accordance with previous studies of canine intestinal IEL.25, 29, 35 Although the percentages of CD4+ cells in both control and IBD dog were comparable to previously published data, percentages of CD8α+ T‐cells were not. Previous studies showed 2‐ to 3‐fold higher CD8α+/CD4 T‐cell ratios in control and IBD dogs compared to previous canine gastroenterology studies in dogs,29, 35 whereas in the present study control dogs had a 5‐fold and IBD dogs nearly a 7‐fold higher CD8α+/CD4 T‐cell ratio. Regional differences may have been caused by altered intestinal bacterial colonization because of different genetic backgrounds or husbandry conditions (eg, breeding, dietary management). Future studies are necessary to elucidate the reasons for the variation in CD8α+/CD4 T‐cell ratio present within IEL.
The IBD dogs had significantly higher percentages of TCRγδ+ T‐cell subsets compared to control dogs. TCRγδ+ IEL (CD8αα+TCRγδ+, CD4−CD8−TCRγδ+) have important regulatory and protective functions in the healthy gut.39 However, in murine IBD models, a pro‐inflammatory role is suspected,40, 41, 42 in which IL‐17‐producing TCRγδ+ T‐cells are able to induce colitis.43 Furthermore, direct correlation between numbers of TCRγδ+ T‐cells in human intestinal mucosa and disease severity in patients suffering from IBD has been demonstrated.44, 45, 46 Similarly, the higher presence of TCRγδ+ lymphocytes in IBD dogs of the present study seems to be linked to greater severity of intestinal inflammation, which was reported in the histopathologic results.
In summary, clinical and histopathologic scores, as well as flow cytometry data for control dogs and dogs with IBD, were compared. The IBD dogs showed significantly higher WSAVA scores as well as CIBDAI scores. An increased percentage of TCRγδ+ T‐cells in IBD dogs was a notable finding, indicating that IEL from IBD dogs express a significantly different immunophenotype compared to control dogs. Future studies are needed to further define the functional relevance of this unique T‐cell subpopulation.
Acknowledgment
Dr Jean Hall is acknowledged for revising this paper. The study was funded by (1) Start‐up grant of the Vetmeduni Vienna (project number PP13010109; title “T‐cell subpopulations in canine chronic enteropathies”) and (2) the grant for doctoral students 2013 of the Vetmeduni Vienna.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
Department for Companion Animals and Horses, Small Animal Clinic, Internal Medicine, University of Veterinary Medicine, Vienna, Austria; Department of Pathobiology, Clinical Pathology Platform, University of Veterinary Medicine, Vienna, Austria; Department of Pathobiology, Institute of Immunology, University of Veterinary Medicine, Vienna, Austria; Department of Pathobiology, Institute of Pathology and Forensic Veterinary Medicine, University of Veterinary Medicine, Vienna, Austria; Department of Biomedical Sciences, Bioinformatics and Biostatistics Platform, University of Veterinary Medicine, Vienna.
Parts of these data have been presented as an abstract at the 2014 American College of Veterinary Internal Medicine Forum, Nashville, TN.
Footnotes
PAA, Pasching, Austria
SAV Liquid Production GmBH, Hochriesstrasse 2, Germany
Carl Roth, Karlsruhe, Germany
Sigma‐Aldrich, Vienna, Austria
Merck, Darmstadt, Germany
BD Biosciences, San Jose, CA
Dako, Glostrup, Denmark
AbD Serotec, Raleigh, NC
Peter F. Moore, California, CA
IBM® Cooperation, Armonk, NY
References
- 1. Allenspach K, Wieland B, Grone A, et al. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J Vet Intern Med 2007;21:700–708. [DOI] [PubMed] [Google Scholar]
- 2. Allenspach K, Rüfenacht S, Sauter S, et al. Pharmacokinetics and clinical efficiacy of cyclosporine treatment of dogs with steroid‐refractory inflammatory bowel diseas. J Vet Intern Med 2006;20:239–244. [DOI] [PubMed] [Google Scholar]
- 3. German AJ, Hall EJ, Day MJ. Chronic intestinal inflammation and intestinal disease in dogs. J Vet Intern Med 2003;17:8–20. [DOI] [PubMed] [Google Scholar]
- 4. Washabau RJ, Day MJ, Willard MD, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med 2010;24:10–26. [DOI] [PubMed] [Google Scholar]
- 5. Gaschen FP, Merchant SR. Adverse food reactions in dogs and cats. Vet Clin North Am Small Anim Pract 2011;41:361–379. [DOI] [PubMed] [Google Scholar]
- 6. Hall EJ. Antibiotic‐responsive diarrhea in small animals. Vet Clin North Am Small Anim Pract 2011;41:273–286. [DOI] [PubMed] [Google Scholar]
- 7. Sartor RB. Mechanisms of disease: Pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 2006;3:390–407. [DOI] [PubMed] [Google Scholar]
- 8. Allenspach K. Clinical immunology and immunopathology of the canine and feline intestine. Vet Clin Small Anim 2011;41:345–360. [DOI] [PubMed] [Google Scholar]
- 9. Simpson KW, Jergens AE. Pitfalls and progress in the diagnosis and management of canine inflammatory bowel disease. Vet Clin North Am Small Anim Pract 2011;41:381–398. [DOI] [PubMed] [Google Scholar]
- 10. Kathrani A, House A, Catchpole B, et al. Polymorphisms in the TLR4 and TLR5 gene are significantly associated with inflammatory bowel disease in German shepherd dogs. PLoS One 2010;5:e15740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fogle JE, Bissett SA. Mucosal immunity and chronic idiopathic enteropathies in dogs. Compend Contin Educ Vet 2007;29:290–302; quiz 306. [PubMed] [Google Scholar]
- 12. McGuckin MA, Eri R, Simms LA, et al. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis 2009;15:100–113. [DOI] [PubMed] [Google Scholar]
- 13. Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011;474:307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suchodolski JS, Dowd SE, Wilke V, et al. 16S rRNA gene pyrosequencing reveals bacterial dysbiosis in the duodenum of dogs with idiopathic inflammatory bowel disease. PLoS One 2012;7:e39333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saurer L, Mueller C. T cell mediated immunoregulation in the gastrointestinal tract. Allergy 2009;64:505–519. [DOI] [PubMed] [Google Scholar]
- 16. Cheroutre H, Madakamutil L. Acquired and natural memory T cells join forces at the mucosal front line. Nat Rev Immunol 2004;4:290–300. [DOI] [PubMed] [Google Scholar]
- 17. van Wijk F, Cheroutre H. Mucosal T cells in gut homeostasis and inflammation. Expert Rev Clin Immunol 2010;6:559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol 2011;11:445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mueller S, Lory J, Corazza N, et al. Activated CD4+ and CD8+ cytotoxic cells are present in increased numbers in the intestinal mucosa from patients with active inflammatory bowel disease. Am J Pathol 1998;152:261–268. [PMC free article] [PubMed] [Google Scholar]
- 20. McVay LD, Li B, Biancaniello R, et al. Changes in human mucosal γδ T cell repertoire and function associated with the disease process in inflammatory bowel disease. Mol Med 1997;3:183–203. [PMC free article] [PubMed] [Google Scholar]
- 21. Junginger J, Schwittlick U, Lemensieck F, et al. Immunohistochemical investigation of Foxp3 expression in the intestine in healthy and diseased dogs. Vet Res 2012;43:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jergens AE, Schreiner CA, Frank DE, et al. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med 2003;17:291–297. [DOI] [PubMed] [Google Scholar]
- 23. Procoli F, Motskula PF, Keyte SV, et al. Comparison of histopathologic findings in duodenal and ileal endoscopic biopsies in dogs with chronic small intestinal enteropathies. J Vet Intern Med 2013;27:268–274. [DOI] [PubMed] [Google Scholar]
- 24. Brunner T, Arnold D, Wasem C, et al. Regulation of cell death and survival in intestinal intraepithelial lymphocytes. Cell Death Differ 2001;8:706–714. [DOI] [PubMed] [Google Scholar]
- 25. Luckschander N, Pfammatter NS, Sidler D, et al. Phenotyping, functional characterization, and developmental changes in canine intestinal intraepithelial lymphocytes. Vet Res 2009;40:58. [DOI] [PubMed] [Google Scholar]
- 26. Poussier P, Ning T, Banerjee D, et al. A unique subset of self‐specific intraintestinal T cells maintains gut integrity. J Exp Med 2002;195:1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol 2001;2:816–822. [DOI] [PubMed] [Google Scholar]
- 28. Keshav S, Vanasek T, Niv Y, et al. A randomized controlled trial of the efficacy and safety of CCX282‐B, an orally‐administered blocker of chemokine receptor CCR9, for patients with Crohn's disease. PLoS One 2013;8:e60094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sonea IM, Jergens AE, Sacco RE, et al. Flow cytometric analysis of colonic and small intestinal mucosal lymphocytes obtained by endoscopic biopsy in the healthy dog. Vet Immunol Immunopathol 2000;77:103–119. [DOI] [PubMed] [Google Scholar]
- 30. Day MJ. Ageing, immunosenescence and inflammageing in the dog and cat. J Comp Pathol 2010;142(Suppl 1):S60–S69. [DOI] [PubMed] [Google Scholar]
- 31. Kleinschmidt S, Meneses F, Nolte I, Hewicker‐Trautwein M. Distribution of mast cell subtypes and immune cell populations in canine intestines: Evidence for age‐related decline in T cells and macrophages and increase of IgA‐positive plasma cells. Res Vet Sci 2008;84:41–48. [DOI] [PubMed] [Google Scholar]
- 32. Hall EJ, German AJ. Inflammatory bowel disease In: Steiner JM, ed. Small Animal Gastroenterology. Hannover: Schlütersche Verlagsgesellschaft GmbH & Co KG; 2008:312–329. [Google Scholar]
- 33. Willard MD, Jergens AE, Duncan RB, et al. Interobserver variation among histopathologic evaluations of intestinal tissues from dogs and cats. J Am Vet Med Assoc 2002;220:1177–1182. [DOI] [PubMed] [Google Scholar]
- 34. Willard MD, Lovering SL, Cohen ND, Weeks BR. Quality of tissue specimens obtained endoscopically from the duodenum of dogs and cats. J Am Vet Med Assoc 2001;219:474–479. [DOI] [PubMed] [Google Scholar]
- 35. Maeda S, Ohno K, Nakamura K, et al. Increased expression of fractalkine and its receptor CX(3)CR1 in canine inflammatory bowel disease and their possible role in recruitment of intraepithelial lymphocytes. Vet Immunol Immunopathol 2012;148:226–235. [DOI] [PubMed] [Google Scholar]
- 36. German AJ, Hall EJ, Day MJ. Analysis of leucocyte subsets in the canine intestine. J Comp Pathol 1999;120:129–145. [DOI] [PubMed] [Google Scholar]
- 37. German AJ, Hall EJ, Moore PF, et al. The distribution of lymphocytes expressing αβ and γδ T‐cell receptors, and the expression of mucosal addressin cell adhesion molecule‐1 in the canine intestine. J Comp Pathol 1999;121:249–263. [DOI] [PubMed] [Google Scholar]
- 38. German AJ, Hall EJ, Day MJ. Immune cell populations within the duodenal mucosa of dogs with enteropathies. J Vet Intern Med 2001;15:14–25. [DOI] [PubMed] [Google Scholar]
- 39. Kuhl AA, Pawlowski NN, Grollich K, et al. Aggravation of intestinal inflammation by depletion/deficiency of γδ T cells in different types of IBD animal models. J Leukoc Biol 2007;81:168–175. [DOI] [PubMed] [Google Scholar]
- 40. Simpson SJ, Hollander GA, Mizoguchi E, et al. Expression of pro‐inflammatory cytokines by TCRαβ+ and TCRγδ+ T cells in an experimental model of colitis. Eur J Immunol 1997;27:17–25. [DOI] [PubMed] [Google Scholar]
- 41. Kawaguchi‐Miyashita M, Shimada S, Kurosu H, et al. An accessory role of TCRγδ+ cells in the exacerbation of inflammatory bowel disease in TCRα mutant mice. Eur J Immunol 2001;31:980–988. [DOI] [PubMed] [Google Scholar]
- 42. Mizoguchi A, Mizoguchi E, de Jong YP, et al. Role of the CD5 molecule on TCR γδ T cell‐mediated immune functions: Development of germinal centers and chronic intestinal inflammation. Int Immunol 2003;15:97–108. [DOI] [PubMed] [Google Scholar]
- 43. Park SG, Mathur R, Long M, et al. T regulatory cells maintain intestinal homeostasis by suppressing γδ T cells. Immunity 2010;33:791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giacomelli R, Parzanese I, Frieri G, et al. Increase of circulating γ/δ T lymphocytes in the peripheral blood of patients affected by active inflammatory bowel disease. Clin Exp Immunol 1994;98:83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yeung MM, Melgar S, Baranov V, et al. Characterisation of mucosal lymphoid aggregates in ulcerative colitis: Immune cell phenotype and TcR‐δγ expression. Gut 2000;47:215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kanazawa H, Ishiguro Y, Munakata A, Morita T. Multiple accumulation of Vdelta2+ gammadelta T‐cell clonotypes in intestinal mucosa from patients with Crohn's disease. Dig Dis Sci 2001;46:410–416. [DOI] [PubMed] [Google Scholar]
- 47. Mason DY, Cordell JL, Tse AGD, et al. The IgM‐associated protein mB 1 as a marker of normal and neoplastic B cells. J Immunol 1991;147:2474–2482. [PubMed] [Google Scholar]
- 48. Rütgen BC, König R, Essler SE, et al. Composition of lymphocyte subpopulations in lymph node aspirates derived from healthy dogs. Vet Clin Pathol 2013. (accepted). [Google Scholar]


