Abstract
Background
Prognostic markers for dogs with thyroid tumors are limited.
Hypothesis/Objectives
To identify clinical, pathologic, and immunohistochemical prognostic factors for dogs with thyroid tumors.
Animals
Seventy dogs with thyroid neoplasia.
Methods
Retrospective study. Dogs with thyroid neoplasia were included when follow‐up information and formalin‐fixed paraffin‐embedded tumor samples were available. Immunohistochemistry (IHC) was performed for thyroglobulin, calcitonin, Ki‐67, and E‐cadherin. Correlation of tumor variables (diameter, volume, localization, scintigraphic uptake, thyroid function, IHC) with local invasiveness and metastatic disease was performed on all tumor samples. Forty‐four dogs treated by thyroidectomy were included in a survival analysis.
Results
Fifty dogs (71%) had differentiated follicular cell thyroid carcinoma (dFTC) and 20 (29%) had medullary thyroid carcinoma (MTC). At diagnosis, tumor diameter (P = .007; P = .038), tumor volume (P = .020), tumor fixation (P = .002), ectopic location (P = .002), follicular cell origin (P = .044), and Ki‐67 (P = .038) were positively associated with local invasiveness; tumor diameter (P = .002), tumor volume (P = .023), and bilateral location (P = .012) were positively associated with presence of distant metastases. Forty‐four dogs (28 dFTC, 16 MTC; stage I–III) underwent thyroidectomy. Outcome was comparable between dogs with dFTC and MTC. Macroscopic (P = .007) and histologic (P = .046) vascular invasion were independent negative predictors for disease‐free survival. Although time to presentation, histologic vascular invasion and Ki‐67 were negatively associated with time to metastases, and time to presentation was negatively associated with time to recurrence, no independent predictors were found. E‐cadherin expression was not associated with outcome.
Conclusions and Clinical Importance
Prognostic factors have been identified that provide relevant information for owners and clinicians.
Keywords: Calcitonin, E‐cadherin, Follicular, Ki‐67, Medullary
Abbreviations
- DFS
disease‐free survival
- dFTC
differentiated follicular cell thyroid carcinoma
- FF‐PE
formalin‐fixed paraffin‐embedded
- FTC
follicular cell thyroid carcinoma
- HE
hematoxylin and eosin
- IHC
immunohistochemistry
- 131I
radioactive iodine‐131
- MTC
medullary thyroid carcinoma
- OS
overall survival
- TM
time to distant metastases
- TR
time to loco‐regional recurrence
- TSH
thyrotropin
- TT4
total thyroxine
- WHO
World Health Organization
Thyroid cancer represents 10–15% of all head and neck neoplasms in the dog.1 Ninety percent of thyroid tumors detected clinically in dogs are carcinomas and up to 38% of dogs with carcinomas present with metastases at the time of diagnosis.2, 3 Thyroidectomy is the preferred treatment modality for tumors that are mobile and well‐circumscribed, whereas unresectable invasive tumors may be treated with external beam radiation or radioactive iodine‐131 (131I) therapy.4, 5, 6
In humans, well‐established prognostic factors for thyroid carcinoma include age, sex, tumor size, stage, histologic type, and grade, vascular invasion, and extrathyroidal tumor extension.7, 8 Low‐risk patients undergo a follow‐up strategy that is considerably different from that of high‐risk patients.9
In dogs, tumor volume >20 cm3, bilateral disease, and cervical vascular invasion have been associated with high metastatic rates.2, 5, 10 However, these associations often were based on necropsy studies or studies in dogs with unresectable tumors, and there are few published studies on prognostic predictors for dogs with operable thyroid tumors. Breed, sex, histologic type, and tumor size did not appear to affect prognosis after surgical resection, whereas bilateral disease and histologic grade of malignancy were prognostic indicators.2, 4, 11, 12 There are few prognostic markers for dogs undergoing thyroidectomy for thyroid neoplasia.
Thyroid carcinoma can arise from thyroid follicular cells (follicular cell thyroid carcinoma, FTC) or from the parafollicular cells (C cells; medullary thyroid carcinoma, MTC).13 According to the World Health Organization (WHO) histologic classification of thyroid tumors in dogs, FTC can be classified as well‐differentiated (dFTC; follicular, compact, follicular‐compact, papillary), poorly differentiated, undifferentiated, or carcinosarcoma.13 In humans, MTC is more aggressive than is dFTC.14 In most veterinary studies, the prevalence of MTC likely is underestimated because these tumors may be difficult to distinguish from dFTC of compact type by microscopic observation alone. Immunohistochemistry (IHC) for calcitonin or for markers of neuroendocrine tissue is required for their identification.15 In 1 study, MTC represented 36% of all thyroid tumors in dogs and was suggested to be more amenable to complete surgical resection and have lower metastatic potential than FTC.15 However, it is still not clear whether canine dFTC and MTC differ with respect to prognosis after thyroidectomy.
E‐cadherin is a transmembrane adhesion glycoprotein of epithelial tissues and plays a role in neoplastic cell behavior as a suppressor of invasion and metastasis.16 In thyroid carcinomas of humans, loss of E‐cadherin expression is an independent prognostic indicator associated with a higher degree of dedifferentiation and higher metastatic potential.16 In dogs with mammary carcinoma, loss of E‐cadherin expression also was found to be related to prognosis.17 The prognostic relevance of E‐cadherin expression in thyroid carcinomas of dogs has not been investigated.
Ki‐67 is a cellular proliferation marker expressed in cell nuclei during all active phases of the cell cycle (G1, S, G2, and mitosis), but not in G0.18 In human dFTC, high Ki‐67 labeling index is associated with higher metastatic rates at diagnosis and shorter disease‐free survival (DFS).19, 20 Although the use of Ki‐67 as a marker for prognosis was shown to have limitations in certain tumors of dogs, its value is well established in mast cell tumors.18, 21 The prognostic relevance of Ki‐67 expression has not yet been examined in thyroid tumors of dogs.
The goals of this study were to identify clinical, pathologic, and immunohistochemical (calcitonin, Ki‐67, and E‐cadherin) prognostic factors for dogs with thyroid neoplasia.
Materials and Methods
Case Selection
The medical record databases of the Companion Animal Clinics of Ghent and Utrecht Universities were searched for dogs diagnosed with thyroid neoplasia from January 1, 1986 to January 1, 2012. For inclusion in the study, the diagnosis had to be confirmed by histopathology. Patients with no follow‐up information and patients for which the paraffin‐embedded tumor samples were not available were excluded.
Medical Records Review
The medical records of the dogs that met the inclusion criteria were reviewed. When complete follow‐up information was not available in the medical record, referring veterinarians and clients were contacted by phone. Information retrieved from medical records included signalment, time to presentation (from detection of cervical mass to diagnosis), owner impression of tumor progression rate, clinical signs, physical examination, tumor mobility (determined by palpation), tumor measurements, imaging results (thoracic radiographs, cervical or thoracic scintigraphy, cervical ultrasonography, computed tomography [CT]), WHO tumor stage, treatment, surgery report, outcome, and necropsy report.22 Whenever possible, dimensions of primary tumors were based on measurements taken immediately after surgical excision or necropsy and alternatively based on measurements taken during CT scan, cervical ultrasonography or physical examination. The volume of each thyroid gland tumor was estimated by the use of an ellipsoid formula:23
When >1 thyroid lobe was affected, the sum of both lobes and ectopic tissue (if present) was used.
Staging was performed according to the WHO staging system (Table 1), based on tumor dimensions, thoracic radiographs, cervical and thoracic scintigraphic examination, and, when available, cytology and histopathology of regional lymph nodes (LNs; mandibular, retropharyngeal, and superficial cervical). In most cases, regional LNs were aspirated or excised (for patients undergoing thyroidectomy) when found to be enlarged at physical examination or surgical exploration. In a subset of patients, cervical ultrasonography and CT scan also aided the evaluation of regional LNs. Regional LN metastases were confirmed by histopathology or by macroscopic evidence of LN invasion during surgery.
Table 1.
World Health Organization's clinical staging system for dogs with thyroid tumors.22
| Stage | Primary Tumor | Regional LN | Distant Metastases |
|---|---|---|---|
| I | T1 a,b | N0 | M0 |
| II | T0 | N1 | M0 |
| T1 a,b | N1 | M0 | |
| T2 a,b | N0 or N1 a | M0 | |
| III | T3 | Any N | M0 |
| Any T | N1 b or N2 b | M0 | |
| IV | Any T | Any N | M1 |
T0, microscopic residual disease; T1, <2 cm; T2, 2–5 cm; T3, >5 cm; N0, no lymph node involvement; N1, ipsilateral lymph node involvement; N2, bilateral lymph node involvement; M0, no evidence of distant metastases; M1, evidence of distant metastases; a, freely movable; b, fixed.
Thyroid function status at the time of diagnosis was determined based on basal circulating total thyroxine (TT4) and thyrotropin (TSH) concentrations and, when available, results of TSH stimulation testing.
Surgical and necropsy reports were reviewed to determine if there was macroscopic evidence of vascular or local invasion by the primary tumor. Macroscopic vascular invasion was defined as evidence of tumor thrombi in the cervical blood vessels. Macroscopic local invasion was defined as evidence of growth of the primary tumor into neighboring tissues (eg, cervical muscles, esophagus, and trachea).
Overall survival (OS), DFS, time to loco‐regional recurrence (TR) and time to distant metastases (TM) were determined for dogs treated by thyroidectomy. OS was defined as time from thyroidectomy to death caused by thyroid neoplasia; patients still alive at the last observation time and patients that died of other causes were censored. If the cause of death could not be determined, it was assumed to be related to thyroid neoplasia. DFS was defined as TR, TM, or time to death caused by thyroid neoplasia, whichever came first. Patients disease‐free at the last observation time and patients that died of other causes were censored. TR was defined as time from thyroidectomy to local recurrence or regional LN metastases, with dogs censored at last follow‐up whenever physical examination disclosed no recurrence or at the time of death if necropsy disclosed no tumor recurrence or LN metastases. For TM, distant metastasis was the event of interest, with dogs censored at the last observation time when thoracic radiographs showed no signs of metastatic disease, or at the time of death if necropsy identified no distant metastases.
Tumor Specimens
Formalin‐fixed paraffin‐embedded (FF‐PE) tissue blocks for each patient were collected from the Departments of Pathology of Ghent and Utrecht Universities and, in some dogs, multiple blocks from 1 tumor site or blocks from multiple sites (local, regional lymph node, distant metastases) were available. All samples were obtained at surgery or necropsy. In total, 304 FF‐PE blocks from 74 patients (52 from Utrecht University, 22 from Ghent University) were available.
Histopathology
Five‐μm sections from each FF‐PE block were stained with hematoxylin and eosin (HE). All HE‐stained slides were reviewed by a single board‐certified pathologist (RD) blinded to clinical information and the previous histopathology report.
The distinction between adenoma and carcinoma was based on histologic evidence of capsular invasion, vascular invasion, or metastases. The histologic type of primary tumors was classified according to the WHO classification as tumors of follicular cell origin (follicular, compact, follicular‐compact, papillary, poorly differentiated, undifferentiated, carcinosarcoma) or C‐cell (medullary) origin.13 Classification of medullary thyroid tumors also was based on IHC for calcitonin. Follicular cell carcinomas classified as poorly differentiated (n = 0), undifferentiated (n = 1) or carcinosarcoma (n = 3) were excluded because of their aggressive biologic behavior.
Primary thyroid tumors were characterized histologically by local invasion (tumor growth into neighboring tissues observed or not observed), vascular invasion (tumor growth into blood vessels observed or not observed), capsular invasion (not observed, invasion into tumor capsule, invasion beyond tumor capsule), necrosis (0, 1–25%, 26–50%, >50%), hemorrhage (0, ≤50%, >50%), nuclear pleomorphism (0, 1–25%, 26–50%, >50%), and mitotic index.24 Estimation of percentage necrosis and percentage hemorrhage was based on the observation of the entire section at 200× magnification. Percentage nuclear pleomorphism and mitotic index were estimated after observation of at least 10 random fields at 400× magnification.
Immunohistochemistry
Immunohistochemistry was performed as previously described.25 Sections were incubated overnight with the primary antibodies in a humidity chamber at 4°C (Table 2). Preliminary evaluation of the optimal concentration of each primary antibody was performed with serial antibody dilutions using the respective positive controls (Table 2); normal canine thyroid tissue was used as negative control. The subset of tumors positive for calcitonin also was stained for thyroglobulin in an automated immunostainer (Dako; S/N S38‐7410‐01).
Table 2.
Antibodies used for immunohistochemistry
| Antibody | Antibody | Antibody Type | Dilution | Positive Control | Negative Control |
|---|---|---|---|---|---|
| Thyroglobulin | A 0251a | Rabbit polyclonal | 1 : 800 | Normal canine thyroid gland (follicular cells) | Normal canine thyroid gland (C cells) |
| Calcitonin | A 0576a | Rabbit polyclonal | 1 : 400 | Normal canine thyroid gland (C cells) | Normal thyroid gland (follicular cells) |
| Ki‐67 | MIB‐1a | Mouse monoclonal | 1 : 200 | Normal canine small intestine | Normal canine thyroid gland |
| E‐cadherin | NCH‐38a | Mouse monoclonal | 1 : 200 | Normal canine thyroid gland |
Dako, Glostrup, Denmark.
Quantification of Immunoreactive Cells
All slides were examined by the same observer (MC), blinded to the clinical information and outcome of each patient. Quantification of Ki‐67 labeling index was performed by evaluating each slide through an optical grid at 400× magnification. In the region of highest positivity of the section, fields were chosen randomly using a minimum of 10 fields per section and counting at least 500 cells per section. Only neoplastic cells with nuclear staining were recorded as positive. The labeling index was calculated as the number of positive cells divided by the number of positive plus negative cells (Fig 1).
Figure 1.
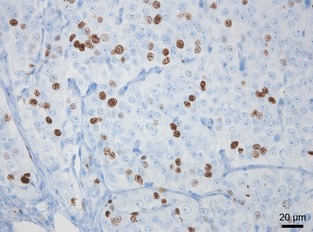
Immunohistochemical staining of Ki‐67 in a canine medullary thyroid carcinoma with a labeling index of 28.4% (400×).
Quantification of E‐cadherin immunolabeling was performed by examining the entire section at 200× magnification and estimating the percentage of neoplastic cells with labeling of membranous E‐cadherin. Tumors were classified according to membranous immunolabeling in 0–5%, 6–30%, 31–60%, 61–90%, and >90% of positive cells (Fig 2).
Figure 2.
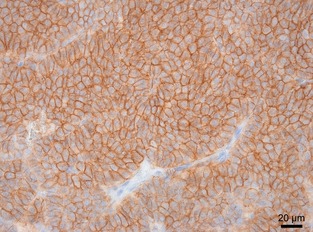
Immunohistochemical staining of E‐cadherin in a canine medullary thyroid carcinoma with >90% of positive cells (400×).
Tumors positive for calcitonin were classified as medullary tumors and tumors negative to calcitonin were classified as follicular cell tumors.15 To ensure the accuracy of this classification, the subset of tumors positive for calcitonin also was stained for thyroglobulin. Calcitonin and thyroglobulin immunolabeling were not quantified. The tumor was considered positive when the cytoplasm of neoplastic cells exhibited a fine granular staining pattern with cell‐to‐cell variation (Fig 3).
Figure 3.
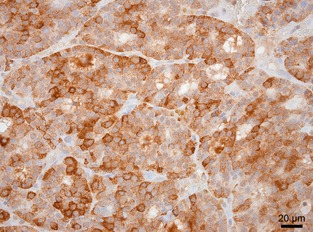
Immunohistochemical staining of calcitonin in a canine medullary thyroid carcinoma (400×).
Statistical Analysis
Correlating Tumor Variables with Local Invasiveness and Metastases at Time of Diagnosis
For binary outcomes, statistical analysis was based on the logistic regression model using exact tests and an exact odds ratio calculation.1 The binary response variables corresponded to macroscopic local invasion, histologic local invasion, and distant metastases at diagnosis. Tumor maximum diameter, tumor volume, tumor localization, scintigraphic uptake, thyroid functional status, histologic type, Ki‐67 labeling index, and E‐cadherin expression were introduced in the binary regression model as covariates. Significance level was set at 5%. For tumor localization, the significance level was adjusted for multiple comparisons (Bonferroni correction).
Comparisons between follicular cell and medullary tumors with respect to the Ki‐67 labeling index and E‐cadherin expression were based on the Mann‐Whitney U‐test.
Survival Analysis
The effect of each variable on OS, DFS, TM, and TR was evaluated with the Cox proportional hazards model.1 Each variable was incorporated in a univariate analysis, either as categorical or continuous. The variables analyzed included: age, time to presentation, weight loss, owner impression of tumor progression rate, body condition (determined during physical examination), tumor mobility, tumor maximum diameter (cm), tumor volume (cm3), thyroid function, tumor scintigraphic uptake, WHO tumor stage, macroscopic vascular invasion, histologic features (histologic type, vascular invasion, capsular invasion, local invasion, percentage necrosis, percentage hemorrhage, nuclear pleomorphism, and mitotic index), Ki‐67 labeling index, E‐cadherin expression, and levothyroxine supplementation. Variables found to have an effect on outcome at the 5% significance level were incorporated in a multivariate analysis to rule out associated effects.
Results
Clinical Data (n = 70)
Seventy dogs were included. Relevant clinical data are summarized in Table 3.
Table 3.
Summary of clinical data organized by histologic tumor type in 70 dogs with thyroid tumors
| Histologic Type | ||
|---|---|---|
| dFTC | MTC | |
| Age | n = 50 | n = 19 |
| Median | 10 | 9 |
| Range | 4–14 | 4–16 |
| Sex | n = 50 | n = 20 |
| Male | 30 (60%) | 8 (40) |
| Female | 20 (40%) | 12 (60%) |
| Weight loss | n = 48 | n = 19 |
| Present | 18 (38%) | 4 (21%) |
| Absent | 30 (62%) | 15 (79%) |
| Body condition | n = 43 | n = 16 |
| Emaciated | 10 (23%) | 2 (13%) |
| Ideal | 27 (63%) | 13 (81%) |
| Obese | 6 (14%) | 1 (6%) |
| Stridor | n = 50 | n = 19 |
| Present | 5 (10%) | |
| Absent | 45 (90%) | 19 (100%) |
| Dyspnea | n = 49 | n = 19 |
| Present | 5 (10%) | |
| Absent | 44 (90%) | 19 (100%) |
| Tumor localization | n = 50 | n = 19 |
| Unilateral | 38 (76%) | 19 (100) |
| Bilateral | 8 (16%) | |
| Ectopic (ventral larynx) | 4 (8%) | |
| Tumor mobility | n = 38 | n = 19 |
| Mobile | 21 (55%) | 11 (69%) |
| Fixed | 17 (45%) | 5 (31%) |
| Tumor diameter (cm) | n = 49 | n = 19 |
| Median | 5 | 4 |
| Range | 1.8–120 | 2.5–8.5 |
| Tumor volume (cm3) | n = 47 | n = 16 |
| Median | 25.7 | 23.2 |
| Range | 2–290 | 4–117 |
| Thyroid function | n = 43 | n = 14 |
| Hypothyroid | 1 (2%) | 1 (7%) |
| Euthyroid | 17 (40%) | 9 (64%) |
| Hyperthyroid | 12 (28%) | |
| Eu/hypothyroid | 13 (30%) | 4 (29%) |
| Tumor scintigraphic uptake | n = 38 | n = 13 |
| Decreased | 8 (21%) | 7 (54%) |
| Normal/increased | 30 (79%) | 6 (46%) |
| Macroscopic local invasion | n = 38 | n = 17 |
| Present | 9 (24%) | |
| Absent | 29 (76%) | 17 (100%) |
| Histologic local invasion | n = 50 | n = 20 |
| Present | 8 (20%) | |
| Absent | 42 (80%) | 20 (100%) |
| Stage | n = 50 | n = 19 |
| I | 1 (2%) | |
| II | 21 (42%) | 10 (52%) |
| III | 17 (34%) | 6 (32%) |
| IV | 11 (22%) | 3 (16%) |
dFTC, differentiated follicular cell thyroid carcinoma; MTC, medullary thyroid carcinoma.
All hyperthyroid dogs for which clinical signs were recorded (13 of 14 dogs) had clinical signs compatible with hyperthyroidism. Total serum calcium concentration was measured in 5 dogs with MTC and was normal in all dogs.
Distant metastases at the time of diagnosis most were frequently located in the lungs (12 dFTC, 2 MTC) and liver (2 FTC). One dog with MTC had signs of metastasis or ectopic thyroid tissue in the mediastinum. Regional LN metastases were identified in 11 dogs.
Forty‐four dogs were treated by thyroidectomy (MST 22 months), 3 dogs underwent debulking (MST 10 months), 4 dogs were treated with thyroidectomy and 131I (MST 13 months), 2 dogs were treated with thyroidectomy and chemotherapy (MST 11 months), 1 dog was treated with thyroidectomy and external beam radiation (survival time 13 months), 6 dogs received no treatment (MST 1.5 months), and 10 dogs were euthanized at diagnosis. Necropsy was performed in 24 dogs.
Eighteen dogs had macroscopic or histologic evidence of local invasion or both; 7 dogs had only macroscopic local invasion and 7 dogs had only histologic local invasion.
All thyroid tumors were carcinomas; 50 (71%) tumors were classified as dFTC and 20 (29%) as MTC after calcitonin immunostaining (Fig 3). The dFTC were classified as follicular (n = 13, 19%), compact (n = 19, 27%), follicular‐compact (n = 17, 24%), and follicular‐papillary (n = 1, 1%). Thyroglobulin staining was negative in all MTC except in 1 dog considered to have a rare variant of MTC with mixed expression of calcitonin and thyroglobulin.26
Clinical Data of Dogs Treated by Thyroidectomy (n = 44)
Tumor mobility was recorded in 36 dogs; 27 dogs had freely movable tumors and 9 had fixed tumors. Dogs were staged as stage I (n = 1), stage II (n = 26), and stage III (n = 16). In 1 dog, although there was no evidence of metastases on imaging, tumor stage (I–III) could not be determined because tumor measurements were not recorded. Thyroidectomy was unilateral in 42 dogs, bilateral in 1 dog, and 1 dog underwent surgical excision of an ectopic thyroid tumor ventral to the larynx. Surgical reports were available for 42 dogs and no dog had macroscopic evidence of local invasion. Median follow‐up time was 11 months (range, 0–60 months).
Of the 44 dogs treated with thyroidectomy, 28 dogs had dFTC (follicular n = 12; follicular‐compact n = 9; compact n = 7) and 16 dogs had MTC. Thirteen dogs with dFTC received lifelong levothyroxine replacement treatment after thyroidectomy.
After thyroidectomy, 4 of 19 dogs with dFTC (21%) and 5 of 12 dogs with MTC (42%) developed distant metastases. Furthermore, 4 of 25 dogs with dFTC (16%) and 3 of 14 dogs with MTC (21%) developed loco‐regional recurrence: locally (1 dFTC, 1 MTC), in regional LNs (3 dFTCs, 1 MTC), and locally and in regional LNs (1 MTC).
Correlating Tumor Variables with Local Invasiveness and Metastases at Time of Diagnosis
The analysis was performed in all 70 dogs (50 dFTCs, 20 MTCs) regardless of treatment modality (Table 4).
Table 4.
Correlating tumor variables with local invasiveness and distant metastases at diagnosis in 70 dogs with thyroid tumors
| Macroscopic Local Invasion | Histologic Local Invasion | Distant Metastases | ||||
|---|---|---|---|---|---|---|
| OR | P Value | OR | P Value | OR | P Value | |
| Tumor diameter | 1.29 | .007 | 1.13 | .038 | 1.29 | .002 |
| Tumor volume | 1.02 | .020 | 1.01 | .139 | 1.01 | .023 |
| Tumor localization a | .156 | .001 | .018 | |||
| Unilateral versus Bilateral | 0.15 | .214 | 0.07 | .027 | 0.10 | .012 |
| Unilateral versus Ectopic | 0.30 | .728 | 0.02 | .002 | 0.50 | .963 |
| Bilateral versus Ectopic | 1.81 | 1.000 | 0.23 | .546 | 4.34 | .546 |
| Tumor mobility (fixed) | 5.86 | .089 | 18.74 | .002 | 2.58 | .310 |
| Scintigraphic uptake (decreased) | 1.24 | 1.000 | 1.23 | 1.000 | 1.24 | 1.000 |
| Thyroid function (hyper thyroidism) | 0.36 | .660 | 0.50 | .920 | 0.29 | .426 |
| Histologic type (dFTC) | 6.89 | .044 | 5.03 | .095 | 1.59 | .743 |
| Ki‐67 | 1.06 | .038 | 1.05 | .210 | 1.01 | .147 |
| E‐cadherin | 1.03 | 1.000 | 1.05 | 1.000 | 1.23 | .528 |
OR, odds ratio; dFTC, differentiated follicular cell thyroid carcinoma.
significance level adjusted for multiple comparisons (Bonferroni correction).
P values in bold show a statistically significant association.
Tumor diameter (P = .007), tumor volume (P = .020), follicular cell origin (P = .044), and Ki‐67 (P = .038) were positively associated with macroscopic local invasion. Tumor diameter (P = .038), ectopic location (P = .002), and tumor fixation on palpation (P = .002) were positively associated with histologic local invasion. Tumor diameter (P = .002), tumor volume (P = .023), and bilateral location (P = .012) were positively associated with presence of distant metastases at diagnosis.
Presence of distant metastases at diagnosis was not significantly different between dogs with dFTC and MTC (P = .743).
E‐cadherin expression, tumor scintigraphic uptake, and thyroid functional status were not associated with either local invasiveness or distant metastases at diagnosis.
E‐cadherin expression was significantly higher (P = .003) in MTC (median immunolabeling, 91–100%) compared to dFTC (median immunolabeling, 31–60%; Fig 2). Ki‐67 labeling index was not significantly different between dFTC and MTC (P = .668).
Survival Analysis
The 44 dogs that underwent thyroidectomy were included in the survival analysis.
Overall survival, DFS, TM, and TR were not significantly different between dogs with dFTC and MTC (Table 5). Given that histologic tumor type and levothyroxine treatment had no significant effect on outcome, all 44 dogs were analyzed together.
Table 5.
Comparison of outcome between 28 dogs with differentiated follicular cell thyroid carcinoma and 16 dogs with medullary thyroid carcinoma treated with thyroidectomy
| dFTC | MTC | Overall | N | P Value | |
|---|---|---|---|---|---|
| OS (months) | |||||
| Median | 17 | 42 | 22 | 44 | 1.00 |
| Range | 1–60 | 0.3–57 | 0.3–60 | ||
| DSF (months) | |||||
| Median | 17 | 15 | 17 | 44 | .58 |
| Range | 0.3–60 | 0.3–45 | 0.3–60 | ||
| TM (months) | |||||
| Median | 60 | 32 | 42 | 31 | .24 |
| Range | 1–60 | 2–42 | 1–60 | ||
| TR (months) | |||||
| Median | >60 | >42 | >60 | 39 | .59 |
| Range | 0.3–60 | 0.5–42 | 0.3–60 | ||
dFTC, differentiated follicular cell thyroid carcinoma; MTC, medullary thyroid carcinoma; OS, overall survival; DSF, disease‐free survival; TM, time to distant metastases; TR, time to loco‐regional recurrence.
Results of the univariate analysis indicated that macroscopic vascular invasion was negatively associated with OS (P = .011, Table 6). Macroscopic (P = .001) and histologic (P = .037) vascular invasion were negatively associated with DFS. Time to presentation (P = .040), histologic vascular invasion (P = .018), and Ki‐67 labeling index (P = .004) were negatively associated with TM. Each month delay in presentation and each 1% increase in Ki‐67 labeling index increased the risk for distant metastases by 14% and 24%, respectively. Time to presentation was negatively associated with TR (P = .038). Each month delay in presentation increased the risk for loco‐regional recurrence by 14%.
Table 6.
Summary of univariate and multivariate survival analyses of 44 dogs with thyroid tumors treated by thyroidectomy
| OS | DFS | TM | TR | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Univariate | ||||||||
| Time to presentation (n = 38) | 1.01 (0.94–1.09) | .763 | 1.03 (0.96–1.11) | .453 | 1.14 (1.01–1.30) | .040 | 1.14 (1.01–1.28) | .038 |
| Macroscopic vascular invasion: Present (n = 4 of 37) | 4.37 (1.39–13.7) | .011 | 10.0 (2.62–38.5) | .001 | a | 0.04 (0.00–5E11) | .837 | |
| Histologic vascular invasion: Present (n = 24 of 44) | 1.89 (0.87–4.11) | .109 | 2.36 (1.05–5.31) | .037 | 12.7 (1.55–105) | .018 | 2.33 (0.51–10.8) | .277 |
| Ki‐67 labeling index (n = 44) | 1.02 (0.98–1.07) | .261 | 1.04 (1.00–1.07) | .066 | 1.24 (1.07–1.44) | .004 | 0.98 (0.86–1.13) | .801 |
| Multivariate | ||||||||
| Macroscopic vascular invasion | 47.5 (2.92–773) | .007 | ||||||
| Histologic vascular invasion | 2.88 (1.02–8.18) | .046 | ||||||
OS, overall survival; DSF, disease‐free survival; TM, time to distant metastases; TR, time to loco‐regional recurrence.
Not performed because only 1 dog in the analysis had macroscopic evidence of vascular invasion.
P values in bold show a statistically significant association.
Given their significant effect on outcome, the above‐mentioned variables were included in a multivariate analysis (Table 6; Fig 4). Macroscopic (P = .007) and histologic (P = .046) vascular invasion were independent negative predictors for DFS. No independent predictors were found for OS, TM, and TR.
Figure 4.
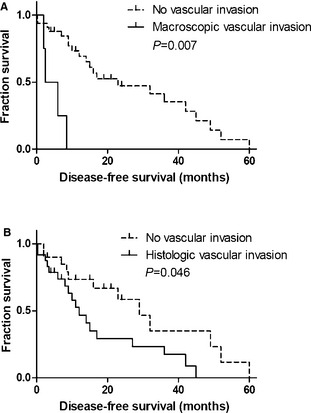
Kaplan‐Meier survival curves for 44 dogs with thyroid gland tumors treated with thyroidectomy. (A) macroscopic vascular invasion n = 4, median disease‐free survival (DFS) 2.5 months; no vascular invasion n = 33, median DFS 23 months; (B) histologic vascular invasion n = 24, median DFS 12 months; no vascular invasion n = 20, median DFS 29 months.
Age, weight loss, tumor progression rate, body condition, stridor, dyspnea, tumor mobility, tumor diameter, tumor volume, stage (I–III), thyroid function, tumor scintigraphic uptake, histologic type, histologic local invasion, capsular invasion, necrosis, hemorrhage, nuclear pleomorphism, mitotic index, and E‐cadherin expression had no significant effect on OS, DSF, TM, or TR.
Discussion
There are few prognostic markers for dogs with thyroid tumors undergoing thyroidectomy. In our exploratory analysis, macroscopic and histologic vascular invasion were independent negative predictors for DFS.
At the time of diagnosis, tumor fixation was associated with histologic local invasion but not with macroscopic local invasion. Furthermore, of 9 dogs with fixed tumors treated by thyroidectomy, no dog had macroscopic evidence of local invasion and only 1 dog had histologic local invasion. In agreement with a previous study, these finding indicate that palpation is not always an accurate predictor of local invasiveness.23
In our study, the prevalence of hyperthyroidism in dFTC (28%) was similar to previous reports.27 It can be hypothesized that functional tumors (in dogs with hyperthyroidism or with preserved scintigraphic uptake) are more differentiated and, therefore, carry a better prognosis. However, patient thyroid function and tumor scintigraphic uptake had no significant effect on outcome.
Time to presentation was negatively associated with TR, which may be a result of delayed diagnosis and treatment. In a recent retrospective study in dogs with thyroid tumors, the effect of duration of clinical signs on survival approached statistical significance.11 In people, time to treatment has been shown to be independently associated with thyroid cancer mortality.28
In agreement with an earlier study, tumor diameter was positively associated with incidence of distant metastases at diagnosis.10 However, after thyroidectomy, tumor diameter was not associated with outcome as previously reported.11, 12 In humans, the risk of thyroid tumor recurrence and cancer‐related mortality increases linearly with tumor size.28
Macroscopic vascular invasion was negatively associated with OS and was an independent negative predictor for DFS. This is in agreement with earlier reports and is not surprising given the extensive degree of neoplastic vessel infiltration necessary for macroscopic observation.2 In humans with FTC, extensive vascular invasion is rare but also is reported to carry a poor prognosis.29
Histologic vascular invasion was negatively associated with TM and was an independent negative predictor for DFS. Our results are in agreement with an earlier study showing the prognostic value of histologic grade of malignancy in dogs with thyroid cancer.12 In that study, vascular invasion was one of the most important histologic criteria used for the overall grade of malignancy. Although in our study histologic vascular invasion was negatively associated with TM and DFS, no association was found with OS. This may be because of the fact that thyroid cancer metastases can have an indolent progression and are not always associated with rapid clinical deterioration. The fact that overall median TM was approximately twice median OS supports this possibility. In humans with dFTC, histologic vascular invasion is an independent predictor of cancer‐related mortality.30 Our study suggests that dogs with this histologic feature are at high risk for metastatic disease after thyroidectomy. Further research is necessary to determine if postoperative adjunctive treatment (eg, 131I) can improve the outcome of these patients.
In this study, 29% of thyroid tumors in dogs were MTC based on IHC for calcitonin. This is in accordance with previous studies in which 16–36% of thyroid carcinomas were MTC.15, 31 Medullary thyroid carcinoma may be difficult to distinguish from dFTC of compact type by microscopic observation alone, and earlier studies likely underestimated their prevalence.15 In fact, 18 of 20 MTC in our study were provisionally classified as dFTC of compact type and 2 as dFTC of follicular‐compact type, before IHC for calcitonin. This emphasizes the importance of routine IHC for identification of MTC.
One of the goals of our study was to investigate the prognostic relevance of differentiating dFTC from MTC with IHC. A previous study suggested that canine MTC may have a less malignant biologic behavior with a higher rate of complete surgical excision and lower incidence of metastases at diagnosis compared to dFTC.15 In accordance with this report, we found that MTC were significantly less likely to be locally invasive at presentation. However, we found no difference in the incidence of metastatic disease at diagnosis. More importantly, after thyroidectomy, OS, DFS, TM, and TR were not significantly different between dogs with dFTC and MTC. This suggests that, although MTC is less invasive and thus more amenable to complete surgical resection, after thyroidectomy the outcome is comparable to dFTC.
Ki‐67 labeling index was negatively associated with TM in our study, but only in the univariate analysis. In humans, Ki‐67 is associated with clinical stage and survival in both dFTC and MTC.20, 32 It has been proposed that human thyroid carcinomas with Ki‐67 labeling index <5% have a more benign clinical course and that tumors with labeling index >15% have a more aggressive biologic behavior.33 Interestingly, at the time of diagnosis, Ki‐67 was positively associated with local invasiveness but not with distant metastases.
In humans with thyroid cancer, loss of E‐cadherin protein expression is a negative prognostic indicator, but we found no association between E‐cadherin expression and outcome.16 Furthermore, no correlation was observed between E‐cadherin expression and local invasiveness or distant metastases at diagnosis. This observation suggests that other factors are more important in the development of local invasion and metastatic disease in dogs with thyroid cancer. Still, it was interesting to note that E‐cadherin expression was significantly higher in MTC compared to dFTC.
The benefit of TSH‐suppressive treatment with levothyroxine is well established in high‐risk human patients with dFTC, and we recommend it routinely in the treatment of canine FTC.34 In our study, because levothyroxine was mainly administered as substitution treatment for dogs with low TT4 concentrations after thyroidectomy, the lack of significant effect on survival might be because of insufficient TSH suppression.
Limitations of our study include its retrospective and exploratory nature. Although review of IHC slides was only performed by 1 observer, which may decrease the accuracy of scoring, it maximizes consistency of comparative scoring between slides.
In conclusion, our study suggests that macroscopic and histologic evidence of vascular invasion are independent negative predictors for DFS in dogs with surgically excised thyroid carcinoma. Canine dFTC and MTC seem to have comparable outcomes after thyroidectomy.
Acknowledgments
The authors thank the Department of Pathobiology of Utrecht University for providing all paraffin‐embedded tumor samples of patients diagnosed and treated at Utrecht University. We also thank the Department of Morphology of Ghent University for their collaboration in sectioning the paraffin‐embedded tumor samples.
This research was supported by the ECVIM‐CA Clinical Studies Fund and the Special Research Fund of Ghent University (grant no. 01J02510).
Conflict of Interest Declaration: The authors disclose no conflict of interest.
This work was performed at Ghent University, Belgium, and Utrecht University, The Netherlands.
Footnotes
SAS 9.3, Cary, NC
References
- 1. Loar AS. Canine thyroid tumors In: Kirk RW, ed. Current Veterinary Therapy. Philadelphia, PA: WB Saunders Co; 1986:1033–1039. [Google Scholar]
- 2. Harari J, Patterson JS, Rosenthal RC. Clinical and pathologic features of thyroid tumors in 26 dogs. J Am Vet Med Assoc 1986;188:1160–1164. [PubMed] [Google Scholar]
- 3. Wucherer KL, Wilke V. Thyroid cancer in dogs: An update based on 638 cases (1995–2005). J Am Anim Hosp Assoc 2010;46:249–254. [DOI] [PubMed] [Google Scholar]
- 4. Klein MK, Powers BE, Withrow SJ, et al. Treatment of thyroid carcinoma in dogs by surgical resection alone: 20 cases (1981–1989). J Am Vet Med Assoc 1995;206:1007–1009. [PubMed] [Google Scholar]
- 5. Theon AP, Marks SL, Feldman ES, et al. Prognostic factors and patterns of treatment failure in dogs with unresectable differentiated thyroid carcinomas treated with megavoltage irradiation. J Am Vet Med Assoc 2000;216:1775–1779. [DOI] [PubMed] [Google Scholar]
- 6. Turrel JM, McEntee MC, Burke BP, et al. Sodium iodide 131I treatment of dogs with nonresectable thyroid tumors: 39 cases (1990–2003). J Am Vet Med Assoc 2006;229:542–548. [DOI] [PubMed] [Google Scholar]
- 7. Gilliland FD, Hunt WC, Morris DM, et al. Prognostic factors for thyroid carcinoma. A population‐based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer 1997;79:564–573. [DOI] [PubMed] [Google Scholar]
- 8. Tubiana M, Schlumberger M, Rougier P, et al. Long‐term results and prognostic factors in patients with differentiated thyroid carcinoma. Cancer 1985;55:794–804. [DOI] [PubMed] [Google Scholar]
- 9. Sipos JA, Mazzaferri EL. Differentiated thyroid carcinoma In: Cooper DS, ed. Medical Management of Thyroid Disease, 2nd ed New York: Informa Healthcare USA, Inc.; 2008:237–295. [Google Scholar]
- 10. Leav I, Schiller AL, Rijnberk A, et al. Adenomas and carcinomas of the canine and feline thyroid. Am J Pathol 1976;83:61–122. [PMC free article] [PubMed] [Google Scholar]
- 11. Nadeau ME, Kitchell BE. Evaluation of the use of chemotherapy and other prognostic variables for surgically excised canine thyroid carcinoma with and without metastasis. Can Vet J 2011;52:994–998. [PMC free article] [PubMed] [Google Scholar]
- 12. Verschueren C. Clinico‐pathological and Endocrine Aspects of Canine Thyroid Cancer. Utrecht: Rijksuniversiteit Utrecht; 1992:11–25. PhD Thesis. [Google Scholar]
- 13. Kiupel M, Capen C, Miller M, et al. Histological classification of the endocrine system of domestic animals In: Schulman FY, ed. WHO International Histological Classification of Tumors of Domestic Animals. Washington, DC: Armed Forces Institute of Pathology; 2008:25–39. [Google Scholar]
- 14. Hundahl SA, Fleming ID, Fremgen AM, et al. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer 1998;83:2638–2648. [DOI] [PubMed] [Google Scholar]
- 15. Carver JR, Kapatkin A, Patnaik AK. A comparison of medullary thyroid carcinoma and thyroid adenocarcinoma in dogs: A retrospective study of 38 cases. Vet Surg 1995;24:315–319. [DOI] [PubMed] [Google Scholar]
- 16. von Wasielewski R, Rhein A, Werner M, et al. Immunohistochemical detection of E‐cadherin in differentiated thyroid carcinomas correlates with clinical outcome. Cancer Res 1997;57:2501–2507. [PubMed] [Google Scholar]
- 17. Matos AJ, Lopes C, Carvalheira J, et al. E‐cadherin expression in canine malignant mammary tumours: Relationship to other clinico‐pathological variables. J Comp Pathol 2006;134:182–189. [DOI] [PubMed] [Google Scholar]
- 18. Zacchetti A, van Garderen E, Teske E, et al. Validation of the use of proliferation markers in canine neoplastic and non‐neoplastic tissues: Comparison of KI‐67 and proliferating cell nuclear antigen (PCNA) expression versus in vivo bromodeoxyuridine labelling by immunohistochemistry. APMIS 2003;111:430–438. [DOI] [PubMed] [Google Scholar]
- 19. Erickson LA, Jin L, Wollan PC, et al. Expression of p27kip1 and Ki‐67 in benign and malignant thyroid tumors. Mod Pathol 1998;11:169–174. [PubMed] [Google Scholar]
- 20. Tallini G, Garcia‐Rostan G, Herrero A, et al. Downregulation of p27KIP1 and Ki67/Mib1 labeling index support the classification of thyroid carcinoma into prognostically relevant categories. Am J Surg Pathol 1999;23:678–685. [DOI] [PubMed] [Google Scholar]
- 21. Maglennon GA, Murphy S, Adams V, et al. Association of Ki67 index with prognosis for intermediate‐grade canine cutaneous mast cell tumours. Vet Comp Oncol 2008;6:268–274. [DOI] [PubMed] [Google Scholar]
- 22. Owen LN. TNM Classification of Tumours in Domestic Animals. Geneva: World Health Organization; 1980:51–52. [Google Scholar]
- 23. Taeymans O, Penninck DG, Peters RM. Comparison between clinical, ultrasound, CT, MRI, and pathology findings in dogs presented for suspected thyroid carcinoma. Vet Radiol Ultrasound 2013;54:61–70. [DOI] [PubMed] [Google Scholar]
- 24. Kirpensteijn J, Kik M, Rutteman GR, et al. Prognostic significance of a new histologic grading system for canine osteosarcoma. Vet Pathol 2002;39:240–246. [DOI] [PubMed] [Google Scholar]
- 25. Campos M, Ducatelle R, Kooistra HS, et al. Immunohistochemical expression of potential therapeutic targets in canine thyroid carcinoma. J Vet Intern Med 2014;28:564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mizukami Y, Nonomura A, Michigishi T, et al. Mixed medullary‐follicular carcinoma of the thyroid gland: A clinicopathologic variant of medullary thyroid carcinoma. Mod Pathol 1996;9:631–635. [PubMed] [Google Scholar]
- 27. Marks SL, Koblik PD, Hornof WJ, et al. 99mTc‐pertechnetate imaging of thyroid tumors in dogs: 29 cases (1980–1992). J Am Vet Med Assoc 1994;204:756–760. [PubMed] [Google Scholar]
- 28. Mazzaferri EL, Jhiang SM. Long‐term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 1994;97:418–428. [DOI] [PubMed] [Google Scholar]
- 29. Nakashima T, Nakashima A, Murakami D, et al. Follicular carcinoma of the thyroid with massive invasion into the cervical and mediastinum great veins: Our own experience and literature review. Laryngoscope 2012;122:2855–2857. [DOI] [PubMed] [Google Scholar]
- 30. Brennan MD, Bergstralh EJ, van Heerden JA, et al. Follicular thyroid cancer treated at the Mayo Clinic, 1946 through 1970: Initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc 1991;66:11–22. [DOI] [PubMed] [Google Scholar]
- 31. Leblanc B, Parodi AL, Lagadic M, et al. Immunocytochemistry of canine thyroid tumors. Vet Pathol 1991;28:370–380. [DOI] [PubMed] [Google Scholar]
- 32. Tisell LE, Oden A, Muth A, et al. The Ki67 index a prognostic marker in medullary thyroid carcinoma. Br J Cancer 2003;89:2093–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mussig K, Wehrmann T, Dittmann H, et al. Expression of the proliferation marker Ki‐67 associates with tumour staging and clinical outcome in differentiated thyroid carcinomas. Clin Endocrinol (Oxf) 2012;77:139–145. [DOI] [PubMed] [Google Scholar]
- 34. Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 2006;16:1229–1242. [DOI] [PubMed] [Google Scholar]


