Abstract
Background
Subaortic stenosis (SAS) is a common congenital heart disease in Boxers. Doppler‐derived aortic peak velocity (AoPV) is a diagnostic criterion for the disease.
Objectives
To investigate the influence of emotional stress during echocardiographic examination on AoPV in normal and SAS‐affected Boxers. To evaluate the effects of aortic root diameters on AoPV in normal Boxers.
Dogs
Two hundred and fifteen normal and 19 SAS‐affected Boxers.
Methods
The AoPV was recorded at the beginning of echocardiographic examination (T0), and when the emotional stress of the dog was assumed to decrease based on behavioral parameters and heart rate (T1). AoPV0–AoPV1 was calculated. In normal dogs, stroke volume index was calculated at T0 and T1. Aortic root diameters were measured and their relationship with AoPV and AoPV0–AoPV1 was evaluated.
Results
In normal dogs, AoPV was higher at T0 (median, 1.95 m/s; range, 1.60–2.50 m/s) than at T1 (median, 1.76 m/s; range, 1.40–2.20 m/s; P < .0001; reduction 9.2%). The stroke volume index at T0 also was greater than at T1 (P < .0001). Weak negative correlations were detected between aortic root size and aortic velocities. In SAS‐affected dogs, AoPV0 was higher than AoPV1 (P < .0001; reduction 7.3%).
Conclusion and Clinical Importance
Aortic peak velocity was affected by emotional stress during echocardiographic examination both in SAS‐affected and normal Boxers. In normal Boxers, aortic root size weakly affected AoPVs, but did not affect AoPV0–AoPV1. Stroke volume seems to play a major role in stress‐related AoPV increases in normal Boxers. Emotional stress should be taken into account when screening for SAS in the Boxer breed.
Keywords: Cardiology, Congenital heart disease, Echocardiography, Subaortic stenosis
Abbreviations
- AA
ascending aorta
- AoA
aortic annulus
- AoPV0
aortic peak velocity time (T) 0
- AoPV1
aortic peak velocity time (T) 1
- AoPV
aortic peak velocity
- HR0
heart rate time (T) 0
- HR1
heart rate time (T) 1
- LVOT
left ventricular outflow tract
- SAS
subaortic stenosis
- STJ
sinotubular junction
- SVI
stroke volume index
- TEE
transesophageal echocardiography
- TTE
transthoracic echocardiography
- VLS
Valsalva sinus
Subaortic stenosis (SAS) is 1 of the most common congenital heart diseases in Boxer dogs.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 The identification of overt lesions in the left ventricular outflow tract (LVOT) by transthoracic echocardiography (TTE) is challenging in mild cases and transesophageal (TEE) or 3‐D echocardiography, which may offer more detailed information, are not routinely available.13 Doppler‐derived aortic peak velocity (AoPV) is an indirect echocardiographic parameter widely accepted for classifying a subject as affected or unaffected by SAS.14 However, a consensus has not yet been reached concerning the cutoff value of AoPV for considering a dog affected by SAS, and the accepted AoPV cutoff value has progressively increased with time.14, 15, 16, 17 Aortic peak velocity values of up to 2.56 m/s are measured in Boxers in the absence of any evidence of discrete lesions in the LVOT by both TTE and TEE.9, 13, 18
Factors that may increase AoPV include sympathetic stimulation, high left ventricular preload, low heart rate or blood viscosity, and certain drugs (e.g., inotropes, arterial vasodilators). On the contrary, the administration of β‐blockers, systolic dysfunction, tachyarrhythmias, and dehydration may decrease AoPV.19, 20, 21, 22 Increases in aortic flow velocity have been reported with noise stimuli as stress factors.23 Boxers may have smaller LVOT areas compared with other breeds, and Boxers with murmurs have statistically smaller sinotubular junction (STJ), ascending aorta (AA), and effective orifice area index compared with values in non‐Boxer controls.21 A relatively smaller LVOT size in Boxers could predispose them to increased ejection velocity, regardless of stroke volume. Moreover, Boxers with murmurs tend to have higher aortic velocity and stroke volume than Boxers without murmurs and non‐Boxer controls.21 Furthermore, the aorto‐septal angle has been shown to influence AoPV in Boxers.24
The objective of this study was to investigate the influence of emotional stress during echocardiographic examination, assessed by behavioral parameters and heart rate (HR), on Doppler‐derived AoPV in normal and in SAS‐affected Boxers. Furthermore, the potential effects of aortic root diameters on AoPV were investigated in normal Boxers.
Materials and Methods
Subjects
Boxers were prospectively recruited from those undergoing mandatory screening for congenital heart disease in Italy.5 Screening included patient history, physical examination, blood pressure measurement by Doppler flow meter, and TTE (M‐mode, 2‐D‐mode, and echo‐Doppler) on both breeder‐ and client‐owned Boxers. Screening was performed on conscious dogs of at least 1 year of age, with a pedigree and implanted microchip, which are registered in the Libro Italiano delle Origini of the Ente Nazionale della Cinofilia Italiano.
Animal care and handling were in compliance with regulations for animal welfare. Informed consent was obtained from all owners.
Inclusion Criteria
Boxers with overt lesions and flow turbulence in the LVOT detected by color‐flow Doppler on TTE were considered SAS‐affected, regardless of AoPV.5, 8, 13, 25 Dogs without obvious structural abnormalities in the LVOT, without flow turbulence in the LVOT assessed by color‐flow Doppler, and with subcostal CW‐Doppler AoPV ≤ 2.3 m/s at Time 1 were considered normal.13 This group included dogs either with or without left basilar heart murmurs (grade 1–3/6).
Exclusion Criteria
Boxers affected by concomitant heart diseases, known systemic diseases, hypertension (systemic systolic arterial blood pressure ≥180 mmHg26), or dogs with echocardiographic evidence of systolic dysfunction (end‐systolic volume index ≥40 mL/m2) were not included in the study. These conditions could influence left ventricular outflow, aortic root morphology and geometry, and AoPV. In addition, cases in which a complete echocardiographic examination in lateral recumbency was not possible (e.g., extreme excitement, restlessness, substantial upper airway disease) were excluded from the study.
Echocardiography
Echocardiographic examination (2‐D, M‐mode, spectral, and color‐flow Doppler) was performed by a single observer (CB) using a machine equipped with a multi‐frequency phased array probe 2.5–3.5 MHz (MyLab 60 machine1 ). Dogs were restrained in lateral recumbency on a soft table with an opening that allowed transducer manipulation and examination from beneath the animal. TTE was performed according to standards from right parasternal, subcostal, left cranial parasternal, and left apical parasternal windows,27 with concurrent continuous electrocardiographic monitoring. High‐quality video clips of standard echocardiographic views and high‐quality M‐mode, PW‐ and CW‐Doppler images of at least 6 cardiac cycles were acquired and stored using the echomachine software (MyLab desk1) for off‐line measurements.
The AoPV was acquired by CW‐Doppler and PW‐Doppler [sample volume located at the aortic annulus (AoA) level] from the subcostal window immediately after patient positioning on the table, when the excitement and anxiety of the dog were assumed to be most pronounced (T0). Then, M‐mode, 2‐D, and color‐Doppler echocardiographic examinations were performed from the right parasternal long‐ and short‐axis planes. The left ventricular outflow was scrutinized for evidence of lesions by 2‐D echocardiography and for the presence or absence of turbulence by color‐flow Doppler. When the dog appeared relaxed and calm, AoPV (T1) was acquired by CW‐Doppler and PW‐Doppler (sample volume located at the AoA level) once again. The time between acquisition of AoPV0 and AoPV1 was measured and recorded by a second observer (PO). Then, the dog was positioned in left recumbency and 2‐D and color‐flow Doppler examination was performed from left parasternal planes.
Because the Boxers included in the study were recruited during mandatory screening for congenital heart disease, CB evaluated stored video clips and images of any individual dog immediately after the examination for reporting purposes. AoPV1 assessed by CW‐Doppler was used for classifying the screened Boxer as affected or unaffected by SAS.
Subsequently, at the end of the recruitment period a third operator (DP) extracted the archived video clips and images of interest for off‐line measurement rendering them anonymous. Five distinct anonymous copies were created for each normal Boxer: 1 with video clips for aortic diameter measurements, and 1 each with images for AoPV0 and AoPV1 measurement (PW‐ and CW‐Doppler). Only 2 anonymous copies of images for CW‐Doppler AoPV0 and AoPV1 were created for SAS‐affected dogs. Then, a sequential number was assigned to each case. The operator (DP) sorted anonymous reports for the increasing ID numbers. In this way, the observer (CB) repeated measurements on acquired clips and images blinded to the dog's previous measurements and timing of AoPVs.
The average PW‐ and CW‐Doppler AoPV was measured over 3 cardiac cycles on periods of regular sinus rhythm both at T0 and at T1. In case of marked sinus arrhythmia, 5 cardiac cycles were averaged. Average HR was calculated over the R‐R intervals preceding the measured spectral signals.
Aortic annulus diameter was measured at the hinge points of the aortic leaflets in early systole from the right parasternal long‐axis LVOT view (Fig 1).
Figure 1.
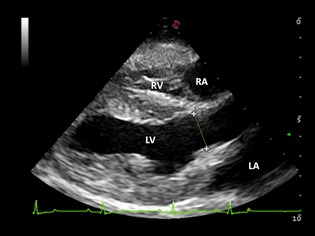
Right parasternal long‐axis view of the left ventricular outflow tract. The aortic annulus diameter is measured at the hinge points of the aortic leaflets in early systole. RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle.
The measurements of VLS, STJ, and AA were taken at end diastole from the inner edge to the inner edge of the aortic root in the long‐axis view, from the left parasternal location. Ascending aortic diameter was measured at a distance from the sinotubular junction equal to the STJ diameter (Fig 2).2, 28 The values for aortic diameters were averaged from 3 high‐quality frames. Aortic diameters were assessed only in normal Boxers.
Figure 2.
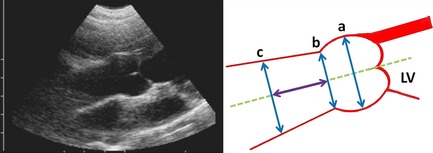
Left parasternal cranial long‐axis view of the aortic root and schematic drawing of aortic root measurements at end‐diastole: (a) Valsalva sinus diameter; (b) sinotubular junction diameter; (c) ascending aortic diameter. The (c) diameter is measured at a distance from the sinotubular junction equal to (b).
Evaluation of Emotional Stress
The decrease in emotional stress was estimated by: decrease in respiratory rate, cessation of panting, whining or vocalization, or some combination of these; decrease in limb muscular tension and reaction to restraining; and, decrease in HR. The observer (CB) estimated HR on the ECG tracings recorded during echocardiography. Emotional stress was considered decreased when the dog showed a regular sinus rhythm or sinus arrhythmia.
Calculations
In normal dogs, measurements of aortic diameters were indexed to the cubic root of the body weight before statistical analysis.29, 30 Stroke volume index was calculated (SVI = area × stroke distance/body surface area) at T0 and T1 at the AoA location. The area was calculated from the average of annular diameters from the right parasternal long‐axis view, and stroke distance from the average of stroke distances obtained from the PW‐Doppler sample volume located at the aortic valve level. The mathematical subtraction between AoPV0 and AoPV1 (AoPV0–AoPV1) and HR0 and HR1 (HR0‐HR1) were calculated both in normal and SAS‐affected Boxers.
Statistical Analysis
Mean, SD, and range values are reported for normally distributed data, whereas median, interquartile range (IQR), and range values are reported for nonnormally distributed data. The Kolmogorov‐Smirnov test was used for assessing the normality of data. Differences between AoPV0 and AoPV1 were tested using the Wilcoxon‐Mann‐Whitney test.
Correlations between aortic root measurements and AoPV were assessed using the Spearman correlation coefficient. Comparison of males and females for aortic root measurements and aortic velocities was performed by the Wilcoxon‐Mann‐Whitney test. The associations between AoPV0‐AoPV1, and aortic root measurements were investigated using linear regression models. For all of the hypotheses tested, 2‐tailed P values <.05 were considered significant. Statistical analysis was performed using SAS Version 9.1.3
Results
Group Characteristics
The study population included 234 Boxer dogs: 215 normal Boxers (110 males; 105 females) and 19 SAS‐affected Boxers (11 males; 8 females), 1–3 years old (22.3 ± 7.4 months) with a median body weight of 29 kg (IQR, 26–33 kg; range, 18.5–43.0 kg). A left basilar heart murmur was auscultated in 80 of 215 normal Boxers (37.2%) and in 100% of SAS‐affected dogs.
Elapsed Time between T0 and T1
Median time from Time 0 to Time 1 was 9.2 minutes (range, 4–15 minutes) in all dogs.
Echocardiography
Table 1 summarizes basic echocardiographic measurements in normal and SAS‐affected Boxers.
Table 1.
Basic echocardiographic measures in normal and SAS‐affected Boxers
| Normal (215 dogs) | SAS‐affected (19 dogs) | |
|---|---|---|
| IVSd (mm) | 9.9 ± 1.6 | 10.1 ± 1.3 |
| LVDd (mm) | 41.8 ± 5.1 | 40 ± 4.7 |
| LVPWd (mm) | 9.4 ± 1.6 | 10.3 ± 0.8 |
| IVSs (mm) | 15 ± 11.6 | 15.9 ± 2.4 |
| LVDs (mm) | 28.5 ± 4.0 | 31.3 ± 5.6 |
| LVPWs (mm) | 16.2 ± 2.6 | 16.2 ± 1.6 |
| LA/Ao | 1.5 ± 0.1 | 1.6 ± 0.3 |
| EDVI (mL/m2) | 86.5 ± 22.4 | 85.5 ± 20.3 |
| ESVI (mL/m2) | 33.8 ± 6.3 | 34.2 ± 5.6 |
| FS (%) | 31.5 ± 3.6 | 31.2 ± 7.1 |
| EF (%) | 59.4 ± 8.0 | 58.6 ± 10.4 |
| CW‐Pulmonic peak velocity (m/s) | 1.4 ± 0.2 | 1.45 ± 0.3 |
Values are reported as mean ± SD. The LA/Ao ratio was calculated from the right parasternal short‐axis view of the heart base according to Hansson et al.35
IVSd, interventricular septum diastole; LVDd, left ventricular diameter diastole; LVPWd, left ventricular posterior wall diastole; IVSs, interventricular septum systole; LVDs, left ventricular diameter systole; LVPWs, left ventricular posterior wall systole; LA/Ao, left atrial/aortic ratio; EDVI, end diastolic volume index; ESVI, end‐systolic volume index; SF, shortening fraction; EF, ejection fraction; CW, continuous‐wave Doppler.
Normal Boxers
The CW‐AoPV was significantly higher at T0 than T1 (P < .0001; median percentage of reduction, 9.2%; Table 2; Fig 3). AoPV0 (P = .802), AoPV1 (P = .293) and AoPV0–AoPV1 (P = .287) were not statistically different between males and females. CW‐AoPV0 and PW‐AoPV0 (P = .762) and CW‐AoPV1 and PW‐AoPV1 (P = .691) were not statistically different.
Table 2.
Median, IQR, and range of AoPV0, AoPV1, AoPV0–AoPV1, HR0, and HR1 in Boxer dogs
| CW AoPV0 (m/s) | CW AoPV1 (m/s) | AoPV0–AoPV1 (m/s) | PW AoPV0 (m/s) | PW AoPV1 (m/s) | HR0 (bpm) | HR1 (bpm) | |
|---|---|---|---|---|---|---|---|
| Normal Boxers (215 dogs) |
1.95 1.81–2.10 (1.60–2.50) |
1.76a
1.66–1.88 (1.40–2.20) |
0.18b
0.11–0.24 (0.02–0.65) |
2.01 1.90–2.18 (1.58–2.65) |
1.78 1.64–1.91 (1.38–2.18) |
97 88–110 (56–135) |
86a
78–100 (58–122) |
|
SAS‐affected (19 dogs) |
3.98 2.69–5.65 (2.28–6.88) |
3.74a
2.44–5.27 (2.15–6.35) |
0.29b
0.21–0.46 (0.13–0.53) |
97 90–110 (68–138) |
85a
76–102 (59–135) |
AoPV0, aortic peak velocity at T0; AoPV1, aortic peak velocity at T1; HR0, heart rate at T0; HR1, heart rate at T1.
Differs significantly from its value at T0.
Differs significantly from zero.
Figure 3.
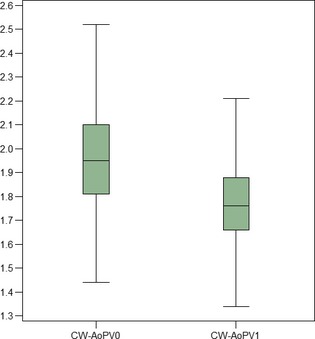
Boxplot of the CW‐AoPV at T0 and T1 in normal Boxers, showing the interquartile range (IQR) and highlighting median, in the box. The whiskers run from the quartile to the minimum/maximum observation below lower/upper fence defined as 1.5 × IQR. Aortic peak velocity at T0 is significantly greater than AoPV1 (P < .0001).
The SVI at T0 (58.88 ± 9.62 mL/m2; range, 35.24–87.32 mL/m2) was significantly higher than SVI at T1 (52.10 ± 10.43 mL/m2; range, 26.85–76.91 mL/m2; P < .0001).
Measurements of aortic root diameters indexed to the cubic root of the body weight of normal Boxers are reported in Figure 4. Weak negative correlations were detected between aortic root diameters indexed to the cubic root of the body weight and CW‐AoPVs (Table 3). Linear regression failed to show any significant association between AoPV0‐AoPV1 and aortic root diameters.
Figure 4.
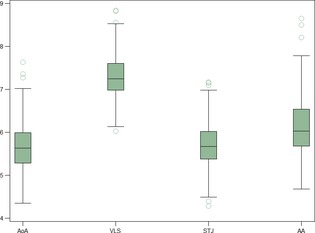
Boxplot of the aortic root measurements indexed to the cubic root of the body weight: aortic annulus (AoA); Valsalva sinus (VLS); sinotubular junction (STJ); ascending aorta (AA). The box shows the interquartile range (IQR) and the median, whiskers run from the quartile to the minimum/maximum observation below lower/upper fence defined as 1.5 × IQR.
Table 3.
Spearman's correlation between aortic root diameters indexed to the cubic root of the body weight (BW1/3) and CW‐AoPV0 or CW‐AoPV1 in normal dogs
| Aortic Root Parameters | AoPV0 (Spearman's Correlation) | P‐Value | AoPV1 (Spearman's Correlation) | P‐Value |
|---|---|---|---|---|
| AoA/BW1/3 | −0.184 | .008 | −0.151 | .031 |
| VLS/BW1/3 | −0.194 | .005 | −0.229 | .001 |
| STJ/BW1/3 | −0.163 | .019 | −0.148 | .034 |
| AA/BW1/3 | −0.164 | .018 | −0.122 | .019 |
AoA, aortic annulus; VLS, Valsalva sinus; STJ, sinotubular junction; AA, ascending aortic diameter.
Heart rate was within normal limits at both T0 and T1, but HR0 was significantly higher than HR1 (P < .0001; Table 2). No association was found between AoPV0 and HR0 (P = .346) or AoPV1 and HR1 (P = .961). The AoPV0–AoPV1 was not associated with HR0 (P = .063), HR1 (P = .621), or with HR0‐HR1 (P = .211).
SAS‐Affected Boxers
The CW‐AoPV was significantly higher at T0 than T1 (P < .0001; median percentage of reduction, 7.3%). HR at T0 was significantly higher than HR1 (P < .0001). No association was found between AoPV0 and HR0 (P = .226) and AoPV1 and HR1 (P = .301). AoPV0–AoPV1 was not associated with HR0‐HR1 (P = .334).
Discussion
Aortic peak velocity is used as a diagnostic criterion for SAS. However, AoPV is affected by several factors such as loading conditions, myocardial inotropy, and total peripheral resistance.19, 20, 21 The results of this study suggest that AoPV is affected by emotional stress during echocardiographic examination both in SAS‐affected and in normal Boxers.
Emotional stress is normal in any sentient being. It consists of an emotional state that occurs in difficult or challenging situations producing several emotions (e.g., fear, anger, anxiety, and pain). It can be considered a normal self‐protective response that helps the individual to cope with a challenging environment.31 Boxer dogs tend to be quite excitable. Most dogs were nervous and afraid at the beginning of the echocardiographic examination because of the manual constraint and struggled. However, most eventually accepted restraint and began to relax within a few minutes (average, 9 minutes).
Stress induces several behavioral and physiological responses, which may be evaluated subjectively and objectively. Behavioral stress signs are not always easy to recognize, and in a setting such as a clinical examination include, among others, lip and nose licking, stress‐associated yawning, panting, excessive shedding, slow or tense movement, shaking, signs of avoidance, excessive whining or other vocalization, restlessness, sweating from paws, dilated pupils, and muscular tension around eyes, mouth and limbs.32, 33 Emotional stress related to excitement or anxiety activates several physiological systems, the sympathetic nervous system being the first. Activation of the sympathetic nervous system increases HR, blood pressure, and hematocrit.22, 31 Moreover, stress stimulates the hypothalamic‐pituitary‐adrenal‐cortical axis which may lead to increases in cortisol and progesterone concentrations.31, 32
Activation of the sympathetic nervous system could increase stroke volume by affecting inotropy but, on the other hand, the decreased diastolic filling time as a consequence of the positive chronotropy potentially could decrease stroke volume by decreasing preload. The overall effect of sympathetic activation seems to be an increase in stroke volume in Boxers, according to the findings of this study. In fact, the higher SVI at T0 suggests that a higher stroke volume could play a role in the stress‐related AoPV increase.
The effect of emotional stress on Doppler‐derived AoPV should be taken into account because it could affect the grading of disease severity in SAS‐affected dogs or lead to a misdiagnosis of mild cases in which overt lesions are not evident on TTE. In our study, 27 (12.5%) normal Boxers would have been misdiagnosed as SAS‐affected when evaluating AoPV0 (cutoff, 2.3 m/s).
Previous studies have shown that Boxers have a relatively smaller LVOT size compared to other breeds, which could predispose them to increased ejection velocity.3, 21, 28 The effect of aortic root diameters on AoPV in normal Boxers found in our study is concordant with data reported in the scientific literature. However, the correlations between aortic root size and aortic velocities are weak, which supports the view that other nonstructural factors are important determinants of AoPV. In fact, AoPV depends on both the anatomical dimension of the aortic root and stroke volume. HR was not associated with aortic velocities and AoPV0–AoPV1 in this study. The HR of Boxers included was in the reference range for dogs at both T0 and T1. It seems that HR does not affect aortic peak velocities in Boxers when within normal limits for dogs. The lack of association could be explained by the fact that the effects of HR variation on left ventricular preload are counteracted by the contrary effect of variation in sympathetic tone and catecholamine output on inotropy.
This study investigated the effect of aortic root diameter on temporal variation in AoPV in normal dogs and failed to show any association between measures of aortic root indexed to body weight and the decrease in AoPV from T0 to T1. This finding suggests that the decrease in velocity cannot be predicted by the size of the aortic root. Moreover, it supports that stress changes velocity independently of the structure of the aortic root.
To summarize, results of this study suggest that emotional stress during echocardiographic examination may increase AoPV, and that increased stroke volume because of sympathetic nervous system activation seems to be the major determinant of this change in velocity. Aortic root diameters weakly affect AoPV, but do not seem to affect the temporal variation in AoPV. In Boxers, AoPV should be measured after the dog is given enough time to acclimate to the environment and to physical restraint to correctly diagnose and grade aortic stenosis.
Study Limitations
A main limitation of the study is the fact that evaluation of the emotional status of the dogs was principally subjective by estimating behavioral responses. However, this evaluation was performed by the same observer (CB), thus minimizing variability. Moreover, the estimation of behavioral responses is accepted as an indicator of emotional status in studies on reactions to stress in the dog.24, 31, 32, 33, 34 The only physiological, thus objective, parameter considered was HR. Blood pressure measurement was not performed at time of AoPV acquisition. Decreased HR and SVI support the subjective assessment that the dogs were more relaxed.
Another limitation is that intra‐observer variability was not assessed. To decrease the risk of underestimation of stenotic lesions, all of the echocardiographic examinations were performed by the same operator, a board‐certified cardiologist, who has used a combination of echocardiographic criteria reported on Boxers in the scientific literature. Two dogs of the SAS‐affected group had overt LVOT lesions but AoPV1 values lower than the cutoff point of 2.3 m/s. There are inherent limits to echocardiographic examination in identifying lesions in the LVOT. Transesophageal echocardiography has the potential to overcome limits, but a study in Boxers failed to show any advantage from TEE in morphologic evaluation of the LVOT.13 No studies have yet compared TTE and 3‐D echocardiography for identification of SAS lesions. The gold standard for the identification of mild lesion remains gross pathology examination, however none of the dogs included in this study was subjected to necropsy. The possible lack of recognition of mild forms of SAS in the normal group of Boxers constitutes another limitation of the study.
The SVI was calculated from PW‐Doppler stroke distances whereas AoPV0 and AoPV1 were derived from CW‐Doppler, thus on different cardiac cycles, which is another limitation. Nevertheless, measurements were made during periods of stable HR, averaged on more cardiac cycles and PW and CW Doppler estimates of AoPV did not differ significantly.
Finally, this study was exclusive to the Boxer breed and therefore the results may not be extrapolated to other breeds of dogs.
Acknowledgment
The study was performed at Clinica Veterinaria Gran Sasso, Milano, Italy.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Footnotes
ESAOTE Biomedica, Firenze, Italy
Bussadori C. Echo patterns in Boxers with subaortic stenosis. Proceedings 18th Ann Meet Vet Med Forum, Seattle, 2000:86–87
SAS Institute, Cary, NC
References
- 1. Patterson DF. Epidemiologic and genetic studies of congenital heart disease in the dog. Circ Res 1968;23:171–202. [DOI] [PubMed] [Google Scholar]
- 2. Patterson DF. Hereditary congenital heart defects in dogs. J Small Anim Pract 1989;30:153–165. [Google Scholar]
- 3. Buchanan JW. Causes and prevalence of cardiovascular disease In: Kirk RW, Bonagura JD, eds. Current Veterinary Therapy, Vol. XI. Philadelphia, PA: WB Saunders; 1992:647–655. [Google Scholar]
- 4. Buchanan JW. Changing breed predispositions in canine heart disease. Canine Pract 1993;18:12–14. [Google Scholar]
- 5. Bussadori C, Pradelli D, Borgarelli M, et al. Congenital heart disease in boxer dogs: Results of 6 years of breed screening. Vet J 2009;181:187–192. [DOI] [PubMed] [Google Scholar]
- 6. Kienle RD. Aortic stenosis In: Kittleson MD, Kienle RD, eds. Small Animal Cardiovascular Medicine. St. Louis, MO: Mosby; 1998:260–272. [Google Scholar]
- 7. Heiene R, Indrebø A, Kvart C, et al. Prevalence of murmurs consistent with aortic stenosis among boxers in Norway and Sweden. Vet Rec 2000;147:152–156. [DOI] [PubMed] [Google Scholar]
- 8. Bussadori CB, Quintavalla C, Capelli A. Prevalence of congenital heart disease in boxers in Italy. J Vet Cardiol 2001;3:7–11. [DOI] [PubMed] [Google Scholar]
- 9. Chetboul V, Trollé JM, Nicolle A, et al. Congenital heart diseases in the boxer dog: A retrospective study of 105 cases (1998–2005). J Vet Med A Physiol Pathol Clin Med 2006;53:346–351. [DOI] [PubMed] [Google Scholar]
- 10. Linde A, Koch J. Screening for aortic stenosis in the Boxer: Auscultatory, ECG, blood pressure and Doppler echocardiographic findings. J Vet Cardiol 2006;8:79–86. [DOI] [PubMed] [Google Scholar]
- 11. Hopfner R, Glaus T, Gardelle O, et al. [Prevalence of heart murmurs, aortic and pulmonic stenosis in boxers presented for pre‐breeding exams in Switzerland]. Schweiz Arch Tierheilkd 2010;152:319–324. [DOI] [PubMed] [Google Scholar]
- 12. Oliveira P, Domenech J, Silva J, et al. Retrospective review of congenital heart disease in 976 dogs. J Vet Intern Med 2011;25:477–483. [DOI] [PubMed] [Google Scholar]
- 13. Quintavalla C, Pradelli D, Domenech O, Bussadori C. Transesophageal echocardiography of the left ventricular outflow tract, aortic valve and ascending aorta in boxer dogs with heart murmurs. Vet Radiol Ultrasound 2006;47:307–312. [DOI] [PubMed] [Google Scholar]
- 14. Bussadori C, Amberger C, Le Bobinnec G, Lombard CW. Guidelines for the echocardiographic studies of suspected subaortic and pulmonic stenosis. J Vet Cardiol 2000;2:15–22. [DOI] [PubMed] [Google Scholar]
- 15. Boon JA. Congenital heart disease In: Boon JA, ed. Manual of Veterinary Echocardiography. Baltimore, MD: Williams & Wilkins; 1998:383–445. [Google Scholar]
- 16. Yuill C, O'Grady M. Doppler‐derived velocity of blood flow across the cardiac valves in the normal dog. Can J Vet Res 1991;55:185–192. [PMC free article] [PubMed] [Google Scholar]
- 17. Lehmkuhl LB. Comparison of transducer placement sites for Doppler echocardiography in dogs with subaortic stenosis. Am J Vet Res 1994;55:192–198. [PubMed] [Google Scholar]
- 18. Bonagura JD, Miller MW, Darke PGG. Doppler echocardiography I. Vet Clin North Am 1998;28:1325–1360. [DOI] [PubMed] [Google Scholar]
- 19. Kirberger RM, Bland‐van den Berg P, Darasz B. Doppler echocardiography in the normal dog. Part II. Factors influencing flow velocities and a comparison between left and right heart blood flow. Vet Radiol Ultrasound 1992;33:380–386. [Google Scholar]
- 20. Sohn S, Kim HS, Han JJ. Doppler flow velocity measurement to assess changes in inotropy and afterload: A study in healthy dogs. Echocardiography 2002;19:207–213. [DOI] [PubMed] [Google Scholar]
- 21. Koplitz SL, Meurs KM, Bonagura JD. Echocardiographic assessment of the left ventricular outflow tract in the boxer. J Vet Intern Med 2006;20:904–911. [DOI] [PubMed] [Google Scholar]
- 22. Höglund K, Hanas S, Carnabuci C, et al. Blood pressure, heart rate, and urinary catecholamines in healthy dogs subjected to different clinical settings. J Vet Intern Med 2012;26:1300–1308. [DOI] [PubMed] [Google Scholar]
- 23. Höglund K, French A, Dukes‐McEwan J, et al. Low intensity heart murmurs in Boxer dogs: Inter‐observer variation and effects of stress testing. J Small Anim Pract 2004;45:178–185. [DOI] [PubMed] [Google Scholar]
- 24. Quintavalla C, Guazzetti S, Mavropoulou A, Bussadori C. Aorto‐septal angle in boxer dogs with subaortic stenosis: An echocardiographic study. Vet J 2010;185:332–337. [DOI] [PubMed] [Google Scholar]
- 25. Schober KE, Fuentes VL. Doppler echocardiographic assessment of left ventricular diastolic function in 74 boxer dogs with aortic stenosis. J Vet Cardiol 2002;4:7–16. [DOI] [PubMed] [Google Scholar]
- 26. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007;21:542–548. [DOI] [PubMed] [Google Scholar]
- 27. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. J Vet Intern Med 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 28. Santilli R, Bussadori C, Borgarelli M. Manuale di cardiologia del cane e del gatto, 1a Edizione Milano: Elsevier; 2012. [Google Scholar]
- 29. Brown DJ, Rush JE, MacGregor J, et al. M‐mode echocardiographic ratio indices in normal dogs, cats, and horses: A novel quantitative method. J Vet Intern Med 2003;17:653–662. [DOI] [PubMed] [Google Scholar]
- 30. Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Vet Intern Med 2004;18:311–321. [DOI] [PubMed] [Google Scholar]
- 31. Hydbring‐Sandberg E, von Walter LW, Höglund K, et al. Physiological reactions to fear provocation in dogs. J Endocrinol 2004;180:439–448. [DOI] [PubMed] [Google Scholar]
- 32. Kalnajs S. The language of dogs ‐ understanding canine body language and other communication signal [CD‐ROM]. Madison: Blue Dog Trainer and Behaviour LLC; 2006. [Google Scholar]
- 33. Lindsay SR. Handbook of Applied Dog Behaviour and Training. Ames, IO: Iowa State University Press; 2001. [Google Scholar]
- 34. Fallani G, Prato Previde E, Valsecchi P. Behavioral and physiological responces of guide dogs to a situation of emotional distress. Physiol Behav 2007;90:648–655. [DOI] [PubMed] [Google Scholar]
- 35. Hansson K, Häggström J, Kvart C, Lord P. Left atrial to aortic root indices using two‐dimensional and M‐mode echocardiography in cavalier King Charles spaniels with and without left atrial enlargement. Vet Radiol Ultrasound 2002;43:568–575. [DOI] [PubMed] [Google Scholar]


