Abstract
Background
In humans, a high concentration of adiponectin is associated with a favorable cardiovascular risk profile whereas, in patients with heart failure (HF), a high concentration of adiponectin is associated with a less favorable prognosis.
Hypothesis/Objectives
To evaluate the physiological determinants of plasma adiponectin concentration in dogs and the influence of heart disease, myxomatous mitral valve disease (MMVD), and dilated cardiomyopathy (DCM).
Animals
One hundred and fourteen client‐owned dogs and 9 Beagles from the research colony of the Clinical Veterinary Unit of the University of Liège.
Methods
We prospectively measured circulating adiponectin concentration in healthy control dogs (n = 77), dogs with MMVD (n = 22) and dogs with DCM (n = 15) of various degrees of severity. Diagnosis was confirmed by Doppler echocardiography. Plasma adiponectin concentration was measured by a canine‐specific sandwich ELISA kit.
Results
An analysis of covariance showed an association between adiponectin concentration and age, neuter status, and heart disease. No association between adiponectin concentration and class of HF, sex, body condition score, body weight, circadian rhythm, or feeding was found. Plasma adiponectin concentration was negatively correlated with age (P = .001). Adiponectin was lower in neutered (P = .008) compared to intact dogs. Circulating adiponectin concentration was increased in dogs with DCM compared to healthy dogs (P = .018) and to dogs with MMVD (P = .014).
Conclusions and Clinical Importance
Age and neutering negatively influence circulating adiponectin concentration. Plasma adiponectin concentration increased in dogs with DCM. Additional research is required to investigate if this hormone is implicated in the pathophysiology of DCM and associated with clinical outcome.
Keywords: Adipokines, Age, Dilated cardiomyopathy, Myxomatous mitral valve disease, Neuter status
Abbreviations
- BCS
body condition score
- BW
body weight
- DCM
dilated cardiomyopathy
- HF
heart failure
- ISACHC
International Small Animal Cardiac Health Council
- MMVD
myxomatous mitral valve disease
White adipose tissue, especially visceral fat, is becoming regarded as an important endocrine organ that secretes a wide variety of hormones, cytokines, and growth factors, collectively labeled adipokines.1 Adiponectin is the most abundant circulating adipokine, exerting insulin‐sensitizing, anti‐inflammatory and anti‐atherosclerotic effects.2 Adiponectin is cardioprotective, promoting myocardial availability of free fatty acids, glucose,3, 4 and cardiomyocyte survival.5
In humans, although adipocytes are responsible for its secretion, plasma adiponectin concentration decreases with increased fat mass, especially with visceral fat distribution in relation with to metabolic syndrome.6 Moreover, in healthy individuals, high plasma adiponectin concentrations are associated with a low cardiovascular risk profile7 whereas, in patients with existing heart failure (HF), high concentrations of this hormone are paradoxically associated with worse outcome.8, 9
In dogs, the inverse relationship between adiponectin and adiposity is highly controversial, with discrepant conclusions resulting perhaps from confounding factors such as sex or neuter status.10 These observations raise the possibility that the physiology of adiponectin is different in dogs compared to humans.11 Moreover, to the best of our knowledge, no information regarding circulating adiponectin concentrations in dogs with HF is available. Cardiac diseases leading to HF differ among species. HF in humans is mostly secondary to myocardial ischemia whereas in dogs HF is mostly secondary to primary myocardial disease or valve degeneration. Therefore, the implication of adiponectin in the pathophysiology and prognosis of heart diseases also may be different.
Therefore, this study was intended to evaluate physiological determinants of circulating adiponectin concentration such as circadian rhythm, age, sex, neuter status, body weight (BW), and body condition score (BCS). Taking these determinants into account, we investigated plasma adiponectin concentrations in dogs at different stages of myxomatous mitral valve disease (MMVD) or dilated cardiomyopathy (DCM).
Material and Methods
The study protocol was approved by the Committee on Animal Experimentation of the University of Liège. Care of experimental animals conformed to the “Guide for the Care and Use of Laboratory Animals” (NIH publication no. 85‐23, National Academy Press, Washington, DC, revised 1996).
Dogs
Healthy control dogs and dogs with heart disease were prospectively recruited from the Clinical Veterinary Unit of the University of Liège. Nine Beagle dogs, belonging to the dog colony of the Clinical Veterinary Unit and fed once daily at 11 am with a maintenance dry diet, were used in a substudy to investigate the effects of feeding and circadian rhythm. Breed, age, sex, neuter status, BW, and BCS (on a 5‐point scale) were noted for each dog. Dogs were considered healthy if they did not have any history of clinical disease with systemic repercussions and had normal physical examination findings. Additional diagnostic tests to establish their state of health were not carried out. In dogs with cardiac disease, diagnosis and assignment to class of HF were made on the basis of history, clinical signs and consistent results of physical examination, thoracic radiography, ECG, and Doppler echocardiography. Standard echocardiography and conventional Doppler examinations were performed by trained observers (1 resident in cardiology or 1 board‐certified cardiologist). All measurements were made by 1 board‐certified cardiologist. A right parasternal window was used to record 2‐dimensional and M‐mode images in the long and short axes. Left ventricular volumes and ejection fraction were calculated by the Simpson's method. The left atrial/aortic diameter ratio was obtained at end‐systole from the 2‐dimensional short axis view at the level of the aorta. Diagnosis of MMVD was based on remodeling of mitral valve leaflets, mitral valve prolapse, and the presence of a systolic regurgitation jet. Dilated cardiomyopathy was diagnosed using published guidelines.12 Dogs with any other cardiac disease or clinically relevant organ‐related or systemic disease were not enrolled. Dogs with heart disease then were classified in HF class I, II, or III according to the International Small Animal Cardiac Health Council (ISACHC) classification system.13
Assay
For each dog, a 2 mL blood sample was collected by jugular puncture into an EDTA‐containing tube. To test diurnal variation and effect of feeding, repeated samples were taken every 6 hours during 24 hours in 9 Beagles. Plasma was separated by centrifugation (10 minutes 1,200 × g at room temperature) within 30 minutes of collection, transferred to cryotubes and frozen at −80°C until assay. Plasma adiponectin concentration was measured in duplicate by a canine‐specific sandwich ELISA kit1 according to the manufacturer's instructions. The use of this assay has been reported previously for canine samples.14 The intra‐assay and inter‐assay coefficients of variation were 4.1 and 4.4%, respectively.
Statistical Analysis
Statistical analysis was performed using the software IBM SPSS 22 and R with the “compute.es” package. Statistical significance was set at P < .05. Normality was tested using the Shapiro–Wilk test. Nonnormally distributed variables were log transformed for analysis or analyzed using nonparametric methods.
Demographic characteristics of control dogs, dogs with MMVD and dogs with DCM were compared using a one‐way analysis of variance or a Kruskall–Wallis test for continuous variables and a chi‐square test for dichotomous variables. The circadian rhythm effect of the adiponectin concentrations was tested by one‐way analysis of variance for repeated measurements. For the analyses thereafter, adiponectin concentrations were log transformed. In healthy dogs, influence on adiponectin concentration of age and BW covariates and of the categorical effects of sex, neuter status and BCS as well as the homogeneity of slopes between the levels of the categorical effects were tested by an analysis of covariance. We performed a second analysis including dogs with heart disease (MMVD and DCM) and adding the categorical effects of cardiac status (healthy, MMVD, DCM) and classes of HF (healthy, ISACHC I, II, and III). Thereafter, the adjusted means of the categorical effects as well as their 95% CI were back‐transformed. To provide standard concentrations of adiponectin according to age in healthy dogs, 3 regressions on age (all of the healthy dogs, intact, and neutered dogs) of the adiponectin concentration were calculated, as well as the 95% CI for the mean of the predicted concentrations. For significant categorical effects, a multiple comparison test on the pairwise adjusted means was performed using the Sidak adjustment method. When the comparison gave a statistically significant difference, the Cohen's d effect size was calculated. This is defined as the difference between 2 means divided by a standard deviation, and therefore the magnitude of Cohen's d gives a normalized measure of the importance of a statistically significant variable.15 Finally, in dogs with heart disease, any relationship between echocardiographic indices (ejection fraction, fractional shortening, left atrial diameter/aortic diameter ratio, left ventricular systolic volume index, left ventricular diastolic volume index), and plasma adiponectin concentration was tested by an analysis of covariance.
Results
Study Population
Demographic characteristics of healthy and cardiac dogs included in the study are described in Table 1. Dogs with heart disease were grouped as HF ISACHC I (n = 14), ISACHC II (n = 15) and ISACHC III (n = 8). Twenty‐two dogs were diagnosed with MMVD and 15 dogs with DCM. In healthy dogs, the most frequently represented breeds were as follows: English Sheepdog (n = 10), followed by Beagle (n = 8), crossbred (n = 6), and French Bulldog (n = 4). In the MMVD group, the most frequently represented breeds were Bichon (n = 5), crossbred (n = 5), and Cavalier King Charles Spaniel (n = 2). In the DCM group, giant and large breeds were most frequently represented, including Labrador (n = 4) and Great Dane (n = 3).
Table 1.
Distribution of dogs included in the study
| Variable | Beagles | Healthy | Heart Disease | ||
|---|---|---|---|---|---|
| ISACHC I | ISACHC II | ISACHC III | |||
| Number | 9 | 77 | 14 | 15 | 8 |
| Male/female | 3/6 | 36/41 | 11/3 | 7/8 | 6/2 |
| Neutered (%) | 0 | 30 | 29 | 53 | 0 |
| Age (years) | 7.6 ± 3.6 | 4.5 ± 3.5 | 9.5 ± 4.1 | 10.8 ± 2.7 | 7.7 ± 3.1 |
| BW (kg) | 14.2 ± 1.5 | 20 ± 8.8 | 14 ± 11.2 | 22 ± 19.4 | 22 ± 11.3 |
| BCS (1/2/3/4/5) | 0/0/7/2/0 | 2/8/45/12/10 | 0/4/6/4/0 | 1/4/3/7/0 | 0/4/2/2/0 |
| MMVD | 11 | 8 | 3 | ||
| DCM | 3 | 7 | 5 | ||
Values are expressed as mean ± SD. BW, body weight; BCS, body condition score (from 1 to 5); MMVD, myxomatous mitral valve disease; DCM, dilated cardiomyopathy; ISACHC, International Small Animal Cardiac Health Council.
A greater proportion (P = .016) of the female population was neutered (43%) than the male population (20%). No significant difference in the sex ratio and in the percentage of neutered dogs was seen between healthy dogs and dogs with heart disease.
Dogs with MMVD (11.0 ± 3.8 years old, [mean ± SD]) were older than healthy dogs (4.5 ± 3.7 years old, P < .001) and dogs with DCM (7.6 ± 2.5 years old, P = .005), whereas dogs with DCM were older than healthy dogs (P = .002).
Dogs with MMVD had a lower BW (6.4 [5.4–11.2] kg, median [interquartile range] than healthy dogs (14.7 [11.0–29.8] kg, P = .002) and dogs with DCM (33.0 [28.4–39.0] kg, P = .007). Dogs with DCM were heavier than healthy dogs (P = .005).
Echocardiographic characteristics of all dogs with heart disease are summarized in Table 2. Because these variables were used to construct the groups, we did not compare the groups statistically. Twenty‐nine of 37 dogs with heart disease (MMVD or DCM) were already under treatment at inclusion. At inclusion, 33 dogs were being managed in the home environment and 4 dogs in DCM ISACHC class III were hospitalized for stabilization.
Table 2.
Echocardiographic data
| Variable | ISACHC | |||||||
|---|---|---|---|---|---|---|---|---|
| DCM | MMVD | |||||||
| All (n = 15) | I (n = 3) | II (n = 7) | III (n = 5) | All (n = 22) | I (n = 11) | II (n = 8) | III (n = 3) | |
| LA/Ao | 2.1 ± 0.4 | 1.6 ± 0.5 | 2.1 ± 2.6 | 2.3 ± 0.2 | 2.0 ± 0.9 | 1.6 ± 0.3 | 2.5 ± 0.6 | 2.2 ± 0.9 |
| LVVId (mL/m2) | 121 ± 39 | 85 ± 43 | 135 ± 3 | 126 ± 29 | 70 ± 38 | 55 ± 33 | 88 ± 31 | 75 ± 60 |
| LVVIs (mL/m2) | 63 ± 35 | 48 ± 29 | 76 ± 45 | 58 ± 27 | 17 ± 14 | 15 ± 10 | 17 ± 8 | 23 ± 21 |
| EF (%) | 49 ± 15 | 46 ± 5 | 46 ± 19 | 54 ± 16 | 75 ± 9 | 74 ± 10 | 82 ± 2.8 | 66 ± 9 |
| FS (%) | 23 ± 15 | 18 ± 5 | 27 ± 16 | 23 ± 20 | 46 ± 9 | 45 ± 13 | 51 ± 6 | 40 ± 7 |
Values are expressed as mean ± SD. LA/Ao, left atrial diameter/aortic diameter; LVVId, diastolic left ventricular volume index; LVVIs, systolic left ventricular volume index; EF, ejection fraction; FS, fractional shortening MMVD, myxomatous mitral valve disease; DCM, dilated cardiomyopathy; ISACHC, International Small Animal Cardiac Health Council.
Plasma Adiponectin Concentration in Healthy Dogs
A preliminary analysis showed no significant effect of sex, BCS or BW on adiponectin concentration. It also showed that the coefficient of regression on age was not significantly different between intact and neutered dogs. The ANCOVA analysis showed that adiponenctin concentration was significantly influenced by age (P = .001) and neutering (P = .008). The adjusted geometric means and 95% CI are given for neutered and intact dogs in Table 3. Cohen's d effect size was calculated on adjusted means from the log of adiponectin concentration for neutered and intact dogs. After the standard of Cohen15 (|d| < 0.2: no practical effect, 0.2 < |d| < 0.5: small effect, 0.5 < |d| < 0.8: medium effect, |d| > 0.8: large effect), neutering had a medium effect on adiponectin concentration. Values for d and its 95% CI are given in Table 3. No significant difference in plasma adiponectin concentration was observed between males and females but neutering led to lower plasma adiponectin concentration that was significant in females (Fig 1). Average adiponectin reference concentrations in units according to age are presented for all healthy dogs (Fig 2A) and separately for healthy intact (Fig 2B) and neutered (Fig 2C) dogs. In a substudy using 9 Beagles, a significant diurnal or feeding effect on plasma adiponectin concentration was not observed (Fig 3).
Table 3.
Analysis of covariance of plasma adiponectin concentration (μg/mL). Adjusted geometric means (admean) for the categorical effect of intact and neutered healthy dogs, ratios between the adjusted geometric means of the neutered and intact dogs, Cohen's d effect size between the neutered and intact dogs calculated from the adjusted means and their 95% confidence intervals (CI)
| Estimate | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|
| admean, intact | 11.34 | 9.49 | 13.54 |
| admean, neutered | 7.10 | 5.36 | 9.41 |
| Ratio neutered‐intact | 0.63 | 0.44 | 0.88 |
| Cohen's d neutered‐intact | −0.71 | −1.15 | −0.26 |
Figure 1.
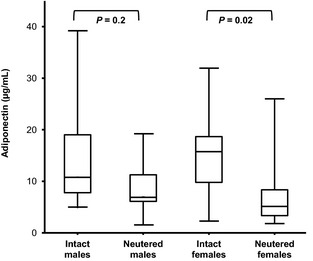
Box and whisker plots of plasma adiponectin concentration in healthy intact and neutered male and female dogs of different breeds. Medians are indicated by the midline; first and third quartiles by the open box; and minima and maxima by the lower, and upper whiskers.
Figure 2.
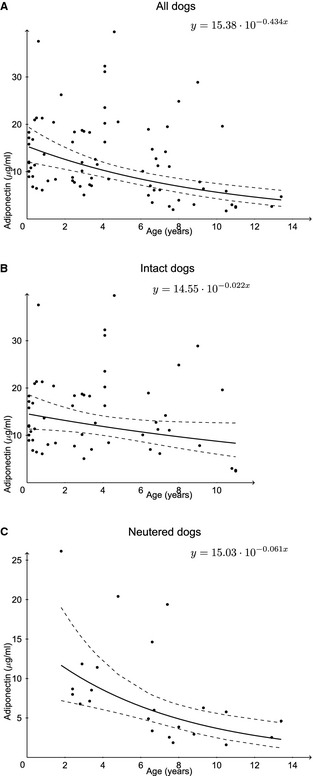
Average adiponectin reference values in units owing to age for all healthy dogs (A), and separately for healthy intact (B), and neutered (C) dogs as well as the 95% CI for the mean of the predicted values.
Figure 3.
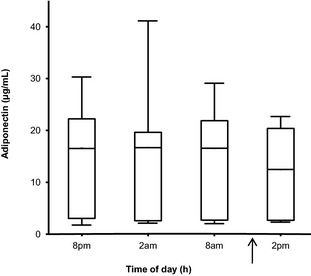
Plasma adiponectin concentration in 9 Beagles during a 24‐hour period. Medians are indicated by the midline; first and third quartiles by the open box; and minima and maxima by the lower and upper whiskers. Arrow represents the feeding time (11 am).
Plasma Adiponectin Concentration in HF Dogs Versus Control Dogs
When control and heart disease dogs were included in the analysis, plasma adiponectin concentration was significantly influenced by neuter status (P < .001), age (P = .014), and heart disease (P = .007). The corresponding adjusted geometric means and their 95% confidence limits are given in Table 4. Age negatively influenced adiponectin concentration without significant difference for all categories of animals; the log back‐transformed regression equation is given in Table 4. A multiple comparison test on the adjusted means showed that plasma adiponectin concentration was higher in dogs with DCM compared to healthy dogs (P = .018) and to dogs with MMVD (P = .014), whereas no significant difference was seen between dogs with MMVD compared to healthy dogs (Fig 4). Pairwise ratios (DCM‐healthy, DCM‐MMVD, MMVD‐healthy) for the adjusted geometric means and their 95% CI are given in Table 4. This table also contains the Cohen's d size effects for differences that are statistically significant. From those numbers and the standard of Cohen, DCM can be seen to have a moderate to large effect on plasma adiponectin concentration. No significant effect of class of HF and no interaction between disease and class of HF were observed. No significant relationship between echocardiographic indices (ejection fraction, fractional shortening, left atrial diameter/aortic diameter ratio, left ventricular systolic volume index, left ventricular diastolic volume index), and plasma adiponectin concentration was found.
Table 4.
Analysis of covariance of plasma adiponectin concentration (μg/ml). Adjusted geometric means (admean) for the categorical effects of intact and neutered healthy and heart failure dogs, ratios of the adjusted geometric means between the different categorical effects and Cohen's d size effects between the statistical significant effects and their 95% confidence intervals (CI)
| Estimate | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|
| admean, intact | 12.91 | 10.75 | 15.51 |
| admean, neutered | 6.56 | 4.98 | 8.63 |
| Ratio neutered‐intact | 0.51 | 0.38 | 0.68 |
| Cohen's d neutered‐intact | −0.82 | −1.21 | −0.43 |
| admean, DCM | 13.95 | 9.70 | 20.05 |
| admean, MMVD | 7.04 | 4.95 | 10.01 |
| admean, healthy | 7.93 | 6.69 | 9.40 |
| Ratio DCM—healthy | 1.76 | 1.08 | 2.87 |
| Ratio DCM—MMVD | 1.98 | 1.12 | 3.51 |
| Ratio MMVD—healthy | 0.89 | 0.54 | 1.47 |
| Cohen's d DCM—healthy | 0.76 | 0.23 | 1.29 |
| Cohen's d DCM—MMVD | 0.86 | 0.19 | 1.52 |
DCM, dilated cardiomyopathy; MMVD, myxomatous mitral valve disease.
Figure 4.
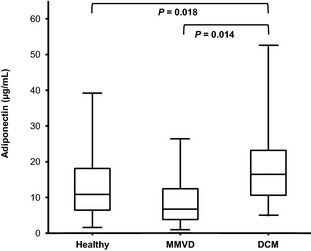
Plasma adiponectin concentration in healthy dogs, and dogs with myxomatous mitral valve disease (MMVD) or dilated cardiomyopathy (DCM). Medians are indicated by the midline; first and third quartiles by the open box; and minima and maxima by the lower and upper whiskers.
Discussion
This study demonstrates that plasma adiponectin is negatively influenced by age and is lower in neutered compared to intact dogs. No effect of BCS and of BW was observed. DCM in dogs (but not MMVD) is associated with higher circulating adiponectin concentrations.
In humans, plasma adiponectin concentration is negatively correlated with body fat content, and a low circulating adiponectin concentration appears to be the strongest predictor of metabolic syndrome.16 In dogs, the implication of adiponectin in the pathogenesis of obesity‐related diseases is under investigation.17 Indeed, the physiology of adiponectin in dogs may differ from that in humans, and diseases associated in humans with obesity such as type 2 diabetes mellitus and atherosclerosis are uncommon in dogs.11 Some studies showed low adiponectin concentrations in obese dogs18, 19 and lower adiponectin concentrations in dogs with obesity‐related metabolic dysfunction compared to obese dogs without metabolic dysfunction20 as well as higher circulating adiponectin concentrations after a period of weight loss.21 On the other hand, several studies have reported that, in dogs, percentage of body fat was not associated with adiponectin concentration.22, 23, 24, 25, 26, 27 Age, sex, and neuter status may be confounding factors when studying the relationship between adiposity and adiponectin.10 Discrepancies also may be related to population differences or assay type.20
Using a covariance analysis and previously mentioned variables, we found that circulating adiponectin concentrations were dependent on age and neuter status but not related to BW, BCS, or to sex. Neutering was associated with a decrease in adiponectin concentration, and the strength of this effect was considered to be moderate to large. This finding is in contrast to 1 study with a small number of dogs (5 individuals per group) that showed a small and nonsignificant decrease in plasma adiponectin concentration only in neutered males but not in neutered females10 and to another showing no effect of orchidectomy on adiponectin concentrations in 5 Beagle dogs at 3 months after surgery.28 In humans, adiponectin concentrations are higher in women than in men and this difference is considered to be related to androgen concentrations.29, 30 Our study supports a negative effect of neutering on circulating adiponectin concentrations and a difference between dogs and humans in the role of androgens on plasma adiponectin concentrations. We also demonstrated that in dogs circulating adiponectin concentrations decrease with age. This finding is in accordance with a recent study in healthy adult client‐owned dogs showing that age was negatively associated with adiponectin concentration.24 In our study, although the age‐neuter status interaction was non‐significant, equations determined separately for neutered and intact dogs suggest a possible more important impact of age in neutered dogs compared to intact dogs. In humans, circulating adiponectin concentrations increase in the elderly.31 Adiponectin concentrations also are greater in centenarians and their offspring32 and lower in healthy offspring of patients with essential hypertension33 suggesting that inherited factors play a role in determining adiponectin concentrations. Several studies in humans have demonstrated that circulating concentrations of adiponectin are highly heritable, and genetic variants implicated in this variability have been identified.34 In our study, plasma adiponectin concentration was in the same range as previously reported for humans6 and dogs.14 We also observed wide variation in plasma adiponectin concentrations with absolute levels ranging from 1.6 to 18 μg/mL in control dogs. Additional studies are needed to investigate the heritability and clinical relevance of this variability.
In the substudy (including 9 dogs), we could not find any evidence of diurnal variation or an effect of feeding on circulating adiponectin concentrations.35
By including dogs with heart disease in the study, we showed that plasma adiponectin concentrations were higher in dogs with DCM compared to healthy dogs and to dogs with MMVD. In human patients with HF, plasma adiponectin concentrations are high even after correction for body mass index.36, 37 In these studies, human patients were diagnosed with a disease of the myocardium such as ischemic or nonischemic dilated cardiomyopathy and hypertensive or primary hypertrophic cardiomyopathy. These findings are in accordance with our study, which showed that dogs with primary myocardial disease have increased circulating adiponectin concentrations. Our study showed for the first time that, in dogs, adiponectin concentrations depend on the type of cardiac disease (primary myocardial disease or endocardial disease). Indeed, in dogs with MMVD, adiponectin concentration remained in the reference range.
In humans, adiponectin concentration increases with the severity of HF. High concentrations of adiponectin are associated with mortality38 and treatment and resolution of decompensated HF are accompanied by a decrease in serum adiponectin concentration39, 40, 41 and a decrease in serum adiponectin concentration in response to treatment predicts good prognosis.42 In our study, circulating adiponectin concentrations were not related to the severity of HF as determined by ISACHC scale. This discrepancy may be related to the small number of dogs in different severity classes leading to a lack of statistical power.
The apparent paradox of adiponectin, a protein known to be cardioprotective, being associated with worse cardiovascular outcomes is referred to “reverse epidemiology,” in which the risk factors for a disease identified in a healthy population are unexpectedly inversely associated with outcomes in a population with the disease.9 The mechanisms behind the phenomenon for adiponectin and HF are still unknown. One hypothesis is that adiponectin concentration is increased in HF as a counter‐regulatory protective response but that unfortunately chronic increases in adiponectin concentration lead to a decrease in adiponectin receptors and functional resistance of adiponectin in target tissues such as heart and muscles.37, 39, 43 This mechanistic hypothesis is supported in dogs by a preliminary study showing an increase in myocardial adiponectin concentration in HF suggesting a greater production of adiponectin in the diseased myocardium.2
A limitation of our study is that control dogs considered “healthy” did not undergo echocardiography. Some of these dogs may have subclinical DCM with no detectable abnormality on physical examination. A second limitation is that the healthy group included some severely underweight and overweight dogs. Anamnesis and clinical examination did not identify any systemic illness, but no other tests were undertaken to explain the origin of the abnormal BCS. Another limitation is that dogs groups were not matched for physiologic parameters but the impact of neuter status and age were taken into account in the statistical analysis. In addition, the small number of dogs in each class of HF and the difference in the number of dogs among classes and diseases may have resulted in an underpowered analysis of the effect of disease severity on plasma adiponectin concentration. Finally, the majority of the dogs were already under treatment for HF before inclusion and some drugs may influence adiponectin concentrations.44
Conclusions
In this study, plasma adiponectin concentration progressively decreased with age, and neutered animals had lower concentrations than intact animals. Sex, BW, BCS, circadian rhythm, and feeding did not influence systemic adiponectin concentrations. Dilated cardiomyopathy was associated with higher adiponectin concentration compared to healthy and MMVD dogs, and the strength of this effect was considered moderate to large. Additional research is required to investigate if increased adiponectin concentrations may be a risk factor for DCM.
Acknowledgment
This work was supported by grants from the Belgian Foundation for Cardiac Surgery, Brussels, Belgium.
Conflict of Interest Declaration: None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of the paper.
Clinical examinations and plasma samples were collected at the Clinical Veterinary Unit of the University of Liège. Adiponectin measurements were made at the Laboratory of Physiology, Université Libre de Bruxelles.
The results were presented at the 2011 ECVIM Congress, Sevilla, September 2011.
Footnotes
Canine Adiponectin ELISA Kit; Millipore, St. Charles, MO
Wood RM, Nelson OL, Häggström J, Höglund K, Ljungvall I, Kvart C. Adiponectin: a protective role in dogs with congestive heart failure? Proceedings of the ACVIM Forum 2011:646 pp
References
- 1. Radin MJ, Sharkey LC, Holycross BJ. Adipokines: A review of biological and analytical principles and an update in dogs, cats, and horses. Vet Clin Pathol 2009;38:136–156. [DOI] [PubMed] [Google Scholar]
- 2. Matsuzawa Y. The metabolic syndrome and adipocytokines. FEBS Lett 2006;580:2917–2921. [DOI] [PubMed] [Google Scholar]
- 3. Fang X, Palanivel R, Cresser J, et al. An APPL1‐AMPK signaling axis mediates beneficial metabolic effects of adiponectin in the heart. Am J Physiol Endocrinol Metab 2010;299:721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ganguly R, Schram K, Fang X, et al. Adiponectin increases LPL activity via RhoA/ROCK‐mediated actin remodelling in adult rat cardiomyocytes. Endocrinology 2011;152:247–254. [DOI] [PubMed] [Google Scholar]
- 5. Konishi M, Haraguchi G, Ohigashi H, et al. Adiponectin protects against doxorubicin induced cardiomyopathy by antiapoptotic effects through AMPK upregulation. Cardiovasc Res 2011;89:309–319. [DOI] [PubMed] [Google Scholar]
- 6. Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose‐specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999;257:79–83. [DOI] [PubMed] [Google Scholar]
- 7. Chen CY, Asakura M, Asanuma H, et al. Plasma adiponectin levels predict cardiovascular events in the observational Arita Cohort Study in Japan: The importance of the plasma adiponectin levels. Hypertens Res 2012;35:843–848. [DOI] [PubMed] [Google Scholar]
- 8. Li Q, Lu Y, Sun L, et al. Plasma adiponectin levels in relation to prognosis in patients with angiographic coronary artery disease. Metabolism 2012;61:1803–1808. [DOI] [PubMed] [Google Scholar]
- 9. Beatty AL, Zhang MH, Ku IA, et al. Adiponectin is associated with increased mortality and heart failure in patients with stable ischemic heart disease: Data from the Heart and Soul Study. Atherosclerosis 2012;220:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verkest K, Rose F, Fleeman L, et al. Adiposity and adiponectin in dogs: Investigation of causes of discrepant results between two studies. Domest Anim Endocrinol 2011;41:34–41. [DOI] [PubMed] [Google Scholar]
- 11. Verkest KR, Bjornvad CR. Guest Editorial: Understanding adiponectin in dogs and cats: A work in progress. Vet J 2012;193:4–5. [DOI] [PubMed] [Google Scholar]
- 12. Dukes‐McEwan J, Borgarelli M, Tidholm A, et al. Proposed guidelines for the diagnosis of canine idiopathic dilated cardiomyopathy. J Vet Cardiol 2003;5:7–19. [DOI] [PubMed] [Google Scholar]
- 13. Fox PR, Sisson D, Moïse NS, et al. Appendix A: Recommendations for diagnosis of heart disease and treatment of heart failure in small animals In: Fox PR, Sisson DD, Moise NS, eds. Textbook of Canine and Feline Cardiology Principles and Clinical Practice, 2nd ed St Louis, MO: WB Saunders Company; 1999:883–901. [Google Scholar]
- 14. Tvarijonaviciute A, Martínez‐Subiela S, Ceron JJ. Validation of 2 commercially available enzyme‐linked immunosorbent assays for adiponectindetermination in canine serum samples. Can J Vet Res 2010;74:279–285. [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen J. Statistical Power Analysis for the Behavioral Sciences, 2nd edn Hillsdale, NJ: Erlbaum; 1988;567 pp. [Google Scholar]
- 16. Trujillo ME, Sherer PE. Adiponectin—Journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med 2005;257:167–175. [DOI] [PubMed] [Google Scholar]
- 17. Ricci R, Bevilacqua F. The potential role of leptin and adiponectin in obesity: A comparative review. Vet J 2012;191:292–298. [DOI] [PubMed] [Google Scholar]
- 18. Gayet C, Leray V, Saito M, et al. The effects of obesity‐associated insulin resistance on mRNA expression of peroxisome proliferator‐activated receptor‐gamma target genes, in dogs. Br J Nutr 2007;98:497–503. [DOI] [PubMed] [Google Scholar]
- 19. Ishioka K, Omachi A, Sagawa M, et al. Canine adiponectin: cDNA structures, mRNA expression in adipose tissues and reduced plasma levels in obesity. Res Vet Sci 2006;80:127–132. [DOI] [PubMed] [Google Scholar]
- 20. Tvarijonaviciute A, Ceron JJ, Holden SL, et al. Obesity‐related metabolic dysfunction in dogs: A comparison with human metabolic syndrome. BMC Vet Res 2012;8:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tvarijonaviciute A, Tecles F, Martinez‐Subiela S, Ceron JJ. Effect of weight loss on inflammatory biomarkers in dogs. Vet J 2012;193:570–572. [DOI] [PubMed] [Google Scholar]
- 22. Grant RW, Vester Boler BM, Ridge TK, et al. Adipose tissue transcriptome changes during obesity development in female dogs. Physiol Genomics 2001;43:295–307. [DOI] [PubMed] [Google Scholar]
- 23. German AJ, Hervera M, Hunter L, et al. Improvement in insulin resistance and reduction in plasma inflammatory adipokines after weight loss in obese dogs. Domest Anim Endocrinol 2009;37:214–226. [DOI] [PubMed] [Google Scholar]
- 24. Mazaki‐Tovi M, Abood SK, Schenck PA. Effect of omega‐3 polyunsaturated fatty acids and body condition on serum concentrations of adipokines in healthy dogs. Am J Vet Res 2012;73:1273–1281. [DOI] [PubMed] [Google Scholar]
- 25. Mitsuhashi Y, Nagaoka D, Ishioka K. Postprandial lipid‐related metabolites are altered in dogs fed dietary diacylglycerol and low glycemic index starch during weight loss. J Nutr 2010;140:1815–1823. [DOI] [PubMed] [Google Scholar]
- 26. Verkest KR, Fleeman L, Morton J, Rand JS. Compensation for obesity‐induced insulin resistance in dogs: Assessment of the effects of leptin, adiponectin, and glucagon‐like peptide‐1 using path analysis. Domest Anim Endocrinol 2011;41:24–34. [DOI] [PubMed] [Google Scholar]
- 27. Wakshlag JJ, Struble AM, Levine CB, et al. The effects of weight loss on adipokines and markers of inflammation in dogs. Br J Nutr 2011;106:11–14. [DOI] [PubMed] [Google Scholar]
- 28. Tvarijonaviciute A, Martínez‐Subiela S, Carrillo‐Sanchez JD. Effects of orchidectomy in selective biochemical analytes in Beagle dogs. Reprod Domest Anim 2011;46:957–963. [DOI] [PubMed] [Google Scholar]
- 29. Böttner A, Kratzsch J, Müller G, et al. Gender differences of adiponectin levels develop during progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab 2004;89:4053–4061. [DOI] [PubMed] [Google Scholar]
- 30. Xu A, Chan KW, Hoo RL, et al. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem 2005;280:18073–18080. [DOI] [PubMed] [Google Scholar]
- 31. Kizer JR, Arnold AM, Strotmeyer ES, et al. Change in circulating adiponectin in advanced old age: Determinants and impact on physical function and mortality. The Cardiovascular Health Study All Stars Study. J Gerontol A Biol Sci Med Sci 2010;65:1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Atzmon G, Pollin TI, Crandall J, et al. Adiponectin levels and genotype: A potential regulator of life span in humans. J Gerontol A Biol Sci Med Sci 2008;63:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Papadopoulos DP, Makris TK, Perrea D, et al. Adiponectin, insulin and resistin plasma levels in young healthy offspring of patients with essential hypertension. Blood Press 2008;17:50–54. [DOI] [PubMed] [Google Scholar]
- 34. Breitfeld J, Stumvoll M, Kovacs P. Genetics of adiponectin. Biochimie 2012;94:2157–2163. [DOI] [PubMed] [Google Scholar]
- 35. Tvarijonaviciute A, Ceron JJ, Tecles F. Serum adiponectin concentration in dogs—Absence of diurnal variation and lack of effect of feeding and methylprednisolone administration. Acta Vet Hung 2012;30:489–500. [DOI] [PubMed] [Google Scholar]
- 36. George J, Patal S, Wexler D, et al. Circulating adiponectin concentrations in patients with congestive heart failure. Heart 2006;92:1420–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yin WH, Wei J, Huang WP, et al. Prognostic value of circulating adipokine levels and expressions of adipokines in the myocardium of patients with chronic heart failure. Circ J 2012;76:2139–2147. [DOI] [PubMed] [Google Scholar]
- 38. Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation 2005;20:1756–1762. [DOI] [PubMed] [Google Scholar]
- 39. Khan RS, Kato TS, Chokshi A, et al. Adipose tissue inflammation and adiponectin resistance in patients with advanced heart failure: Correction after ventricular assist device implantation. Circ Heart Fail 2012;5:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ohara T, Hashimura K, Asakura M, et al. Dynamic changes in plasma total and high molecular weight adiponectin levels in acute heart failure. J Cardiol 2011;58:181–190. [DOI] [PubMed] [Google Scholar]
- 41. Schulze PC, Biolo A, Gopal D. Dynamics in insulin resistance and plasma levels of adipokines in patients with acute decompensated and chronic stable heart failure. J Card Fail 2011;17:1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matsumoto M, Lee‐Kawabata M, Tsujino T, et al. Decrease in serum adiponectin levels in response to treatment predicts good prognosis in acute decompensated heart failure. J Clin Hypertens 2010;12:900–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van Berendoncks AM, Garnier A, Beckers P, et al. Functional adiponectin resistance at the level of the skeletal muscle in mild to moderate chronic heart failure. Circ Heart Fail 2010;3:185–194. [DOI] [PubMed] [Google Scholar]
- 44. Chang LC, Huang KC, Wu YW, et al. The clinical implications of blood adiponectin in cardiometabolic disorders. J Formos Med Assoc 2009;108:353–366. [DOI] [PubMed] [Google Scholar]


