Abstract
Intracranial neoplasia is commonly diagnosed in dogs and can be treated by a variety of methods, but formal comparisons of treatment efficacy are currently unavailable. This review was undertaken to summarize the current state of knowledge regarding outcome after the treatment of intracranial masses in dogs, with the aim of defining optimal recommendations for owners. This review summarizes data from 794 cases in 22 previously published reports and follows PRISMA guidelines for systematic review. A Pubmed search was used to identify suitable articles. These then were analyzed for quality and interstudy variability of inclusion and exclusion criteria and the outcome data extracted for summary in graphs and tables. There was a high degree of heterogeneity among studies with respect to inclusion and exclusion criteria, definition of survival periods, and cases lost to follow‐up making comparisons among modalities troublesome. There is a need for standardized design and reporting of outcomes of treatment for brain tumors in dogs. The available data do not support lomustine as an effective treatment, but also do not show a clear difference in outcome between radiotherapy and surgery for those cases in which the choice is available.
Keywords: Craniotomy, Glioma, Meningioma, Outcome, Radiation, Radiotherapy
Abbreviations
- CT
computed tomography
- MRI
magnetic resonance imaging
- TRMMW
treatment‐related morbidity, mortality, and withdrawal from treatment
When advanced imaging (computed tomography [CT] in the 1980s, magnetic resonance imaging [MRI] in the 1990s) became widely available to veterinarians1, 2, 3, 4 it was recognized that brain tumors were a common cause of clinical neurological signs in older dogs.5, 6 In response, many small animal specialty centers soon began attempts at treatment, often mimicking the approaches that had been well established in the treatment of similarly affected human patients.7, 8, 9, 10, 11, 12 The outcome was that surgical excision via craniotomy and external beam radiation therapy have become widely practiced treatments in veterinary medicine during the past 25 years.
Although brain tumor treatment is commonly discussed at veterinary meetings, there is limited information comparing outcomes after different types of treatment. Furthermore, most data pertain to small case series and retrospective analyses, which carry inherent sources of bias.13 In human medicine, formal and informal comparisons between surgical and radiation therapy approaches have been made over several decades,14, 15 allowing defined protocols to be established for tumor type‐specific treatment.16 In contrast, it is difficult for veterinarians confidently to give advice to clients regarding treatment decisions because there have been no robustly designed formal prospective clinical trials comparing various treatment options for intracranial tumors.
It is unlikely that a randomized clinical trial of one treatment versus another will be carried out in dogs in the near future because of ethical and financial limitations in veterinary research. Although imperfect, systematic review of previously published literature provides an alternative means of answering questions regarding relative efficacy of competing therapies. Here, we present a systematic review of the outcomes after treatment of intracranial mass lesions in dogs. Our primary aim was to compare outcomes of the various treatment modalities, focusing on surgery versus radiation therapy for intracranial masses. The outcome associated with symptomatic management was used as a baseline for comparison.
Materials and Methods
Pubmed was used to search for published literature on the treatment of intracranial mass lesions in dogs using the following search terms: “dog brain tumor radiation,” “dog brain neoplasia radiation,” “dog brain mass radiation,” “dog brain tumor surgery,” “dog brain neoplasia surgery,” “dog brain mass surgery,” “dog brain tumor chemotherapy,” “dog brain neoplasia chemotherapy,” “dog brain mass chemotherapy,” “dog hydroxyurea,” “dog lomustine,” “dog craniotomy,” “dog craniectomy,” “dog brain Bagley,” “dog brain Lecouteur,” “dog brain Sturges,” “dog brain Rossmeisl,”, “dog brain Glass,” “dog brain Klopp,” “dog brain Jeffery,” “dog brain Meij,” “stereotactic radiotherapy dog,” “stereotactic radiosurgery dog,” “dog glioma,” “dog oligodendroglioma,” “dog pituitary mass,” “dog pituitary tumor,” “dog choroid plexus,” and “dog meningioma.” The references in several review articles were scanned for additional primary source material: Lecouteur,12 Snyder et al,17 Mayer and Treuil,18 Motta et al 2012.19 One additional original article20 and one review21 were published during the preparation of material for this review, and data from these sources also were included.
Reports on any treatment modality were eligible for inclusion in the analysis provided that at least 10 cases in total were included and information on survival time was available. Case series of dogs treated symptomatically using palliative corticosteroids and antiseizure medications also were included to provide a baseline against which the outcome of more aggressive therapies could be compared. Series dealing exclusively with functional pituitary microadenomas were excluded because this category constitutes a specific group of tumors with a distinctly different set of clinical signs (ie, hyperadrenocorticsm), prognosis and treatment options. Series that only provided survival rate without reporting the exact number of days, or that were not written in English, also were excluded.
In analysis, studies were first classified into 1 of 9 levels of evidence associated with their specific study design,22, 23 ranging from Level Ia for a systematic review of homogenous randomized controlled trials to Level V for expert opinion without explicit critical appraisal. Next, data was extracted on 6 treatment modalities or treatment combinations: (i) surgery; (ii) radiation therapy; (iii) chemotherapy; (iv) symptomatic management (ie, corticosteroids, anticonvulsant drugs, or both); (v) surgery plus radiation; and (vi) surgery plus chemotherapy. For studies in which survival information on several treatment modalities was reported, the effects of each modality were analyzed separately whenever possible. For each treatment modality, we extracted information on inclusion and exclusion criteria, median survival time, censored cases (ie, missing data or patient surviving at the time of reporting), complications and adverse effects and, lastly, reported treatment‐related morbidity, mortality and withdrawal from treatment before completion (abbreviated to “TRMMW”). This TRMMW group represents all reported dogs that died or were euthanized during general anesthesia or surgery, failed to recover from surgery, or failed to complete radiation therapy or chemotherapy because of death, euthanasia, or early withdrawal.
The original intention was to carry out systematic review followed by meta‐analysis. However, the bias inherent in retrospective studies, inconsistency in data collection and reporting, and inability to extract all the necessary information from all studies rendered statistical analysis inappropriate. Therefore, all extracted data are reported in summary tables and scatter plots. Outliers in this analysis were defined as studies in which survival time was >50% longer than the next highest value or <50% shorter than the next lowest value and were examined in detail in an attempt to determine what may have contributed to their unusual outcomes.
Results
After exclusion of irrelevant or experimental studies, and of the studies that arose repeatedly in our Pubmed searches using different search phrases and studies that contained fewer than 10 cases, we identified 29 articles that fulfilled the initial inclusion criteria. An additional 5 were excluded because they dealt with pituitary microadenomas alone and 2 reports were excluded because insufficient data were available on survival. This left 22 reports (Fig 1) that met all inclusion and exclusion criteria.
Figure 1.
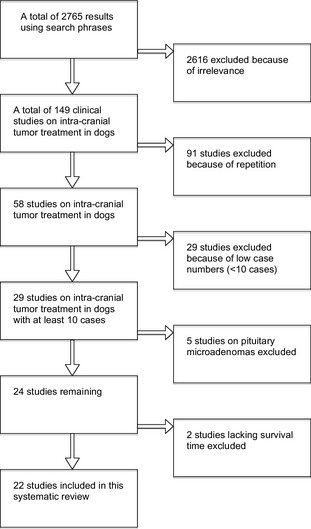
Flowchart to explain selection of articles for analysis.
There was a substantial degree of heterogeneity among the 22 studies in terms of precise mode of treatment, case inclusion criteria and follow‐up periods. For instance, survival time was not clearly defined in 4 reports, was defined as the period from imaging diagnosis to death in 3 reports,24, 25, 26 as “progression‐free” survival time in 1 report,27 and in the remaining 14/22 studies it was defined as the period between the start of treatment and death. There also was variation in the proportion of cases that had histological confirmation of lesion character. In 6/22 series,27, 28, 29, 30, 31, 32 all lesions were histologically confirmed as tumors (mostly meningiomas); most of these were reports on surgical interventions. Only 2 of the studies24, 33 were clearly defined as prospective, although 2 others27, 34 were suggestive of a prospective design; the remainder were retrospective collections of cases that had presented over a variable timed period before the report. According to a standard 9‐level classification method used for assessment of interventions for brain tumor treatment in humans,22, 23 0/22 studies were any type of Level I; 4 were Level IIb; and the remaining 18 were Level IV.
Data from all 22 studies were pooled to extract information on signalment, inclusion and exclusion criteria, median survival time, censored and TRMMW cases, complications and adverse effects; this information is summarized in Tables 1, 2, 3. Of the cases in which sex was reported (n = 536), 53% of dogs (n = 286) were male and 47% (n = 250) were female. The median age of the dogs among all studies was 10 years old (range of medians, 6–11 years old) and the median weight was 25 kg (range of medians, 21–27 kg).
Table 1.
Summary of number of dogs treated by each treatment modality
| Treatment | Total | Censored | TRMMW (% of Total) | Net |
|---|---|---|---|---|
| Surgery | 109 | 26 | 10 (9.2) | 73 |
| Radiation | 449 | 91 | 42 (9.4) | 316 |
| Symptomatic | 141 | 12 | 1 (0.7) | 128 |
| Surgery and radiation | 26 | 2 | 4 (15.4) | 20 |
| Surgery and chemotherapy | 8 | 4 | 0 (0) | 4 |
| Chemotherapy | 61 | 11 | 0 (0) | 50 |
TRMMW – treatment‐related morbidity, mortality and withdrawal from treatment before completion.
Table 2.
Reported median survival time and complications associated with each treatment modality
| Treatment Modality | Number of Studies (Cases) | Survival Time Median (Range) Days | Reported Complications and Adverse Effects |
|---|---|---|---|
| Symptomatic | 4 (138) | 65 (6–359) | Polyuria, polydipsia, polyphagia, and sedation |
| Surgical | 6 (108) | 312 (27–2104) | Aspiration pneumonia, bronchopneumonia, pneumocranium, uncontrollable seizures postop, middle cerebral artery thrombosis, arterial cerebral circle hemorrhage, hyponatremia, hypo‐osmolality, large temperature fluctuation |
| Radiation | 13 (428) | 351 (139–906) | Aspiration pneumonia, pulmonary thromboembolism, altered mentation (acute central nervous system toxicity), late‐onset radiation necrosis, risks associated with repeated anesthesia, permanent skin epilation, atrophy of temporal muscle, keratitis with corneal vascularization, bilateral cataracts, and deafness |
Table 3.
Median survival time of dogs with masses at specific locations
| Tumor Location | Number of Studies (Total Cases) | Median Survival Time (Range of Medians) in Days |
|---|---|---|
| Extra‐axial | 17 (335) | 348 (46–2104) |
| Intra‐axial | 10 (127) | 226 (60–437) |
| Pituitary | 6 (104) | 351 (of those reaching median) (118–688a) |
The study by Kent et al35 reported a median survival of more than 2000 days.
Symptomatic management
Five of the 22 studies reported on symptomatically managed cases, of which 410, 24, 25, 35 reported median survival times. Median survival times in these reports, which pertained to data from 128 dogs, ranged from 6 days (n = 4510) to 359 days (n = 2735; pituitary masses) with a median among reports of 65 days. Adverse effects and complications were predominantly those associated with corticosteroids and anticonvulsants (Table 2).
One study10 did not clearly define the survival period and censored individuals were not removed from the cohort for the final analysis of survival time; in the remaining 3 studies survival time was defined as the period between diagnosis and death. It is not clear whether the censored group was included in survival time analysis in 1 study,24 although the censored group represents only a small portion of the medically managed cohort (ie, 4/51 animals). We reanalyzed the data reported in 1 study25 using the methods stated above for censored and TRMMW groups because the raw dataset was available in the published article.
Surgery
Six of the 22 studies reported median survival times for cases (n = 109) treated by surgery (Table 1 and Fig 2). The range of median survival times was 27 days10 (n = 16) to 2104 days31 (n = 27) and the median among reports was 312 days. The study on survival of dogs with endoscopy‐assisted surgically treated meningiomas31 reported forebrain and brainstem or cerebellar meningiomas as 2 separate subgroups; the 2 survival times are presented as 2 separate data points in our analysis (Fig 2) because the original data were not available.
Figure 2.
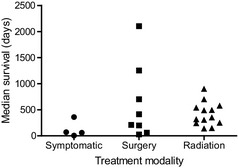
Median survival times associated with treatment using different modalities. This plot was compiled from data included in references.10, 11, 20, 24, 25, 26, 27, 28, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 45, 46 The data included in references31 and 32 have been subdivided into two points because the summary data was unavailable.
One study31 reported an unusually high median survival time of 2104 days for endoscopy‐assisted surgically treated forebrain meningiomas, which by our definition, is an outlier. In this study, only animals that survived the first week postsurgery were included; 4 of the 27 treated dogs died within this period and were excluded from survival time analysis and 4 other cases received radiotherapy at a later date. The survival curve in this study illustrates that 7 dogs were censored within the first 500 days and, in all, 16 meningioma cases were censored.
Similarly, a notably prolonged median survival time of 1254 days was reported in another study30 in which rostro‐tentorial (bilateral or unilateral) meningiomas were excised using a surgical aspirator. Three of 20 dogs were lost to follow‐up after discharge and the report concerns only 17 cases. Of these, 14 survived to discharge, the other 3 died from disease or complications. In the survival plot, several dogs were censored before 2 years, indicating that the reported median survival time in this study corresponds to the death of 1 of the 2 remaining dogs at 1254 days.
Radiation therapy
Median survival times were available from 13 studies on radiation therapy (including those included in 1 study36 in which 20 dogs had radiation therapy alone), comprising a total of 428 animals (Table 1 and Fig 2); these ranged from 139 days34 (n = 47) to over 900 days27 (n = 20) with a median of 351 days. In 1 additional study on pituitary masses35 (n = 19) >50% of dogs survived for >2000 days and thus a median value was not available. Most series reported on megavoltage or cobalt‐60 irradiation, but 1 publication11 reported the results of orthovoltage irradiation in a small number of dogs that had completed the prescribed course (this group included 4/13 dogs that also had undergone surgery). The median survival times of the radiation‐treated cases fell within a narrower range (139–906 days) than those treated by surgery (27–2104 days).
The longest median survival after treatment by radiotherapy was achieved in a study35 in which >50% of treated dogs (n = 19) with pituitary masses survived >2000 days. Not all of these dogs had neurologic signs at the time of treatment and some masses were small (median height, 9 mm). One study37 reported a median all‐cause mortality survival time of 699 days for 46 cases, substantially longer than reported in many other series. The study further divided the subjects by the location of intracranial tumors with their respective median survival time reported separately (ie, 726 days for extra‐axial, 430 for intra‐axial, 688 for pituitary macroadenomas). Inclusion criteria included completion of the course of treatment, dogs that had received previous surgery were excluded and none of the dogs were reported to have any evidence of concomitant life‐threatening diseases when treatment was started.
One study38 reported a median survival time of 577 days for meningiomas (ie, extra‐axial masses) in 21 dogs (11 after excluding censored and TRMMW groups). Of these, 10 previously had surgery. Another study39 reported a median survival time of 539 days (from the beginning of treatment) for pituitary macroadenomas (>1 cm diameter) in 12 dogs. The pretreatment clinical signs exhibited by these animals were mild.
Another study34 reported a very short median survival time (133 days) from the commencement of radiation therapy in 47 cases. This study separately reported the survival of all dogs that entered the study (133 days) and that of dogs that completed the 6‐fraction course of radiotherapy (230 days). Many other studies reported results only on dogs that completed the course of treatment.
Location effect
Information on the location of the lesion was available for 566 animals among the 22 studies. Of these, 59% (n = 335) had extra‐axial tumors (predominantly presumptive meningiomas based on imaging or histopathological examination), 22% (n = 127) had intra‐axial tumors (predominantly presumptive gliomas based on imaging or histopathological examination), and the remaining 18% (n = 104) had pituitary macroadenomas (based on imaging).
The medians of the reported median survival times, where available, of extra‐axial, intra‐axial and pituitary masses were 348, 233, and 343 days, respectively (Table 2 and Fig 3). Animals included in the “outlier” study31 discussed above were included in these data.
Figure 3.
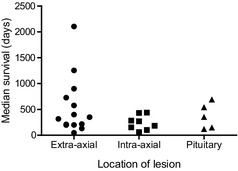
Median survival time associated with masses located at different sites within the skull. This plot was compiled from data included in references.20, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 37, 38, 39, 40, 45 Reference35 (Kent et al is not included here because the treated group did not have a median survival).
The lack of reporting consistency among reports in terms of inclusion and exclusion criteria, singular versus combination treatment modalities and measurement of survival time (mean versus median) rendered the extraction of survival information particularly challenging. For instance, whereas 1 study31 excluded all animals that died within the first week after surgery for meningiomas, another study38 included all animals that died within a minimum of 6 months after completion of radiation therapy for meningiomas, but they also included animals treated by both radiation and surgery.
Treatment for extra‐axial tumors (presumptive meningioma)
Extra‐axial mass lesions, often presumptive meningiomas, constituted the most commonly reported intracranial tumors in dogs in the 22 studies included in our analysis. Fifteen of the 22 studies reported on the treatment of extra‐axial tumors (total n = 197), of which median survival time was available in 4 studies for surgically treated cases (n = 63), and 9 for radiation therapy‐treated cases (n = 134, Fig 4). Despite the limited number of data points, the range of the median survival times of the surgically treated group (198–2104 days) was substantially longer than that of the radiation‐treated group (130–900 days, with a median of 444 days, Fig 4). Again, there were many differences in study design and reporting among these different studies.
Figure 4.
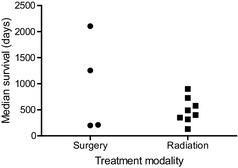
Median survival times of dogs with meningiomas treated with surgery or radiotherapy. This plot was compiled from data included in references.11, 20, 27, 28, 30, 31, 32, 34, 37, 38, 40, 45
Other treatment modalities
The same information on 3 other treatment options (chemotherapy, surgery with radiation, and surgery with chemotherapy) was extracted from all 22 studies, where available. These treatment modalities are not discussed in detail in this review because of the low number of cases that could be identified and analyzed from the reports (n = 50, 20 and 4, respectively) and the limited availability of median survival times. Similarly, hyperthermia was reported in one study36, but its effects are not analyzed here because of low case numbers. In the largest study on chemotherapy, 56 dogs were treated, resulting in median survival of 75 days.25 This survival period was not significantly different from that of a medically treated group described in the same article, although dogs were not randomly assigned to different therapies.
These issues are further complicated by problems associated with reporting, not dissimilar to those mentioned earlier. In 1 study28 the median disease‐specific survival time of 14 dogs treated with surgery alone was reported to be 210 days, compared with 495 days for 12 dogs treated with surgery and radiation. In contrast, in a study on meningiomas,38 similar survival periods were reported for surgery plus radiotherapy and radiotherapy‐alone groups, although the number in the combination therapy group was small (10 dogs). Neither of these studies provided information regarding how dogs were selected to be treated by surgery alone or to receive combination therapy.
Complications of different treatment modalities
Table 2 lists the reported complications associated with each of the modalities. Direct comparison among complication rates of different treatment modalities is not possible based on the available data, largely because of the differences in the period during which animals were directly observed, methods of case detail recording, and lack of consistent censoring criteria among studies. For instance, although some studies28, 31 excluded animals that died within 1 week postsurgery from the survival time analysis, another study40 included all the cases that died during the peri‐operative period. Although direct comparison of the complication rates among various treatment modalities is not possible, the TRMMW group provides information on the likelihood of an animal completing the full course of a particular treatment (Table 1), which indirectly provides an estimate of the inherent risks and complications associated with each treatment. Surgery and radiation therapy appear to have very similar TRMMW rates as a percentage of the total number of cases (ie, 9.2% versus 9.4%). These rates are much higher than the dropout rate in symptomatic case series (0.7%).
Bone marrow suppression (leukopenia and thrombocytopenia) is a commonly reported adverse effect associated with chemotherapy.41 Occasional emesis with rare need for anti‐emetic drugs also is mentioned in some studies without specific case numbers. One study25 reported that lomustine was titrated using CBC evaluation, but the study did not report how many cases showed toxicity.
Discussion
The aim of this review was to determine whether, in the absence of a formal randomized trial, there is reliable published evidence to support specific modes of treatment for the treatment of brain tumors in dogs, either generally, or of specific subtypes. Theoretically, direct comparison among differently treated series of cases should provide reasonable evidence regarding the relative effectiveness of the different available therapies. This review found many obstacles to reaching such a conclusion. The main difficulties were the variability in reporting of inclusion and exclusion criteria (including variation in constituent cases by histologic type or location) and the description of outcomes, including adverse effects. Moreover, median survival times were not readily extracted from several reports and there were very low case numbers in several studies, thereby precluding the possibility of any meaningful statistical analysis. The high proportion of censoring in some studies, particularly those already containing low case numbers, inevitably increases the likelihood of finding extreme results that cannot be generalized.13 Of particular note is the lack of inclusion of cases that died at early time points, such as within the first 7 days (for surgical patients) or exclusion from analysis of cases that died before completion of treatment (for radiotherapy patients). Therefore, legitimate comparisons among published studies are difficult to make, especially comparisons to palliative therapy alone, because such “early‐death” cases would not be excluded from those series. In human medicine, there are clear definitions of end‐points defined by intention‐to‐treat and per protocol treatment and this convention has not been followed in veterinary reports. In this analysis, we aimed to mitigate this lack of information by using the summary term of TRMMW to highlight the proportion of animals that were unaccounted for in the final analysis of each report.
Despite these caveats, based on the published data available in this systematic review, there is evidence of a beneficial effect of both radiation and surgery compared with symptomatic therapy alone. In general, there appears to be little difference overall between survival after surgery and radiotherapy, even for meningiomas, for which the largest body of information exists. Radiotherapy appears to be associated with as a long a survival period as surgery and there is little evidence to suggest that the adverse effects and morbidity are any worse in radiotherapy‐treated cases. However, the 2 series reporting the longest median survival periods30, 31, both involved surgical treatments, suggesting the possibility that meticulous surgical excision, as reported in these 2 studies, may have survival benefit. On the other hand, closer examination of these 2 series suggests that the apparent prolonged survival may not be a robust finding; both series had high rates of censoring for cases that are lost to follow‐up or still alive at early time intervals. For instance, in a study in which dogs were treated using an ultrasonic aspirator,30 it was suggested that this method may extend survival, but the reported median survival period is dependent upon analysis of only approximately 3 surviving cases. These 2 series also are most susceptible to possible publication bias (ie, bias toward publication of articles in which outcomes are positive) and small sample bias (in which small samples are more likely to produce extreme results). These issues are problematic for much veterinary clinical research, but are especially relevant to these studies.
Another major inconsistency among reports is whether “all‐cause” or “cause‐specific” mortality is presented. There is merit to both approaches, depending upon the answer that is sought. For instance, from an owner's point‐of‐view it would be preferable to see the data for all‐cause mortality because they do not always know whether there are other lesions in their dog that might contribute to the animal's death within a specified period. On the other hand, veterinarians likely are more interested in cause‐specific mortality because they want to determine which treatment to recommend for an individual dog. Nevertheless, without consistency in data reporting, direct comparison is impossible.
There is insufficient data to comment on modes of treatment other than radiotherapy and surgery because only a small number of chemotherapy cases have been reported, despite much anecdotal commentary about this modality. One study25 has already provided moderately strong evidence that lomustine given at a dosage of 90 mg/m2 seems to provide no survival benefit over symptomatic therapy alone, but dogs in that study were not randomized to treatment. There has long been a suggestion that combinations of treatment, notably surgery and radiotherapy, provide the best mode of treatment for brain tumors (particularly meningiomas),42 largely based on data provided in the articles analyzed here. However, looking at the data as a whole, this conclusion appears inappropriate because the evidence would suggest that adding radiotherapy to surgery has a large impact,28 whereas adding surgery to radiotherapy has no impact,38 suggesting that radiotherapy is the effective modality and surgery may have little additive effect.
Is there any reason to be skeptical about the results reported for radiotherapy? In these studies there are many caveats about which cases are included in the series. For instance, it is commonly stated that specific cases were not included unless they completed the course of radiotherapy. Such exclusion will omit an unknown number of cases that are unsuccessfully treated, including those with substantial pretreatment morbidity, which could artificially inflate the reported survival times. This observation suggests that there is a need for better presentation of data, especially to take into account the need for intention‐to‐treat analyses and to record all animals that were considered, but excluded from specific types of treatment. Exclusion of early mortality also occurs in reports on surgery with many publications stating that cases that died within 7 days were excluded from analysis. Another question that might be asked is whether or not the newer modes of radiotherapy (eg, gamma knife, conformational) are superior to older delivery systems. There currently is insufficient information with which to answer that question because there are only 2 case series that report specifically on newer modes of radiotherapy. In our analysis we included all types of radiotherapy together.
Unfortunately, it is not possible to sum the results of all of the studies together to give an overall statement regarding the value of the different therapies, or even suggesting whether or not different treatments might be preferred for specific types or locations of tumors. This information could be obtained by adherence to publishing guidelines43 for the reporting of methods and results so that different approaches can be contrasted. It would be helpful if sufficient detail were included to permit independent analysis of the published data, or at least for such data to be available as supplementary information when published. Similar recommendations have been made in human medicine to prevent the waste of resources that is inevitable if data are not made widely available.44
Although ideal, it is unlikely that a randomized trial to compare different modalities across all types of tumor or even for selected sub‐groups will be carried out unless the funding environment for veterinary medicine changes. Therefore, a good opportunity for identifying the best treatments might be through registries of brain tumor treatment that could be banked by a central organization. At present, for all the effort expended on treatment of brain tumors in dogs there remains only approximately 700 published treated cases available for analysis, and the data from many of these cases are not available for re‐analysis.
Acknowledgments
Conflict of Interest Declaration Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration Authors declare no off‐label use of antimicrobials.
Funding There was no grant support for this study.
Contributions to this review were made by each author at their respective institutions.
This work has not been presented at any meetings.
References
- 1. Fike JR, LeCouteur RA, Cann CE, Pflugfelder CM. Computerized tomography of brain tumors of the rostral and middle fossas in the dog. Am J Vet Res 1981;42:275–281. [PubMed] [Google Scholar]
- 2. Turrel JM, Fike JR, LeCouteur RA, Higgins RJ. Computed tomographic characteristics of primary brain tumors in 50 dogs. J Am Vet Med Assoc 1986;188:851–856. [PubMed] [Google Scholar]
- 3. Stewart WA, Parent JM, Towner RA, Dobson H. The use of magnetic resonance imaging in the diagnosis of neurological disease. Can Vet J 1992;33:585–590. [PMC free article] [PubMed] [Google Scholar]
- 4. Kraft SL, Gavin PR, DeHaan C, et al. Retrospective review of 50 canine intracranial tumors evaluated by magnetic resonance imaging. J Vet Intern Med 1997;11:218–225. [DOI] [PubMed] [Google Scholar]
- 5. Foster ES, Carrillo JM, Patnaik AK. Clinical signs of tumors affecting the rostral cerebrum in 43 dogs. J Vet Intern Med 1988;2:71–74. [DOI] [PubMed] [Google Scholar]
- 6. Moore MP, Bagley RS, Harrington ML, Gavin PR. Intracranial tumors. Vet Clin North Am Small Anim Pract 1996;26:759–777. [DOI] [PubMed] [Google Scholar]
- 7. Oliver JE. Surgical approaches to the canine brain. Am J Vet Res 1968;29:353–378. [Google Scholar]
- 8. Turrel JM, Fike JR, LeCouteur RA, et al. Radiotherapy of brain tumors in dogs. J Am Vet Med Assoc 1984;184:82–86. [PubMed] [Google Scholar]
- 9. Kostolich M, Dulisch ML. A surgical approach to the canine olfactory bulb for meningioma removal. Vet Surg 1987;16:273–277. [DOI] [PubMed] [Google Scholar]
- 10. Heidner GL, Kornegay JN, Page RL, et al. Analysis of survival in a retrospective study of 86 dogs with brain tumors. J Vet Intern Med 1991;5:219–226. [DOI] [PubMed] [Google Scholar]
- 11. Evans SM, Dayrell‐Hart B, Powlis W, et al. Radiation therapy of canine brain masses. J Vet Intern Med 1993;7:216–219. [DOI] [PubMed] [Google Scholar]
- 12. LeCouteur RA. Current concepts in the diagnosis and treatment of brain tumours in dogs and cats. J Small Anim Pract 1999;40:411–416. [DOI] [PubMed] [Google Scholar]
- 13. Button KS, Ioannidis JP, Mokrysz C, et al. Power failure: Why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 2013;14:365–376. [DOI] [PubMed] [Google Scholar]
- 14. Rutigliano MJ, Lunsford LD, Kondziolka D, et al. The cost effectiveness of stereotactic radiosurgery versus surgical resection in the treatment of solitary metastatic brain tumors. Neurosurgery 1995;37:445–453. [DOI] [PubMed] [Google Scholar]
- 15. Hart MG, Grant R, Walker M, Dickinson H. Surgical resection and whole brain radiation therapy versus whole brain radiation therapy alone for single brain metastases. Cochrane Database Syst Rev 2005; (1):CD003292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ryken TC, Kalkanis SN, Buatti JM, Olson JJ; AANS/CNS Joint Guidelines Committee . The role of cytoreductive surgery in the management of progressive glioblastoma: A systematic review and evidence‐based clinical practice guideline. J Neurooncol 2014;118:479–488. [DOI] [PubMed] [Google Scholar]
- 17. Snyder JM, Shofer FS, Van Winkle TJ, Massicotte C. Canine intracranial primary neoplasia: 173 cases (1986–2003). J Vet Intern Med 2006;20:669–675. [DOI] [PubMed] [Google Scholar]
- 18. Mayer MN, Treuil PL. Radiation therapy for pituitary tumors in the dog and cat. Can Vet J 2007;48:316–318. [PMC free article] [PubMed] [Google Scholar]
- 19. Motta L, Mandara MT, Skerritt GC. Canine and feline intracranial meningiomas: An updated review. Vet J. 2012;192:153–165. [DOI] [PubMed] [Google Scholar]
- 20. Griffin LR, Nolan MW, Selmic LE, et al. Stereotactic radiation therapy for treatment of canine intracranial meningiomas. Vet Comp Oncol 2014. doi:10.1111/vco.12129 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21. Dickinson PJ. Advances in diagnostic and treatment modalities for intracranial tumors. J Vet Intern Med 2014;28:1165–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Proescholdt MA, Macher C, Woertgen C, Brawanski A. Level of evidence in the literature concerning brain tumor resection. Clin Neurol Neurosurg 2005;107:95–98. [DOI] [PubMed] [Google Scholar]
- 23. Oxford Centre for Evidence‐based Medicine – Levels of Evidence (March 2009). http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed March 16, 2015.
- 24. Rossmeisl JH Jr, Jones JC, Zimmerman KL, Robertson JL. Survival time following hospital discharge in dogs with palliatively treated primary brain tumors. J Am Vet Med Assoc 2013;242:193–198. [DOI] [PubMed] [Google Scholar]
- 25. Van Meervenne S, Verhoeven PS, de Vos J, et al. Comparison between symptomatic treatment and lomustine supplementation in 71 dogs with intracranial, space‐occupying lesions. Vet Comp Oncol 2014;12:67–77. [DOI] [PubMed] [Google Scholar]
- 26. Fulton LM, Steinberg HS. Preliminary study of lomustine in the treatment of intracranial masses in dogs following localization by imaging techniques. Semin Vet Med Surg (Small Anim) 1990;5:241–245. [PubMed] [Google Scholar]
- 27. Théon AP, Lecouteur RA, Carr EA, Griffey SM. Influence of tumor cell proliferation and sex‐hormone receptors on effectiveness of radiation therapy for dogs with incompletely resected meningiomas. J Am Vet Med Assoc 2000;216:701–707. [DOI] [PubMed] [Google Scholar]
- 28. Axlund TW, McGlasson ML, Smith AN. Surgery alone or in combination with radiation therapy for treatment of intracranial meningiomas in dogs: 31 cases (1989‐2002). J Am Vet Med Assoc 2002;221:1597–1600. [DOI] [PubMed] [Google Scholar]
- 29. Jeffery N, Brearley MJ. Brain tumors in the dog: Treatment of 10 cases and review of recent literature. J Small Anim Pract 1993;34:367–372. [Google Scholar]
- 30. Greco JJ, Aiken SA, Berg JM, et al. Evaluation of intracranial meningioma resection with a surgical aspirator in dogs: 17 cases (1996–2004). J Am Vet Med Assoc 2006;229:394–400. [DOI] [PubMed] [Google Scholar]
- 31. Klopp LS, Rao S. Endoscopic‐assisted intracranial tumor removal in dogs and cats: Long‐term outcome of 39 cases. J Vet Intern Med 2009;23:108–115. [DOI] [PubMed] [Google Scholar]
- 32. Niebauer GW, Dayrell‐Hart BL, Speciale J. Evaluation of craniotomy in dogs and cats. J Am Vet Med Assoc 1991;198:89–95. [PubMed] [Google Scholar]
- 33. Théon AP, Feldman EC. Megavoltage irradiation of pituitary macrotumors in dogs with neurologic signs. J Am Vet Med Assoc 1998;213:225–231. [PubMed] [Google Scholar]
- 34. Norman A, Ingram M, Skillen RG, et al. X‐ray phototherapy for canine brain masses. Radiat Oncol Investig 1997;5:8–14. [DOI] [PubMed] [Google Scholar]
- 35. Kent MS, Bommarito D, Feldman E, Theon AP. Survival, neurologic response, and prognostic factors in dogs with pituitary masses treated with radiation therapy and untreated dogs. J Vet Intern Med 2007;21:1027–1033. [DOI] [PubMed] [Google Scholar]
- 36. Thrall DE, Larue SM, Powers BE, et al. Use of whole body hyperthermia as a method to heat inaccessible tumours uniformly: A phase III trial in canine brain masses. Int J Hyperthermia 1999;15:383–398. [DOI] [PubMed] [Google Scholar]
- 37. Bley CR, Sumova A, Roos M, Kaser‐Hotz B. Irradiation of brain tumors in dogs with neurologic disease. J Vet Intern Med 2005;19:849–854. [DOI] [PubMed] [Google Scholar]
- 38. Keyerleber MA, McEntee MC, Farrelly J, et al. Three‐dimensional conformal radiation therapy alone or in combination with surgery for treatment of canine intracranial meningiomas. Vet Comp Oncol 2013. doi:10.1111/vco.12054 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 39. de Fornel P, Delisle F, Devauchelle P, Rosenberg D. Effects of radiotherapy on pituitary corticotroph macrotumors in dogs: A retrospective study of 12 cases. Can Vet J 2007;48:481–486. [PMC free article] [PubMed] [Google Scholar]
- 40. Brearley MJ, Jeffery ND, Phillips SM, Dennis R. Hypofractionated radiation therapy of brain masses in dogs: A retrospective analysis of survival of 83 cases (1991‐1996). J Vet Intern Med 1999;13:408–412. [DOI] [PubMed] [Google Scholar]
- 41. Heading KL, Brockley LK, Bennett PF. CCNU (lomustine) toxicity in dogs: A retrospective study (2002‐07). Aust Vet J 2011;89:109–116. [DOI] [PubMed] [Google Scholar]
- 42. Taylor SM. Chapter 65 Intracranial disorders In: Nelson RW, Couto CG, eds. Small Animal Internal Medicine, 4th edn St Louis, MO: Mosby Elsevier; 2009:1019–1026. [Google Scholar]
- 43. The STROBE Statement . http://www.strobe-statement.org/. Accessed March 16, 2015.
- 44. Chan AW, Song F, Vickers A, et al. Increasing value and reducing waste: Addressing inaccessible research. Lancet 2014;383:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mariani CL, Schubert TA, House RA, et al. Frameless stereotactic radiosurgery for the treatment of primary intracranial tumours in dogs. Vet Comp Oncol 2013. doi: 10.1111/vco.12056. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 46. Spugnini EP, Thrall DE, Price GS, et al. Primary irradiation of canine intracranial masses. Vet Radiol Ultrasound 2000;41:377–380. [DOI] [PubMed] [Google Scholar]


