Abstract
Background
Dogs with a chronic enteropathy (CE) have a lower vitamin D status, than do healthy dogs. Vitamin D status has been associated with a negative clinical outcome in humans with inflammatory bowel disease.
Objectives
To examine the relationship between serum 25 hydroxyvitamin D (25(OH)D) concentrations at diagnosis and clinical outcome in dogs with a CE.
Animals
Forty‐one dogs diagnosed with CE admitted to the Royal Dick School of Veterinary Studies, Hospital for Small Animals between 2007 and 2013.
Methods
Retrospective review. Serum 25(OH)D concentrations were compared between dogs which were alive at follow up or had died because of non‐CE‐related reasons (survivors) and dogs which died or were euthanized due to their CE (non‐survivors). A binary logistic regression analysis was performed to determine significant predictors of death in dogs with CE.
Results
Serum concentrations of 25(OH)D at the time a CE was diagnosed were significantly lower in nonsurvivors (n = 15) (median nonsurvivors 4.36 ng/mL, interquartile range 1.6–17.0 ng/mL), median survivors (n = 26) (24.9 ng/mL interquartile range 15.63–39.45 ng/mL, P < .001). Serum 25(OH)D concentration was a significant predictor of death in dogs with CE (odds ratio 1.08 [95% CI 1.02–1.18)]).
Conclusions
Serum 25(OH)D concentrations at diagnosis are predictive of outcome in dogs with CE. The role of vitamin D in the initiation and outcome of chronic enteropathies in dogs is deserving of further study.
Keywords: 25 (OH)D, Prognostic, Inflammatory bowel disease
Abbreviations
- 25(OH)D
25 hydroxyvitamin D
- AIC
akaike information criteria
- ALT
alanine transaminase
- ALP
alkaline phosphatase
- IBD
inflammatory bowel disease
- CE
chronic enteropathy
- CPA
Clinical Pathology Accreditation
- CIBDAI
canine inflammatory bowel disease activity index
- HPLC
high performance liquid chromatography
- (TNF)‐α
tumor necrosis factor alpha
- VDR
vitamin D receptor
- WSAVA
World Small Animal Veterinary Association
Chronic enteropathies in dogs are a major cause of morbidity and mortality.1 A diagnosis of chronic enteropathy (CE) is made in dogs which have a several week history of gastrointestinal signs such as weight loss, vomiting, and diarrhea, and the absence of an underlying etiology based on diagnostic evaluation and the presence of an inflammatory infiltrate within gastrointestinal biopsies.2 The pathogenesis of CE in dogs is considered to be multifactorial and includes factors such as abnormal mucosal immunity, disrupted epithelial barrier function, altered intestinal microbial flora, environment, and genetics.2, 3
Dogs with CE have lower serum concentrations of 25 hydroxyvitamin D (25(OH)D), which is the vitamin D metabolite widely used to assess vitamin D status, than do healthy dogs and hospitalized dogs with nongastrointestinal illnesses.4, 5 In addition, the severity of the clinical signs, as assessed by the canine inflammatory bowel activity index (CIBDAI), correlates with serum 25(OH)D concentrations in dogs with a CE.5 However, the prognostic significance of serum 25(OH)D concentrations has not been investigated in dogs with a CE.
In contrast, the relationship between vitamin D status and IBD has been extensively explored in human medicine. In human patients with IBD, notably ulcerative colitis and Crohn's disease, vitamin D deficiency is a frequent finding.6, 7, 8, 9 Higher predicted vitamin D status has been associated with a reduced risk of developing Crohn's disease.10 Among people with IBD, 25(OH)D concentrations are related to disease severity scores and patient quality of life.11, 12, 13 Low plasma 25(OH)D concentrations have also been associated with an increased risk of surgery and longer hospitalization periods in patients with either Crohn's disease or ulcerative colitis.14 In addition, normalization of 25(OH)D concentrations in patients with Crohn's disease has been associated with a reduction in the risks associated with surgery.14 Vitamin D status has also been linked to IBD treatment outcomes, as low pretreatment vitamin D status has been associated with reduced durability of response to antitumor necrosis factor (TNF)‐α treatment.15 Studies have also demonstrated improvements in disease activity scores and quality of life scores in Crohn's disease patients supplemented with oral vitamin D.16, 17
The hypothesis of this study was that the vitamin D status would not be different in dogs that died or were euthanized because of the complications associated with their CE compared to dogs which were alive at follow up or had died because of diseases unrelated to their CE. The aim of this study was to measure serum concentrations of 25(OH)D, alongside a range of clinical and biochemical variables, in dogs with a confirmed diagnosis of CE and known clinical outcome.
Material and Methods
Study Population
The records of dogs referred to the Hospital for Small Animals, Royal Dick School of Veterinary Studies for investigation of chronic gastrointestinal disease (more than 3 weeks in duration) between 2007 and 2013 were retrospectively reviewed. Dogs were considered eligible for inclusion in the study if they had presenting clinical signs consistent with a CE, which included any of the following: vomiting, diarrhea, increased borborygmi, abdominal pain, increased or decreased appetite, and weight loss. All dogs were considered eligible if they had histopathological evidence of inflammation within the small or large intestine and there were no clinically relevant abnormalities detected on hematology, biochemistry, or abdominal ultrasonography, which were not attributable to CE. In addition, a stored frozen serum sample from each dog, collected at the time of diagnosis was required for retrospective analysis of 25(OH)D concentrations. Hematology variables (total white blood cell count, mature neutrophils, band neutrophils lymphocytes, monocytes, eosinophils, basophils, total red blood cell counts, packed cell volume, hemoglobin, mean cell volume, mean cell hemoglobin concentration, and platelet number) were measured on ADVIA(r) 2120i System with Autoslide.1 Biochemistry variables (albumin, alanine transaminase [ALT], alkaline phosphatase [ALP], bile acids, bilirubin, total calcium, creatinine, globulin, phosphate, potassium, sodium, chloride, urea, and glucose) were measured on an ILab650 biochemistry analyzer.2 Clinical records were also reviewed for the results of fecal parasitology. Canine inflammatory bowel disease activity index (CIBDAI) scores were calculated as follows: appetite, activity levels, vomiting, fecal consistency, fecal frequency, and weight loss were each scored from 0 to 3. A score of 0 indicated no changes were present, a score of one indicated mild changes, 2 moderate changes, and 3 severe changes. In addition, a score of one point was added if the feces contained blood or mucus. The area of intestinal tract which was biopsied (duodenum or duodenum and colon) was determined based on the presenting clinical signs and was at the discretion of the primary clinician managing the case. A diagnosis of CE was made if there was histological evidence of intestinal inflammation and no clear underlying cause.
Vitamin D Analysis
Serum samples retained for 25(OH)D measurement were frozen after being used for routine biochemical analysis. They were stored at −70°C before being sent to the laboratory for analysis on dry ice. Serum 25(OH)D has been shown to be stable under these conditions.18 Serum concentrations of 25(OH)D were measured as previously described in detail.19, 20 Samples were extracted using acetonitrile and applied to C18 Silica Sep‐paks. Separation of metabolites was by straight phase high performance liquid chromatography (HPLC)3 using a Hewlett‐Packard Zorbax‐Sil Column4 eluted with hexane:propan‐2‐ol:methanol (92 : 4 : 4). Serum 25(OH)D2 and 25(OH)D3 were measured separately by application to a second Zorbax‐Sil Column eluted with hexane:propan‐2‐ol (98 : 2) and quantified by ultraviolet absorbance at 265 nm and corrected for recovery (sensitivity 5 nmol/l, intra‐ and interassay coefficients of variation 3.0 and 4.2%, respectively).21 Total 25(OH)D was defined as the sum of 25(OH)D2 and 25(OH)D3. This laboratory is accredited by CPA UK (CPA number 0865) and has been certified as proficient by the international Vitamin D Quality Assurance Scheme (DEQAS).
Histopathology
Where available, the slides of the original duodenal biopsies were reviewed by a single veterinary pathologist. A qualitative scoring system (World Small Animal Veterinary Association [WSAVA] Standards for the Diagnosis of Gastrointestinal Inflammation in Endoscopic Biopsy Samples)22 was used to assess the degree of inflammation. The template for this system assesses the following histological changes (villous stunting, epithelial injury, crypt distension, lacteal dilatation, and mucosal fibrosis) and inflammatory infiltrates (intraepithelial lymphocytes, lamina propria lymphocytes and plasma cells, lamina propria eosinophils, and lamina propria neutrophils). The changes for each of the variables listed were graded as normal (0), mild (1), moderate (2), or severe (3). The sums of all these variables were added together to determine an intestine inflammatory score which ranged from 0 (normal) to 30 (very severe).
Outcome
For each dog enrolled, follow‐up data was obtained by reviewing clinical records and by telephone contact with referring veterinary surgeons and owners. Outcome was recorded as survivors if the dogs were alive at follow up or had died because of a non‐CE‐related illnesses or nonsurvivors if dogs had died or were euthanized because of the complications associated with CE.
Statistical Analysis
Univariable measures were compared between dogs which survived and nonsurviving dogs using a Mann–Whitney U‐test. A Fisher's exact test was used to compare the sex of surviving and nonsurviving dogs. A binary logistic regression model was used to estimate the association between outcome (survivors versus non‐survivors) and serum 25(OH)D concentrations conditional on a range of other candidate predictors. Stepwise selection of variables was used to minimize Akaike Information Criteria (AIC), which is a parameter‐penalized measure of model fit. Duodenal histology scores were classified into 3 approximate tertiles (low <7, medium 7–8, high >8) for the regression model. The statistical analysis was performed using R statistical system (R Development Core Team 2012).
Ethical Review
Informed consent for the use of residual clinical blood samples for research purposes was obtained at admission for each dog enrolled. Ethical approval for the study was obtained from the University of Edinburgh's Veterinary Ethical Review Committee.
Results
Signalment
Forty‐one dogs were included in the study. There were 15 nonsurvivors and 26 survivors. In the nonsurvivors group, 2 dogs were intact males, 7 were neutered males, 1 was an intact female, and 5 were neutered females. In the survivors group, 7 dogs were intact males, 11 were neutered males, and 8 were neutered females. Breeds in the nonsurvivors groups included Border Collie (n = 1), Boxer (n = 3), Cavalier King Charles Spaniel (n = 1), Cross Breed (n = 3), German Short Haired Pointer (n = 1), Hungarian Vizsla (n = 1), Italian Greyhound (n = 1), Pyrenees Mountain Dog (n = 1), Springer Spaniel (n = 1), Staffordshire Bull Terrier (n = 1), and West Highland White Terrier (n = 1). In the survivors group, breeds included Border Terrier (n = 1), Boxer (n = 7), Cocker Spaniel (n = 1), Cavalier King Charles Spaniel (n = 1), Chinese Crested (n = 1), Cross Breed (n = 1), Irish Setter (n = 2), Labrador Retriever (n = 2), Lurcher (n = 2), Rottweiler (n = 1), Shar‐pei (n = 1), Shetland Sheep Dog (n = 1), Springer Spaniel (n = 1), Staffordshire Bull Terrier (n = 1), Toy Poodle (n = 2), and Yorkshire Terrier (n = 1).
Clinical Findings
The median duration of clinical signs at diagnosis was 3 months in the nonsurvivors (range 1–10 months) and 3.5 months (range 0.75–24 months) in the survivors group. Hematology, biochemistry, and abdominal ultrasonography findings did not reveal any clinically relevant abnormalities in any of the 41 dogs which could not be attributed to their CE. Fecal parasitology was performed in 36 dogs and did not reveal evidence of parasitic infection in any of the samples. Twelve dogs underwent gastroduodenoscopy and 29 dogs had both gastroduodenoscopy and colonoscopy.
Histopathological examination of duodenal biopsies in the nonsurvivors revealed lymphoplasmacytic enteritis (5) and mixed lymphoplasmacytic and eosinophilic enteritis (10). In the survivor group histopathological diagnosis based on duodenal biopsies included lymphoplasmacytic enteritis (8) and mixed lymphoplasmacytic and eosinophilic enteritis (18). Follow up for the survivor group ranged from 18 to 75 months (median 27 months). In the nonsurvivors group the follow up ranged from 4 days to 24 months (median 2 months).
Outcome
Fifteen dogs died or were euthanized as a result of CE (Tables 1 and 2). The age of the dogs at presentation which subsequently died or were euthanized because of their CE ranged from 9 to 114 months (median 96 months). Dogs which subsequently died had been treated with dietary changes and antibiotics (n = 2), dietary changes, prednisolone and antibiotics (n = 7), and dietary changes, antibiotics, prednisolone and other immunosuppressive medications (n = 6). Twenty‐six dogs did not die as a result of gastrointestinal disease (Tables 1 and 2). Sixteen were alive at follow up and 10 had died because of nongastrointestinal diseases. The age of the dogs in the survivors group ranged from 6 to 96 months (median 60.5 months). Dogs in this group were treated with dietary changes and antibiotics (n = 10), dietary changes and prednisolone (n = 1), dietary changes, prednisolone and antibiotics (n = 3) and dietary changes, prednisolone, antibiotics and other immunosuppressive drugs (n = 1), diet alone (n = 10), and gastroprotectants (n = 1).
Table 1.
Univariable analysis of clinical and biochemical variables in surviving and nonsurviving dogs. The data show the median value for each variable and the interquartile range
| Survivors (n = 26) | Nonsurvivors (n = 15) | P Value | |
|---|---|---|---|
| Age (months) | 60.5 (36–73) | 96.0 (60–108) | .004 |
| Calcium mg/dL | 9.68 (9.04–10.12) | 8.24 (6.76–9.04) | .002 |
| Calcium mmol/L | 2.42 (2.26–2.53) | 2.06 (1.69–2.26) | |
| Albumin mg/dL | 3.34 (2.73–3.52) | 2.45 (1.3–2.69) | .0007 |
| Albumin g/L | 33.35 (27.3–35.2) | 24.50 (13–26.9) | |
| CIBDAI | 6 (5–9) | 10 (7–12) | .0022 |
| 25(OH)D ng/mL | 24.90 (15.63–39.45) | 4.3 (1.6–17.0) | .0003 |
| Male/female | 18/8 | 9/6 | .73 |
Table 2.
Impact on model akaike information criteria (AIC) after addition of dropped predictors. An increase in AIC represents a poorer parameter‐penalized model fit
| Added Predictor | AIC |
|---|---|
| Final model which includes 25(OH)D and age | 36.97 |
| Albumin | 38.97 |
| Calcium | 38.81 |
| Sex | 38.60 |
| Histology tertile | 40.31 |
Univariable analysis revealed that serum 25(OH)D, albumin and total calcium concentrations were significantly lower in nonsurvivors compared to survivors (Table 1, Fig 1). Age and CIBDAI scores were significantly higher in nonsurvivors compared to survivors (Table 1). There was no significant difference in the number of male and female dogs between the 2 groups (Table 1).
Figure 1.
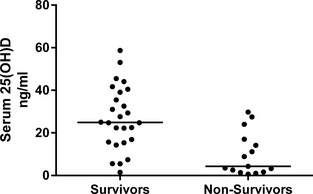
Serum 25 hydroxyvitamin D concentrations in surviving and nonsurviving dogs with a chronic enteropathy.
A binary logistic regression model was performed to estimate the association between outcome and serum 25(OH)D concentrations conditional on a range of other candidate predictors. Canine inflammatory bowel disease activity index was not included in this model as it has previously been shown that this measure strongly correlates with serum 25(OH)D concentrations.5 In this analysis duodenal histology severity scores were also included which were available for 34 of the 41 dogs. There was no significant difference in the histopathology scores between dogs which died compared to the ones which survived (P = .06). There were 10 dogs with a score of <7, 10 dogs with a score of 7 or 8, and 14 dogs with a score of 9 or greater. The initial model included age, sex, histopathology score as a tertile, serum calcium, albumin, and 25(OH)D concentrations. After stepwise AIC selection, the optimal predictive final model used serum 25(OH)D concentrations and age demonstrating that vitamin D status was an independent predictor of mortality in dogs with CE. The odds ratio for death was 1.08 (95% confidence interval 1.02–1.18) for vitamin D status and for age was 0.97 (95% confidence 0.93–1.00). Table 2 shows the consequences on model fit following the reintroduction of discarded predictors, demonstrating that the model based on 25(OH)D concentrations and age was optimal.
Discussion
The main finding of this study is that serum 25(OH)D concentrations are significantly lower at the time of diagnosis in dogs which died or were euthanized as a result of a CE. This is an important finding as it is presently difficult to predict outcomes in dogs with a CE. Older age, hypoalbuminemia, and higher CIBDAI scores are predictive of a poorer outcome in dogs with a CE, findings which are further supported by our research.1, 23, 24 This study, which demonstrates that 25(OH)D concentration at point of diagnosis is a prognostic marker in dogs with a CE, will provide additional prognostic information to owners and veterinarians managing dogs with a CE. Further work is needed to determine the optimal cut‐off value for vitamin D as a prognostic marker.
The mechanism(s) underlying a hypovitaminosis D state in canine CE is unknown. Reduced dietary intake of vitamin D in dogs with a CE maybe an important cause of hypovitaminosis D especially as dogs do not cutaneously produce vitamin D.25 As low vitamin D status in dogs with a CE has been associated with a decreased appetite5, this may, in part, explain the reduced serum 25(OH)D concentrations that are frequently observed in dogs with a CE. However, dogs with a CE were found to have a lower 25(OH)D concentrations than hospitalized ill dogs with nongastrointestinal illnesses, many of which also have reduced appetites.5 Consequently, the influence of appetite on the vitamin D status in dogs with a CE remains unclear.
Circulating 25(OH)D is bound to vitamin D binding protein and albumin.26 Enteric loss of albumin is regarded to be a significant problem in many dogs with a CE, notably in cases of protein losing enteropathies.27 Consequently, loss of protein‐bound vitamin D into the gastrointestinal tract could account for the low vitamin D status in some dogs with a CE. This may explain our earlier finding of a correlation between serum albumin concentration and vitamin D status in dogs with a CE.5 Similarly, albumin concentrations has also been shown to be a predictor of serum 25(OH)D concentration in humans with IBD.28 Malabsorption might also contribute to low vitamin D status in human patients with Crohn's disease and this could potentially influence serum 25(OH)D concentrations in dogs with CE. However, while vitamin D absorption appears to be reduced in patients with Crohn's disease compared to healthy controls, there seems to be substantial variation in absorption of vitamin D in these patients.29 Further studies are needed in both people and dogs with CE to clarify the potential role of vitamin D malabsorption in driving a hypovitaminosis D state.
Although hypovitaminosis D in CE has traditionally been considered to be a result of intestinal disease, there is growing evidence that hypovitaminosis D may contribute to the initiation of intestinal inflammation. Supporting evidence for a link between hypovitaminosis D and CE comes from rodent models which have demonstrated that vitamin D receptor knock out (VDR−/−) mice are more susceptible to experimental forms of inflammatory bowel disease. For example, experimentally induced colitis in VDR−/− mice was significantly more severe compared to wild‐type mice.30, 31 Furthermore it has also been demonstrated that supplementing wild‐type mice with vitamin D can decrease the severity of gastrointestinal inflammation with chemical induced colitis.30 In addition, it has been shown that feeding mice a vitamin D‐restricted diet can predispose to IBD.32 A recent meta‐analysis of animal and human trials concluded that while many studies reported an improvement with supplementing vitamin D, there is insufficient evidence to currently recommend vitamin D treatment for human cases of IBD.33 Therefore, further research is needed into the potential role of vitamin D in the management of these diseases.
The vitamin D receptor is expressed on many immune cell types and it is clear that vitamin D can modulate both the innate and acquired immune responses via effects on monocytes, macrophages, dendritic cells, and lymphocytes.34, 35 Vitamin D has also been shown to profoundly modulate proinflammatory responses.36, 37 Therefore, lack of vitamin D might drive abnormal inflammatory and immune processes.
Loss of tolerance to normally harmless bacterial and dietary antigens is hypothesized to be important for the development of canine CE.3 Vitamin D may also be important in regulating the immune response to commensal gut flora and maintaining normal bacterial populations. For example, dysbiosis was also reported in VDR−/− mice and Cyp27B1−/− mice38 and dysbiosis may contribute to CE in dogs.39
There is growing evidence linking vitamin D deficiency with disrupted intestinal mucosal barrier function. It has been proposed that altered epithelial barrier function, resulting in increased epithelial permeability, leads to increased exposure of the mucosal immune system to luminal antigens and that this may contribute to the initiation and perpetuation of chronic inflammation.40 This hypothesis is supported by the observation that intestinal permeability is increased in people with naturally occurring inflammatory bowel disease and their unaffected relatives.41 Similar findings have been reported in dogs with naturally occurring CE where increased paracellular permeability has been demonstrated by lactulose to rhamnose absorption tests.42, 43 In vitro active vitamin D metabolite, 1,25‐dihydroxyvitamin D3 [1,25(OH)2D3], markedly enhances tight junctions by increasing tight junction protein expression.44 The same study also demonstrated that VDR−/− mice were more susceptible to mucosal injury than wild‐type mice. These results suggest that vitamin D might be important in mucosal integrity and gastrointestinal barrier function which could contribute to CE.
Limitations of this study include the lack of standardization in treatment regime, which is a result of the retrospective nature of the study design.
In summary, this study demonstrates the serum vitamin D concentrations are predictive of clinical outcome in dogs with CE. Although, causality cannot be inferred from these results, the finding that low serum 25(OH)D concentrations are negatively correlated with outcome highlights the need to further examine the relationship between vitamin D homeostasis and disease development and outcome in dogs with CE.
Acknowledgments
The authors acknowledge all the clinicians, nurses, and owners of the dogs who contributed toward the study. RJM is supported by a Wellcome Trust Intermediate Clinical Fellowship.
Funding: The study was not supported by a grant.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Siemens Medical Solutions Diagnostics Ltd, Los Angeles, CA
Diamond Diagnostics, Los Angeles, CA
Waters Associates, Milford, MA
Hichrom, Reading, UK
References
- 1. Craven M, Simpson J, Ridyard A, et al. Canine inflammatory bowel disease: Retrospective analysis of diagnosis and outcome in 80 cases (1995–2002). J Small Anim Pract 2004;45:336–342. [DOI] [PubMed] [Google Scholar]
- 2. Hall EJ, German AJ. Disease of the Small Intestine Textbook of Veterinary Internal Medicine. London: Ettinger and Feldman Saunders Co; 2010;II:1526–1572. [Google Scholar]
- 3. German AJ, Hall EJ, Day MJ. Chronic intestinal inflammation and intestinal disease in dogs. J Vet Intern Med 2003;17:8–20. [DOI] [PubMed] [Google Scholar]
- 4. Mellanby R, Mellor P, Roulois A, et al. Hypocalcaemia associated with low serum vitamin D metabolite concentrations in two dogs with protein‐losing enteropathies. J Small Anim Pract 2005;46:345–351. [DOI] [PubMed] [Google Scholar]
- 5. Gow AG, Else R, Evans H, et al. Hypovitaminosis D in dogs with inflammatory bowel disease and hypoalbuminaemia. J Small Anim Pract 2011;52:411–418. [DOI] [PubMed] [Google Scholar]
- 6. Siffledeen JS, Siminoski K, Steinhart H, et al. The frequency of vitamin D deficiency in adults with Crohn's disease. Can J Gastroenterol 2003;17:473–478. [DOI] [PubMed] [Google Scholar]
- 7. Dumitrescu G, Mihai C, Dranga M, et al. Serum 25‐hydroxyvitamin D concentration and inflammatory bowel disease characteristics in Romania. World J Gastroenterol 2014;20:2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suibhne TN, Cox G, Healy M, et al. Vitamin D deficiency in Crohn's disease: Prevalence, risk factors and supplement use in an outpatient setting. J Crohns Colitis 2012;6:182–188. [DOI] [PubMed] [Google Scholar]
- 9. Kuwabara A, Tanaka K, Tsugawa N, et al. High prevalence of vitamin K and D deficiency and decreased BMD in inflammatory bowel disease. Osteoporos Int 2009;20:935–942. [DOI] [PubMed] [Google Scholar]
- 10. Ananthakrishnan AN, Khalili H, Higuchi LM, et al. Higher predicted vitamin D status is associated with reduced risk of Crohn's disease. Gastroenterology 2012;142:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blanck S, Aberra F. Vitamin D deficiency is associated with ulcerative colitis disease activity. Dig Dis Sci 2013;58:1698–1702. [DOI] [PubMed] [Google Scholar]
- 12. Ulitsky A, Ananthakrishnan AN, Naik A, et al. Vitamin D deficiency in patients with inflammatory bowel disease: Association with disease activity and quality of life. J Parenter Enteral Nutr 2011;35:308–316. [DOI] [PubMed] [Google Scholar]
- 13. Hlavaty T, Krajcovicova A, Koller T, et al. Higher vitamin D serum concentration increases health related quality of life in patients with inflammatory bowel diseases. World J Gastroenterol 2014;20:15787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ananthakrishnan AN, Cagan A, Gainer VS, et al. Normalization of plasma 25‐hydroxy vitamin D is associated with reduced risk of surgery in Crohn's disease. Inflamm Bowel Dis 2013;19:1921–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zator ZA, Cantu SM, Konijeti GG, et al. Pretreatment 25‐hydroxyvitamin D levels and durability of anti‐tumor necrosis factor–α therapy in inflammatory bowel diseases. J Parenter Enteral Nutr 2014;38:385–391. [DOI] [PubMed] [Google Scholar]
- 16. Yang L, Weaver V, Smith JP, et al. Therapeutic effect of vitamin D supplementation in a pilot study of Crohn's patients. Clin Transl Gastroenterol 2013;4:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miheller P, Műzes G, Hritz I, et al. Comparison of the effects of 1, 25 dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn's disease patients. Inflamm Bowel Dis 2009;15:1656–1662. [DOI] [PubMed] [Google Scholar]
- 18. Agborsangaya C, Toriola AT, Grankvist K, et al. The effects of storage time and sampling season on the stability of serum 25‐hydroxy vitamin D and androstenedione. Nutr Cancer 2010;62:51–57. [DOI] [PubMed] [Google Scholar]
- 19. Mawer EB, Hann J, Berry JL, et al. Vitamin D metabolism in patients intoxicated with ergocalciferol. Clin Sci 1985;68:135–141. [DOI] [PubMed] [Google Scholar]
- 20. Mawer EB, Berry JL, Cundall JP, et al. A sensitive radioimmunoassay using a monoclonal antibody that is equipotent for ercalcitriol and calcitriol (1, 25‐dihydroxy vitamin D 2 and D 3). Clin Chim Acta 1990;190:199–209. [DOI] [PubMed] [Google Scholar]
- 21. Berry JL, Martin J, Mawer EB. 25‐Hydroxyvitamin D assay kits: Speed at cost of accuracy? In: Norman AW, Bouillion R, Thomasset M, eds. Vitamin D Endocrine System: Structural, Biological, Genetic and Clinical Aspects. Riverside, CA: Univer‐ sity of California; 2000:797‐800. [Google Scholar]
- 22. Day M, Bilzer T, Mansell J, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: A report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol 2008;138:S1–S43. [DOI] [PubMed] [Google Scholar]
- 23. R Core Team , R: A language and environment for statistical computing version 3.0.2. http://www.r-project.org/. 2012.
- 24. Allenspach K, Wieland B, Gröne A, et al. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J Vet Intern Med 2007;21:700–708. [DOI] [PubMed] [Google Scholar]
- 25. Okanishi H, Sano T, Yamaya Y, et al. The characteristics of short‐ and long‐term surviving Shiba dogs with chronic enteropathies and the risk factors for poor outcome. Acta Vet Scand 2013;55:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. How K, Hazewinkel H, Mol J. Dietary vitamin D dependence of cat and dog due to inadequate cutaneous synthesis of vitamin D. Gen Comp Endocrinol 1994;96:12–18. [DOI] [PubMed] [Google Scholar]
- 27. Chun RF, Peercy BE, Orwoll ES, et al. Vitamin D and DBP: The free hormone hypothesis revisited. J Steroid Biochem Mol Biol 2014;144 Part A:132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dossin O, Lavoue R. Protein‐losing enteropathies in dogs. Vet Clin North Am Small Anim Pract 2011;41:399–418. [DOI] [PubMed] [Google Scholar]
- 29. Pappa HM, Gordon CM, Saslowsky TM, et al. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics 2006;118:1950–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Farraye F, Nimitphong H, Stucchi A, et al. Use of a novel vitamin D bioavailability test demonstrates that vitamin D absorption is decreased in patients with quiescent Crohn's disease. Inflamm Bowel Dis 2011;17:2116–2121. [DOI] [PubMed] [Google Scholar]
- 31. Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol 2007;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Froicu M, Weaver V, Wynn TA, et al. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol 2003;17:2386–2392. [DOI] [PubMed] [Google Scholar]
- 33. Lagishetty V, Misharin AV, Liu NQ, et al. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology 2010;151:2423–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nicholson I, Dalzell AM, El‐Matary W. Vitamin D as a therapy for colitis: A systematic review. J Crohns Colitis 2012;6:405–411. [DOI] [PubMed] [Google Scholar]
- 35. Prietl B, Treiber G, Pieber TR, et al. Vitamin D and immune function. Nutrients 2013;5:2502–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwalfenberg GK. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol Nutr Food Res 2011;55:96–108. [DOI] [PubMed] [Google Scholar]
- 37. Wobke TK, Sorg BL, Steinhilber D. Vitamin D in inflammatory diseases. Front Physiol 2014;5:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yin K, Agrawal DK. Vitamin D and inflammatory diseases. J Inflamm Res 2014;7:69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ooi JH, Li Y, Rogers CJ, et al. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate‐induced colitis. J Nutr 2013;143:1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suchodolski JS, Dowd SE, Wilke V, et al. 16S rRNA gene pyrosequencing reveals bacterial dysbiosis in the duodenum of dogs with idiopathic inflammatory bowel disease. PLoS One 2012;7:e39333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chichlowski M, Hale LP. Bacterial‐mucosal interactions in inflammatory bowel disease—An alliance gone bad. Am J Physiol Gastrointest Liver Physiol 2008;295:G1139–G1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hollander D, Vadheim CM, Brettholz E, et al. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med 1986;105:883–885. [DOI] [PubMed] [Google Scholar]
- 43. Allenspach K, Steiner JM, Shah BN, et al. Evaluation of gastrointestinal permeability and mucosal absorptive capacity in dogs with chronic enteropathy. Am J Vet Res 2006;67:479–483. [DOI] [PubMed] [Google Scholar]
- 44. Quigg J, Brydon G, Ferguson A, et al. Evaluation of canine small intestinal permeability using the lactulose/rhamnose urinary excretion test. Res Vet Sci 1993;55:326–332. [DOI] [PubMed] [Google Scholar]
- 45. Kong J, Zhang Z, Musch MW, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 2008;294:G208–G216. [DOI] [PubMed] [Google Scholar]


