Abstract
Background
Canine epileptoid cramping syndrome (CECS) is a paroxysmal movement disorder of Border Terriers (BTs). These dogs might respond to a gluten‐free diet.
Objectives
The objective of this study was to examine the clinical and serological effect of a gluten‐free diet in BTs with CECS.
Animals
Six client‐owned BTs with clinically confirmed CECS.
Methods
Dogs were prospectively recruited that had at least a 6‐month history of CECS based on the observed phenomenology (using video) and had exhibited at least 2 separate episodes on different days. Dogs were tested for anti‐transglutaminase 2 (TG2 IgA) and anti‐gliadin (AGA IgG) antibodies in the serum at presentation, and 3, 6, and 9 months after the introduction of a gluten‐free diet. Duodenal biopsies were performed in 1 dog.
Results
Serum TG2 IgA titers were increased in 6/6 BTs (P = .006) and AGA IgG titers were increased in 5/6 BTs at presentation compared to those of controls (P = .018). After 9 months, there was clinical and serological improvement in all BTs with CECS strictly adhering to a gluten‐free diet (5/5). One dog had persistently increased antibody titers. This dog scavenged horse manure. On the strict introduction of a gluten‐free diet this dog also had an improved clinical and serological response. The diet‐associated improvement was reversible in 2 dogs on completion of the study, both of which suffered a relapse of CECS on the re‐introduction of gluten.
Conclusions
Canine epileptoid cramping syndrome in BTs is a gluten‐sensitive movement disorder triggered and perpetuated by gluten and thus responsive to a gluten‐free diet.
Keywords: Dyskinesia, Gluten hypersensitivity, Movement disorder, Neurology, Paroxysmal nonkinesigenic dyskinesia
Abbreviations
- AGA IgG
anticanine gliadin‐IgG
- BT
Border Terrier
- CD
celiac disease
- CECS
canine epileptoid cramping syndrome
- NCGS
nonceliac gluten sensitivity
- PNKD
paroxysmal nonkinesigenic dyskinesia
- TG
transglutaminase
- TG2 IgA
anticanine transglutaminase‐2‐IgA
Canine epileptoid cramping syndrome (CECS) is a paroxysmal movement disorder of Border Terriers (BTs) that is analogous to paroxysmal nonkinesigenic dyskinesias (PNKD) in humans.1, 2 Canine epileptoid cramping syndrome is characterized by attacks of involuntary movements (dystonia, chorea, ballism, tremor, and athetosis) occurring at rest with no loss of consciousness.2 Episodes occur in dogs as young as 6 weeks, but can develop up to 7 years of age.2 Border Terriers experience paroxysms, often in response to stress or excitement, affecting the extremities, head and neck, often associated with borborygmi.2 Although mild episodes consist of tightening of muscles in the extremities, followed by choreoathetosis and involuntary postures (dystonia), involuntary movements might be severe and prevent functional use of the limbs.2 Episodes last from minutes to hours and might occur as frequently as several times each day, or be interspersed by months or years.2 The cause of CECS is unknown, and treatment is often unsatisfactory with no medication being reported to have a beneficial effect.2 Neurological examination between episodes is normal and cross‐sectional imaging of the brain and spinal fluid analysis did not detect abnormalities. A clinical diagnosis relies on the exclusion of other causes for paroxysmal episodes by recognition of the features of CECS during an episode.2 Video footage is frequently invaluable in the assessment of CECS. Anecdotal reports among owners and breeders suggest that the replacement of a gluten‐free diet might reduce the frequency and severity of CECS episodes. This view was further supported by the finding that over 50% of BTs with CECS responded to a gluten‐free or hypoallergenic diet according to their owners.2
Celiac disease (CD) is an immune‐mediated enteropathy triggered in genetically susceptible individuals by the ingestion of gluten.3 However, this term should be reserved for those patients with gluten‐sensitive enteropathy; characterized by an abnormal duodenal biopsy of an increased density of intraepithelial lymphocytes to villous atrophy, in the presence of transglutaminase‐2 antibodies (autoantibodies against an enzyme that breaks down gliadin) in serum.3 The more recently coined term, nonceliac gluten sensitivity (NCGS), comprises a spectrum of conditions in humans, all of which are characterized by an immune response to the ingestion of gluten, but with diverse manifestations4 such as an enteropathy5 (distinct to CD), dermatopathy (dermatitis herpetiformis)6; and neurological disorders such as gluten ataxia.7 In patients with neurological manifestations of NCGS, antibodies against gliadin (the protein responsible for gluten sensitivity) are the best diagnostic markers.8, 9
Our first objective was to establish any therapeutic effects of a gluten‐free diet in BTs with CECS. Our second objective was to provide serological evidence that an association exists between CECS and gluten sensitivity.
Materials and Methods
Animals
Serum samples were collected from 6 BTs with a clinical diagnosis of CECS presented to Davies Veterinary Specialists between March 2012 and July 2014. Owners were asked to sign an informed consent document before inclusion of their dog in the study.
Criteria for Case Selection
A video was obtained of a typical episode from each dog and was evaluated by 2 of the authors (ML, LSG). Based on the observed phenomenology in conjunction with the medical history, a diagnosis of CECS was made. This is in accordance with the diagnosis of CECS in a previous study.2
Information obtained and recorded from the medical records of CECS dogs consisted of signalment (age and sex); history (first onset, progression, frequency and duration of episodes); signs suggestive of gastrointestinal disease (specifically vomiting, diarrhea, persistent borborygmi); and concurrent medical conditions. Dogs with a history of receiving a gluten‐free diet before accession were excluded from the study. All dogs had to have at least a 6‐month history of CECS and have exhibited at least 2 separate episodes on different days.
Each dog was subjected to a complete general physical and neurological examination by a veterinary neurologist (ML, LSG). A minimum database for each dog consisted of the following: complete blood cell count (CBC), serum biochemistry profile, routine urinalysis, and dynamic bile acid concentrations. In selected dogs, additional testing was performed including magnetic resonance imaging of the brain (using a 0.4T magnet1), cerebrospinal fluid analysis (collected from the cerebellomedullary cistern) by cytological review and total protein assay, and gastroduodenoscopy with the collection of endoscopic biopsy samples from the stomach and descending duodenum attained with flexible endoscopic biopsy forceps.
Dietary Trial
All dogs were then fed an exclusively gluten‐free diet.2 Participants were instructed to switch their dog to the new diet progressively, over 7 days, by gradually increasing the proportion of new diet fed. Instructions also were given about ensuring that the chosen diet was fed exclusively by avoiding consumption of treats or table scraps, preventing outdoor scavenging and avoiding access to food of other dogs in the household by feeding individual dogs separately and lifting up food bowls when not in use. No other medication was given throughout the trial. During this time, owners were asked to keep a diary and record any episodes.
Follow‐Up
A re‐examination was scheduled 3, 6, and 9 months after starting the gluten‐free diet, in which a full general physical and neurological examination was performed alongside a CBC and serum biochemistry profile.
Sample Collection
A serum sample was collected at presentation and at 3, 6, and 9 months in all dogs. Serum samples were also collected from BTs with no history of CECS having medical investigations for conditions unrelated to neurological or gastrointestinal disease to serve as controls. All serum samples were stored at −80°C until assayed.
Serology
All serum samples were tested for serologic evidence of anticanine transglutaminase‐2‐IgA (TG2 IgA) autoantibodies and anticanine gliadin‐IgG (AGA IgG) antibodies utilizing a commercial laboratory,3 These markers were selected owing to their utility as diagnostic markers of gluten sensitivity in humans.3, 8, 9
For detection of antibodies against canine tissue, transglutaminase‐2, the antigen was coated onto a microtiter plate. The plate surface was blocked with bovine serum albumin prior to use with canine sera and control samples. Bound antibodies against canine TG2 were detected by incubation with peroxidase‐conjugated secondary antibody against canine IgA. In the last step, the peroxidase converts a substrate (tetramethylbenzidine) into a blue product, which upon addition of the stop solution (0.5 M H2SO4) turns yellow. Negative control values were recorded using a buffer and conjugate.
Regarding IgG antigliadin antibodies, the gliadin‐coated microtiter plate from the ELISA for the determination of antibodies (IgG) against gliadin from Steffens Biotechnische Analysen was used.4 Detection of bound antibodies against gliadin was obtained by incubation with peroxidase‐conjugated secondary antibody against canine IgG1. Negative control values were recorded using a buffer and conjugate.
Statistical Analysis
Analyses of correlations between the CECS dogs and the control group were performed using the Mann–Whitney test. A P‐value of <.05 was considered to be significant. All statistical tests were performed using a commercial software package.5
Results
Animals
A total of 6 BTs were included in this study. The mean age of the BTs at episode onset was 2.6 years (median 3 years; range, 1–4 years). Three BTs were female (50%; 2/3 neutered) and 3 were male (50%; 3/3 neutered).
Dyskinesia Episodes
Border Terriers had shown the clinical signs for a mean of 1.4 years at presentation (median 1.5 years; range, 6 months–2 years). Mean episode frequency was 1 per month (median 1 episode every 2 months; range, 1 episode every 3 months up to 1 episode per week). The mean duration of an episode was 15 minutes (median 5 minutes; range, 2–60 minutes).
Concurrent Clinical Signs
In 2/6 BTs, owners reported signs suggestive of gastrointestinal disease. In 1 dog this included borborygmi and occasional vomiting. In the other dog, this was reported as frequent soft stools.
Investigations
Results of general physical examination, neurological examination, CBC, serum biochemistry profile, and dynamic bile acid concentrations did not detect any abnormalities (n = 6). In 4 dogs, an MRI scan was performed with spinal fluid analysis, yielding normal results. In 1 dog (dog 1) with signs suggestive of gastrointestinal disease (borborygmi and occasional vomiting) gastroduodenoscopy with collection of endoscopic biopsies of the stomach and duodenum did not reveal abnormalities.
Response
Three BTs (dogs 1, 4, and 5) had no further episodes of CECS after commencing the dietary trial. Two BTs (dogs 2 and 3) had 1 and 2 episodes, respectively, within 4 weeks of starting the trial, but have subsequently experienced no further episodes. The final dog (dog 6) continued to have episodes throughout the study period.
Antibody Titers
Serum samples were available from all 6 dogs at presentation and in 5 control BTs. At presentation, all 6 BTs had increased values of antitransglutaminase‐2 antibodies (TG2 IgA; median 0.808; range 0.714–1.154) compared to the control group (median 0.216; range 0.129–0.285; P = .006). Buffer and conjugate negative control values were 0.219 and 0.213 respectively. In 5 dogs, serological evidence of elimination of TG2 IgA was observed, correlating with clinical remission of CECS episodes in these dogs (Fig 1). All 6 owners claimed to have adhered to the gluten‐free diet for their dogs, but one of these BTs (dog 6) had persistently high TG2 IgA and continued to experience episodes of CECS. On further questioning of the owner, it became apparent that this dog had been scavenging horse manure during the study. Therefore, the owner was asked to eliminate this from the dog's oral intake and 3 months after commencing the proper gluten‐free diet, a serological response was observed and no further CECS episodes were reported. Subsequently this dog was removed from the study because of protocol violation. Furthermore, at the end of the trial, 2 owners unwittingly re‐introduced gluten‐containing “treats” to their dog's diet. On doing so, both dogs relapsed with an episode of CECS. They duly returned to using the gluten‐free diet and have subsequently been faring well, although autoantibodies were not measured at the time of relapse.
Figure 1.
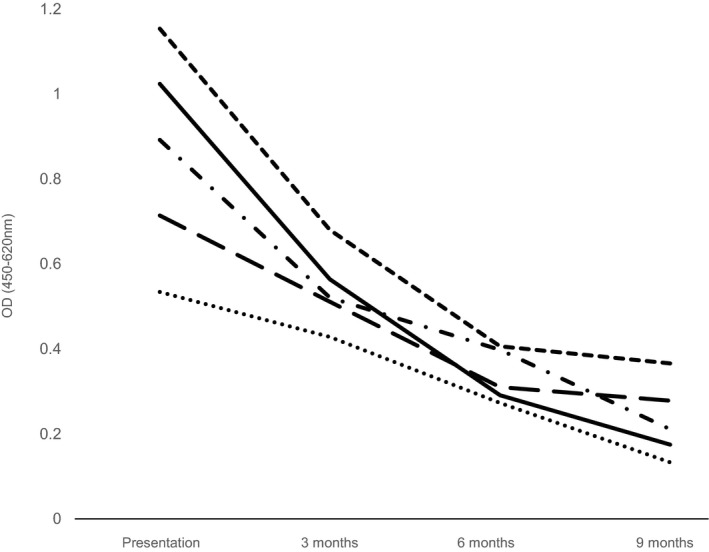
Chart demonstrating the serological response of antitransglutaminase‐2 IgA to a gluten‐free diet in 5 dogs with canine epileptoid cramping syndrome (dog 6 excluded due to failure to adhere to methods). The time periods of monitoring were initial presentation, 3, 6, and 9 months after commencement of a gluten‐free diet. Each dog is indicated with a different line.
Anti‐gliadin antibodies (AGA IgG) were also significantly increased (median 0.398; range 0.175–0.641) compared to control dogs at presentation (median 0.121; range 0.092–0.162; P = .018), but the scope of increase was not such as to be of clinical importance. Buffer and conjugate negative control values were 0.096 and 0.105, respectively. However, titers decreased on institution of a gluten‐free diet in all 5 dogs that clinically responded and ultimately in the sixth dog once the owner was compliant with the gluten‐free diet (Fig 2).
Figure 2.
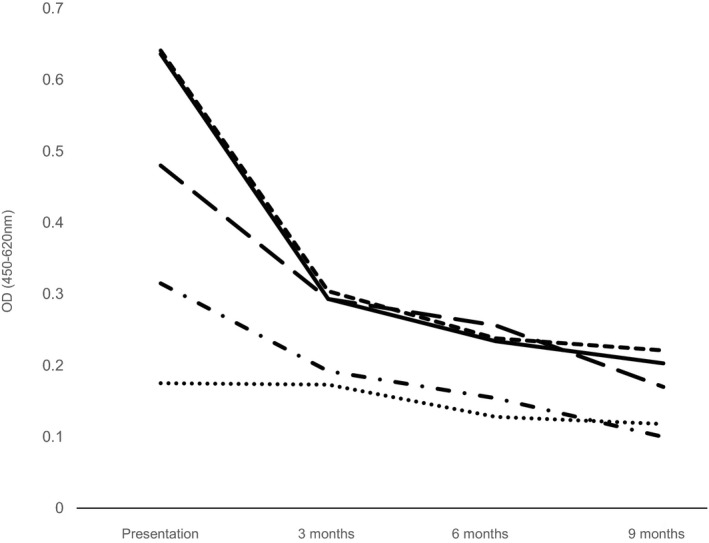
Chart demonstrating the serological response of anti‐gliadin IgG to a gluten‐free diet in 5 dogs with canine epileptoid cramping syndrome (dog 6 excluded because of failure to adhere to methods). The time periods of monitoring were initial presentation, 3, 6, and 9 months after commencement of a gluten‐free diet. Each dog is indicated with a different line.
Discussion
These investigations support the hypothesis that CECS is a manifestation of gluten sensitivity, making this the first paroxysmal movement disorder in veterinary medicine with a serological link to gluten.
Gluten comprises both gliadin and glutenin proteins with evidence suggesting that the gliadin fraction induces disease.10 Gliadin is a unique protein in that it contains a large number of glutamine residues. Tissue transglutaminase is an enzyme that, among its other functions, catalyzes the deamidation of glutamine residues to glutamic acid.11 This process might result in the creation of new epitopes that could play a pivotal role in the immunopathogenesis of gluten sensitivity.12 At present, a causative link between transglutaminase activity and gluten sensitivity has not been definitely established though evidence supporting this hypothesis is strong.13
In recent years, the view of CD has undergone a profound revision. The first indication that gluten can cause extra‐intestinal signs came from a study that described a gluten‐dependent skin rash (known as dermatitis herpetiformis) that could occur even without histological evidence of intestinal involvement.6 Neurological signs occur in patients with histologically confirmed CD.14 Recognition of neurological manifestations of NCGS have since increased, the most common being gluten ataxia7 and peripheral neuropathy.15 However, signs of chorea,16 stiff person syndrome,17 seizures,18 and a recently described clinical entity of myoclonic ataxia19 are less common. Tentative links of a PNKD in a 10‐year‐old girl responding to a gluten‐free diet were not supported by serological assays.20
Initially, the assumption had been that neurological signs associated with NCGS were secondary to a malabsorption with subsequent vitamin deficiencies in view of the concurrent enteropathy in these patients.14 However, neurological signs emerged even in the absence of enteropathy, suggesting an alternative etiology.21 Current evidence suggests that neurologic manifestations are immune‐mediated. This is based on histopathological findings from patients with gluten ataxia that demonstrate macroscopic cerebellar atrophy visible on MRI with diffuse infiltration of T lymphocytes within the cerebellar white matter and perivascular cuffing with inflammatory cells.22 Although the presence of the anti‐gliadin antibody is historically considered to be an important hallmark of CD, lower figures for its sensitivity and specificity in comparison to TG2‐IgA have led to a diminished utility of the marker for diagnosis.3 However, the emergence of NCGS identified that AGA IgG are more robust markers than TG2‐IgA for the extra‐intestinal manifestations of gluten sensitivity.21 Given that only 40% of the patients with neurological manifestations due to gluten sensitivity responsive to a gluten‐free diet will have an enteropathy21, it has become standard practice to measure both markers in people and it seems appropriate to adhere to a similar method in dogs.
The association of gluten with CECS might give an explanation as to why a proportion of dogs with CECS exhibit signs of gastrointestinal disease.2 In gluten ataxia <10% of patients will have signs of gastrointestinal disease, but up to one‐third will have evidence of enteropathy on biopsy.23 One dog in the present study with borborygmi and occasional vomiting had no histological evidence of enteritis. The owner of the second dog with signs suggestive of gastrointestinal disease did not consent to endoscopic examination. Further studies are required to assess the prevalence and nature of chronic enteropathy in BTs with CECS to determine if these dogs have a neurological manifestation of CD or a NCGS.
The addition of recombinant canine TG2 antigen to the samples before testing them in the ELISA is required to exclude non‐specific effects. However, the measurement of canine anti‐TG2 antibodies has previously been performed in the cerebrospinal fluid of dogs presenting with inflammatory meningoencephalitis in which verification of the ELISA was performed successfully.24 Measurement in the serum has not previously been undertaken.
The evidence for the existence of gluten‐responsive CECS presented in this study is the presence of anti‐gliadin and TG2 antibodies in multiple serum samples and the clinical and serological response to a gluten‐free diet. Furthermore, the re‐introduction of a gluten diet in 2 dogs resulted in a relapse of CECS. However, a larger number of dogs will be required to ascertain the clinical importance and utility of these observations.
Our study design might be associated with bias—for example, owners might be more likely to default from the diet if they see no immediate improvement in signs. However, we emphasized to the owners that improvement on the diet, if at all, was likely to be slow and to take several months. An additional source of bias was that 1 investigator undertook all clinical assessments and was not blinded to the treatment state of the dogs. Furthermore, owners might have been able to reduce exposure to inciting causes such as excitement following counseling by a veterinary neurologist which could, in part, explain the reduction in the frequency of episodes following the instigation of the diet. A double‐blinded randomized placebo‐controlled trial is required to overcome some of these limitations. Furthermore, future studies will verify the clinical utility of these results by the addition of canine TG2 antigen to the samples before testing them in the ELISA, in order to exclude nonspecific effects.
Conclusions
Serological testing for anti‐transglutaminase‐2 and anti‐gliadin antibodies might represent an useful diagnostic tool for CECS and for monitoring dietary compliance, especially in dogs who are nonadherent to a gluten‐free diet. Determination of gluten sensitivity and switching to a gluten‐free diet should be considered in BTs exhibiting dyskinesia.
Acknowledgments
Conflict of Interest Declaration: None of the other authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of this paper.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Aperto permanent magnet, Hitachi, Tokyo, Japan
Hypoallergenic Canine Dry, Royal Canin, Camargue, France
ZEDIRA GmbH, Darmstadt, Germany
Steffens Biotechnische Analysen GmbH, Ebringen, Germany
XLStats; Excel, Windows
References
- 1. Jankovic J, Demirkiran M. Classification of paroxysmal dyskinesias and ataxias. Adv Neurol 2002;89:387–400. [PubMed] [Google Scholar]
- 2. Black V, Garosi L, Lowrie M, et al. Phenotypic characterisation of canine epileptoid cramping syndrome in the Border terrier. J Small Anim Pract 2013;55:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Catassi C, Fasano A. Celiac disease. Curr Opin Gastroenterol 2008;24:687–691. [DOI] [PubMed] [Google Scholar]
- 4. Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten‐related disorders: Consensus on new nomenclature and classification. BMC Med 2012;10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sapone A, Lammers KM, Mazzarella G, et al. Differential mucosal IL‐17 expression in two gliadin‐induced disorders: Gluten sensitivity and the autoimmune enteropathy celiac disease. Int Arch Allergy Immunol 2002;152:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marks J, Shuster S, Watson AJ. Small‐bowel changes in dermatitis herpetiformis. Lancet 1966;10:120–1282. [DOI] [PubMed] [Google Scholar]
- 7. Hadjivassiliou M, Grünewald R, Sharrack B, et al. Gluten ataxia in perspective: Epidemiology, genetic susceptibility and clinical characteristics. Brain 2003;126:685–691. [DOI] [PubMed] [Google Scholar]
- 8. Hadjivassiliou M, Davies‐Jones GAB, Sanders DS, et al. Dietary treatment of gluten ataxia. J Neurol Neurosurg Psychiatry 2003;74:1221–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Volta U, Tovoli F, Cicola R, et al. Serological tests in gluten sensitivity (nonceliac gluten intolerance). J Clin Gastroenterol 2012;46:680–685. [DOI] [PubMed] [Google Scholar]
- 10. Van de Kamer JH, Weijers HA, Dicke WK. Coeliac disease. IV. An investigation into the injurious constituents of wheat in connection with their action on patients with coeliac disease. Acta Paediatr 1953;42:223–231. [DOI] [PubMed] [Google Scholar]
- 11. Sjostrom H, Lundin KE, Molberg O, et al. Identification of a gliadin T‐cell epitope in coeliac disease: General importance of gliadin deamidation for intestinal T‐cell recognition. Scand J Immunol 1998;48:111–115. [DOI] [PubMed] [Google Scholar]
- 12. Dietrich W, Essligner B, Trapp D, et al. Cross linking to tissue transglutaminase and collagen favours gliadin toxicity in coeliac disease. Gut 2006;55:478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klöck C, Diraimondo TR, Khosla C. Role of transglutaminase 2 in celiac disease pathogenesis. Semin Immunopathol 2012;34:513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cooke WT, Thomas‐Smith W. Neurological disorders associated with adult coeliac disease. Brain 1966;89:683–722. [DOI] [PubMed] [Google Scholar]
- 15. Luostarinen L, Himanen SL, Luostarinen M, et al. Neuromuscular and sensory disturbances in patients with well treated coeliac disease. J Neurol Neurosurg Psychiatry 2003;74:490–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pereira AC, Edwards MJ, Buttery PC, et al. Choreic syndrome and coeliac disease: A hitherto unrecognized association. Mov Disord 2004;19:478–482. [DOI] [PubMed] [Google Scholar]
- 17. Hadjivassiliou M, Aeschlimann D, Grünewald RA, et al. GAD antibody‐associated neurological illness and its relationship to gluten sensitivity. Acta Neurol Scand 2011;123:175–180. [DOI] [PubMed] [Google Scholar]
- 18. Cronin CC, Jackson LM, Feighery C, et al. Coeliac disease and epilepsy. QJM 1998;91:303–308. [DOI] [PubMed] [Google Scholar]
- 19. Sarrigiannis PG, Hoggard N, Aeschlimann D, et al. Myoclonus ataxia and refractory coeliac disease. Cerebellum Ataxias 2014;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hall DA, Parsons J, Benke T. Paroxysmal nonkinesigenic dystonia and celiac disease. Mov Disord 2007;22:708–710. [DOI] [PubMed] [Google Scholar]
- 21. Hadjivassiliou M, Sanders DS, Grünewald RA, et al. Gluten sensitivity: From gut to brain. Lancet Neurol 2010;9:318–330. [DOI] [PubMed] [Google Scholar]
- 22. Hadjivassiliou M, Grünewald R, Chattopadhyay AK, et al. Clinical, radiological, neurophysiological, and neuropathological characteristics of gluten ataxia. Lancet 1998;352:1582–1585. [DOI] [PubMed] [Google Scholar]
- 23. Hadjivassiliou M, Sanders DS, Woodroofe N, et al. Gluten ataxia. Cerebellum 2008b;7:494–498. [DOI] [PubMed] [Google Scholar]
- 24. Tanaka M, Inoue A, Yamamoto K, et al. Transglutaminase 2: A novel autoantigen in canine idiopathic central nervous system inflammatory diseases. J Vet Med Sci 2012;74:733–737. [DOI] [PubMed] [Google Scholar]


