Abstract
Background
The Precision Xtra® meter is a promising low cost electrochemical point‐of‐care unit for measuring blood glucose concentration ([gluc]) in cattle blood. The meter uses an algorithm that assumes the intra‐erythrocyte [gluc] equals the plasma [gluc] on a molal basis, and that the hematocrit is similar in humans and cattle.
Objectives
The primary objective was to determine the accuracy of the meter for measuring plasma [gluc] in dairy cattle. Secondary objectives were to characterize the influence of hematocrit and sample temperature on the measured value for [gluc].
Animals
A total of 106 periparturient Holstein‐Friesian cattle.
Methods
Blood and plasma samples (1,109) were obtained and Deming regression and Bland–Altman plots were used to determine the accuracy of the meter against the reference method (plasma hexokinase assay). Multivariable regression and linear regression were used to determine the effect of hematocrit and sample temperature on the plasma [gluc] measured by the meter.
Results
Intra‐erythrocyte [gluc] was 18% of plasma [gluc] on a molar basis. Sample temperature had a significant linear effect on plasma [gluc] as measured by the meter for 3/5 plasma samples when measured [gluc] > 160 mg/dL.
Conclusions and clinical importance
The meter utilizes an algorithm that is optimized for human blood and is inaccurate when applied to bovine blood. Until a cattle‐specific algorithm is developed, we recommend using plasma as the analyte instead of blood and calculating plasma [gluc] using the equation: [gluc] = 0.66 × [gluc]p‐meter + 15, where [gluc]p‐meter is the value reported by the meter. If blood is measured, then we recommend using the equation: [gluc] = 0.90 × [gluc]b‐meter + 15.
Keywords: hyperglycemia, hyperketonemia, hypoglycemia, ketosis
Abbreviations
- [gluc]b, molal
molal blood glucose concentration measured by the meter
- [gluc]b‐meter
molar blood glucose concentration measured by the meter
- [gluc]e, molal
molal intra‐erythrocyte glucose concentration measured by the meter
- [gluc]
glucose concentration
- [gluc]p,molal
molal plasma glucose concentration measured by the meter
- [gluc]p‐meter
molar plasma glucose concentration measured by the meter
- Hct
hematocrit
- PP
plasma protein
Introduction
Glucose requirements in dairy cows increase from approximately 1.0 kg/day during late gestation to 2.5 kg/day during early lactation.1, 2 Glucose in the plasma of adult ruminants is primarily derived from gluconeogenesis and in periparturient dairy cattle an inability to provide the required amount of glucose can result in hypoglycemia and hyperketonemia (Type I ketosis).3, 4 An early and accurate diagnosis of hypoglycemia may therefore help in the diagnosis, differentiation, and treatment of Type I and II ketosis in dairy cattle and quantitation of the magnitude of negative energy balance in early lactation.
The Precision Xtra® 1 unit is an electrochemical point‐of‐care meter that uses specialized electrochemical test strips that are screen‐printed to monitor glucose and β‐OH butyrate concentrations in whole blood.5 The first layer of the test strip contains mesh and surfactants that facilitate retention of erythrocytes and passage of plasma by capillary action to an underlying layer that employs an enzymatic reaction, with the reaction products being detected by generation of an electrical current.6, 7, 8, 9 Fundamental assumptions of this methodologic approach are that the measured value for blood [gluc] is minimally affected by changes in hematocrit (Hct) and sample temperature. Changes in Hct have been widely documented to influence the measured value for blood [gluc] when used to analyze human blood.10, 11, 12 It has been known for more than 100 years that intra‐erythrocyte [gluc] is lower than plasma [gluc] in cattle, horses, pigs, and dogs, but not in humans and other primates.13, 14, 15, 16 The low intra‐erythrocyte [gluc] in domestic animals has been attributed to their ability to synthesize ascorbic acid from glucose and lack of expression of the GLUT‐1 receptor on erythrocytes from adult animals,17 which serve as the major transporter for ascorbic acid into erythrocytes.18 A recent study demonstrated that using the Precision Xtra® meter produced lower measured values for blood [gluc] when blood samples were <32°C, presumably because the rate of the enzymatic reaction in the test strip was temperature dependent.19 A potential effect of sample temperature on the measured [gluc] has not been explored in method comparison studies using human blood because blood samples for testing are usually obtained from the finger pad and immediately analyzed and are therefore assumed to be at approximately 37°C.
The low cost of the Precision Xtra® meter and test strips, coupled with its ease of use and rapid test results, potentially provides a clinically useful and practical on farm method to measure [gluc] in lactating dairy cattle. Method comparison studies evaluating the performance of the meter using cattle blood have raised concerns about the unit's accuracy, with the source of errors being poorly understood.20, 21 Voyvoda and Erdogan (2010) reported that [gluc] measured by the meter varied from 19 mg/dL (1.0 mmol/L) above to 9 mg/dL (0.5 mmol/L) below the true value. Wittrock and colleagues (2013) reported a similar range of variability with the meter measuring 20 mg/dL (1.1 mmol/L) above to 21 mg/dL (1.2 mmol/L) below the true value. Neither of these studies investigated the effect of Hct on the test performance nor employed a wide range of values for blood [gluc]. More extensive method comparison studies utilizing sheep and goat blood have identified proportional and constant biases for the meter relative to reference methods.22, 23, 24
The methodology and algorithm used by the Precision Xtra® meter to calculate plasma [gluc] from the measured value in blood are optimized for blood samples from humans. We hypothesized that the algorithm used by the meter to calculate plasma [gluc] was inaccurate when analyzing bovine blood because intra‐erythrocyte [gluc] is less than plasma [gluc] and the median Hct of bovine blood is considerably lower than the median value for human blood (43%).12, 25 The primary objective of the study reported here was to determine the accuracy of the meter for the measurement of blood and plasma [gluc] in dairy cattle. The secondary objective was to confirm findings that intra‐erythrocyte [gluc] is much lower than plasma [gluc] in adult cattle. We planned to use the information obtained during this study to develop an equation that characterized the influence of Hct on the measured value for [gluc].
Materials and Methods
All methods were evaluated and approved by the Purdue Animal Care and Use Committee.
Measurement of Blood Glucose Concentration
Blood samples were obtained from 106 late periparturient Holstein‐Friesian cattle (34 primiparous and 72 multiparous) from the Purdue University Dairy Research and Education Center between May 2012 and March 2013. Cows were fed an acidogenic total mixed ration (dietary cation‐anion difference = −10 mEq/100 g of dry matter = ([Na+] + [K+])—([Cl−] + [S2−]) based on formulations recommended by the National Research Council26 for close up cows. Primiparous and multiparous cattle were fed an acidogenic close up ration starting six and 3 weeks before parturition, respectively. After calving, all cows were switched to a lactating cow total mixed ration. The ration was fed once daily between 08:00 and 09:30 am. Cattle were given ad libitum access to water at all times.
Blood samples were obtained daily at approximately 09:00 am from the coccygeal vein or artery on days −4, −3, −2, −1, 0, 1, 2, 3, 7, 14, 21, and 28 days relative to calving (day 0) using 20G vacutainer needles, vacutainer holders, and 10 mL lithium heparin blood collection tubes. The proposed puncture site at the ventrum of the tail was cleared from debris and feces by swabbing the site with gauze containing 70% isopropyl alcohol. Immediately after blood collection, blood [gluc] was measured in a drop of nonheparinized blood from the tip of the vacutainer needle using an electrochemical point‐of‐care meter.1 Coded glucose test strips that required a calibrator strip packaged in the box of test strips were used for the first 5 months of the study, after which noncoded glucose test strips were used.
At the reaction area on the test strip, 0.6 μL of plasma reacted with glucose oxidase to form gluconolactone. The presence of plasma in the reaction area completed a circuit between three electrodes (working, reference, and auxillary which is a fill trigger). Gluconolactone reacted with potassium ferricyanide to create potassium ferrocyanide, which then reacted with the electrode's metal creating a small electric current that was directly proportional to the concentration of glucose in the sample. After 5 seconds of reaction time, the meter used a proprietary algorithm to calculate [gluc] from the generated current, with the calculated value displayed on a screen.
Measurement of Plasma Glucose Concentration, Hematocrit, and Plasma Protein Concentration
Heparinized blood samples from the 106 cows were transferred to a climate‐controlled laboratory area within 15 minutes of collection. Hematocrit was measured in triplicate using plain capillary tubes after centrifugation for 5 minutes at 14,800 × g. The capillary tubes were then broken to obtain a small volume of plasma, which was measured using the meter in the same manner as that described above for blood; the time interval between measuring blood and plasma concentrations was always <30 minutes. Plasma total protein concentration was then measured in triplicate on the same small volume of plasma using a handheld analog refractometer.2 The remainder of the heparinized blood sample was then centrifuged within 30 minutes of collection for 5 minutes at 1,400 × g. Plasma was harvested and transferred into duplicate polypropylene vials within 1 hour of centrifugation and stored at −20°C.
The effect of sample temperature on the value for plasma glucose concentration measured by the meter ([gluc]p‐meter) was investigated by purposively selecting nine plasma samples with a [gluc]p‐meter ranging from 30 to 409 mg/dL when measured on the day of parturition in the climate‐controlled laboratory at the dairy at unknown sample temperature. The measured [gluc]p‐meter values were ordered from lowest to highest, and a stored sample vial with sufficient plasma was selected from those samples with a [gluc]p‐meter value closest to 30, 60, 100, 130, 160, 210, 260, 310, 360 (unavailable), and 410 mg/dL. The nine stored plasma samples were thawed at room temperature and placed in a water bath at 7, 12, 17, 22, 27, 32, 37, and 42°C for 30 minutes and then immediately analyzed in duplicate using the meter as previously described.
Reference Method for Measuring Plasma Glucose Concentration
A plasma sample from the day of parturition for 102 of the 106 cows was analyzed at the Veterinary Diagnostic Laboratory at the University of Illinois Urbana‐Champaign using an autoanalzer3; insufficient volume was present in 4 of the 106 samples, thereby precluding analysis. Samples from the day of parturition were used because of the large range in the plasma glucose concentration on this day due, in part, to maternal hypercortisolemia. The reference method employed hexokinase to react with glucose in the presence of adenosine triphosphate (ATP) and magnesium ions (Mg2+) to form glucose‐6‐phosphate (G‐6‐P) and adenosine diphosphate (ADP). Glucose‐6‐P was then oxidized by G‐6‐P dehydrogenase to 6‐phosphogluconate while the co‐enzyme NAD+ was reduced to NADH, with the change in NADH concentration being detected spectrophotometrically.
A second stored plasma sample was available from the day of parturition for 89 of the 102 cows that had plasma [gluc] measured using the hexokinase reference method. To remove any effect of sample temperature on the measured [gluc]p‐meter value, the 89 samples were thawed at room temperature, vortexed, placed in a water bath at 37°C for 30 minutes, and measured using the Precision Xtra® meter.
Calculations
Direct reading point‐of‐care glucose meters such as the Precision Xtra® meter sense the content of reactive glucose in whole blood and therefore measure glucose content in terms of molality (mmol/kg of free water). Because it is preferable to present glucose content as a molar concentration in plasma ([gluc]p,molar in units of mmol/L of plasma),12 glucose molarity is currently calculated from glucose molality in whole blood ([gluc]b,molal in units of mmol/kg of free water) assuming fixed values for Hct and plasma protein concentration ([PP]) of human blood,10, 12 such that:
| (1) |
Equation (1) was developed for human blood and assumes that the free water content of the erythrocyte (f e) is 0.71 times the intra‐erythrocyte volume, and that the free water content of plasma = 0.93 L/kg of plasma based on a plasma protein concentration ([PP]) of 7 g/dL. When Hct = 43% (median value for adult humans), [gluc]p,molar = 1.11 × [gluc]b,molal, and this is the standard correction factor applied when human blood is analyzed by point‐of‐care meters that do not have Hct‐correction.10, 11, 12 In other words:
| (2) |
where [gluc]b‐meter is the molar value reported by the meter (units of mg/dL) when blood is analyzed.
Equation (1) needs to be revised for use in cattle because bovine blood has a different median value for [PP] and f e than human blood.27 Experimentally determined values for f e range from 0.52 to 0.74 because of species differences in the amount of intra‐erythrocyte protein (predominantly hemoglobin), and f e has been experimentally determined to equal 0.65 times the intra‐erythrocyte volume in cattle.27 Accordingly, the factors 0.93 and 0.71 in equation (1) must be revised if the equation is to be applied to cattle blood. A potentially more accurate equation for bovine blood is therefore:
| (3) |
Based on a plasma protein concentration ([PP]) of 6 g/dL and Hct of 33% for periparturient cattle, equation (3) predicts that [gluc]p,molar = [gluc]b‐meter = 1.11 × [gluc]b,molal. Because this estimate is identical to the standard correction factor applied by many point‐of‐care glucose meters in equation (2), equation (3) suggests that the Precision Xtra® meter should provide an accurate measure of [gluc]p,molar when blood from adult cattle is analyzed.
It should be noted that equations (1) and (3) assume that the glucose concentration in intra‐erythrocyte water expressed in terms of molality ([gluc]e,molal) is equivalent to the glucose concentration in plasma water expressed in terms of molality ([gluc]p,molal), such that [gluc]e,molal = [gluc]p,molal. This appears to be true for humans and nonhuman primates where glucose enters the erythrocyte primarily via carrier‐mediated transport through GLUT‐1 receptors.28, 29, 30 In contrast, insulin does not bind to erythrocytes from adult cattle17, 31 and many other domestic animals, and as a consequence [gluc]e,molal < [gluc]p,molal. 13, 14, 15, 16 Accordingly, a more generalizable equation is therefore:
| (4) |
where:
| (5) |
It should be noted that in equation (4), when r = 1, f e = 0.71, Hct = 43%, and [PP] = 7 g/dL (reference values for human blood), then [gluc]p,molar = [gluc]b‐meter = 1.11 × [gluc]b,molal.
Combining equations (2) and (4) and algebraic rearrangement provides the following equivalent expression for blood:
| (6) |
Equation (6) simplifies to the following equation when the meter is used to analyze plasma because Hct = 0:
| (7) |
If the meter is used to analyze a blood and plasma sample obtained from the same blood vessel, then [gluc]p,molar is the same in equations (6) and (7). Dividing equation (6) by equation (7) therefore provides:
| (8) |
Equation (8) can be rearranged to provide an expression in terms of r, such that:
| (9) |
A median estimate for r was therefore obtained for blood from periparturient cattle by measuring the [gluc] (using the meter), Hct and [PP] in blood, and the [gluc] (using the meter) in plasma, using the same venous blood sample. In equation (5), r is expressed as a ratio of molal concentrations (mg/kg of water). Because of differences in the free water content of erythrocytes and plasma, the highest theoretical value for r in molar units is approximately 0.75, which agrees with experimental findings.13, 15 To facilitate comparison of our results to other studies, the intra‐erythrocyte [gluc] was calculated in molar units ([gluc]e,molar) using the following equation and expressed as a percentage of the plasma [gluc] measured by the reference method.
| (10) |
Sensitivity of Meter Reading to Changes in Hematocrit, Plasma Protein Concentration, and r:
Sensitivity of the dependent variable (percent error reading by meter assuming a correction factor of 1.11) to the 4 independent factors hematocrit, [PP], r, and f e in equation (5) were conveyed by a spider plot, which graphically depicted the relationship between the dependent variable and percentage change in 1 independent factor while the remaining 3 independent factors were held constant at typical values.32 The spider plot was created using equation (5) and typical values for the blood of healthy humans (Hct = 43%, [PP] = 7.0 g/dL; r = 1.0; f e = 0.71).
Statistical Analysis
Data are expressed as median and range and P < 0.05 was assigned as statistically significant. Linear regression was used to characterize the relationship between [gluc]p‐meter and temperature for 9 plasma samples obtained on the day of parturition. Deming regression was used to evaluate the relationship between [gluc]b‐meter and the plasma [gluc] determined by the reference method ([gluc]hexokinase), as well as between [gluc]p‐meter and [gluc]hexokinase. The agreement between the meter and reference method was also examined using Bland–Altman difference plots.33 Deming regression was also used to evaluate the relationship between [gluc]b‐meter and [gluc]p‐meter to confirm the implicit assumption in equation (9) that the relationship was linear with zero asymptote. Multivariable regression was used to investigate the effect of the independent variables [gluc]hexokinase, Hct, and the interaction between [gluc]hexokinase and Hct on the dependent variable ([gluc]b‐meter). The interaction term was dropped from the analysis if Hct and the interaction between [gluc]hexokinase and Hct were not significant. Statistical analyses were performed using SAS 9.3,4 Analyse‐it2.26,5 and an Excel spreadsheet.6
Meter performance was also evaluated by expressing [gluc]b‐meter as a percentage of plasma [gluc] determined by the reference method ([gluc]hexokinase) or as an absolute difference. The American Diabetes Association has recommended that glucose meters agree with the reference method to within ± 15% at all concentrations.34 Draft recommendations from the Food and Drug Administration published in early 2014 state that 95% and 99% of all measured blood glucose values must be within 15% and 20% of the reference value, respectively. The 2013 standard (ISO 15197‐2013) from the International Standards Organization states that 95% of the individual glucose results for the meter should fall within 15 mg/dL of the reference method value when plasma [gluc] < 100 mg/dL and within 15% of the reference value when plasma [gluc] > 100 mg/dL. The American Society for Veterinary Clinical Pathology recommended a total allowable error of 10% in hypoglycemic samples and 20% in hyperglycemic samples.35 Alternative meter performance criteria are available.36
Results
The intra‐observer coefficient of variation (CV) from 20 consecutive analyses of blood samples with a mean low (46 mg/dL) and moderate (102 mg/dL) values for [gluc]b‐meter was 5.5% and 4.4%, respectively; CV values of plasma samples with a mean moderate (80 mg/dL) and high (128 mg/dL) values for [gluc]p‐meter were both 3.0%.
Effect of Temperature
Variation in sample temperature from 7 to 42°C had no detectable linear effect on the value for [gluc]p‐meter in 4/4 samples when the sample [gluc]p‐meter was < 160 mg/dL (Fig. 1; Table 1). In comparison, sample temperature had a linear effect on the measured value for [gluc]p‐meter in 3/5 samples, where the value exceeded 160 mg/dL.
Figure 1.
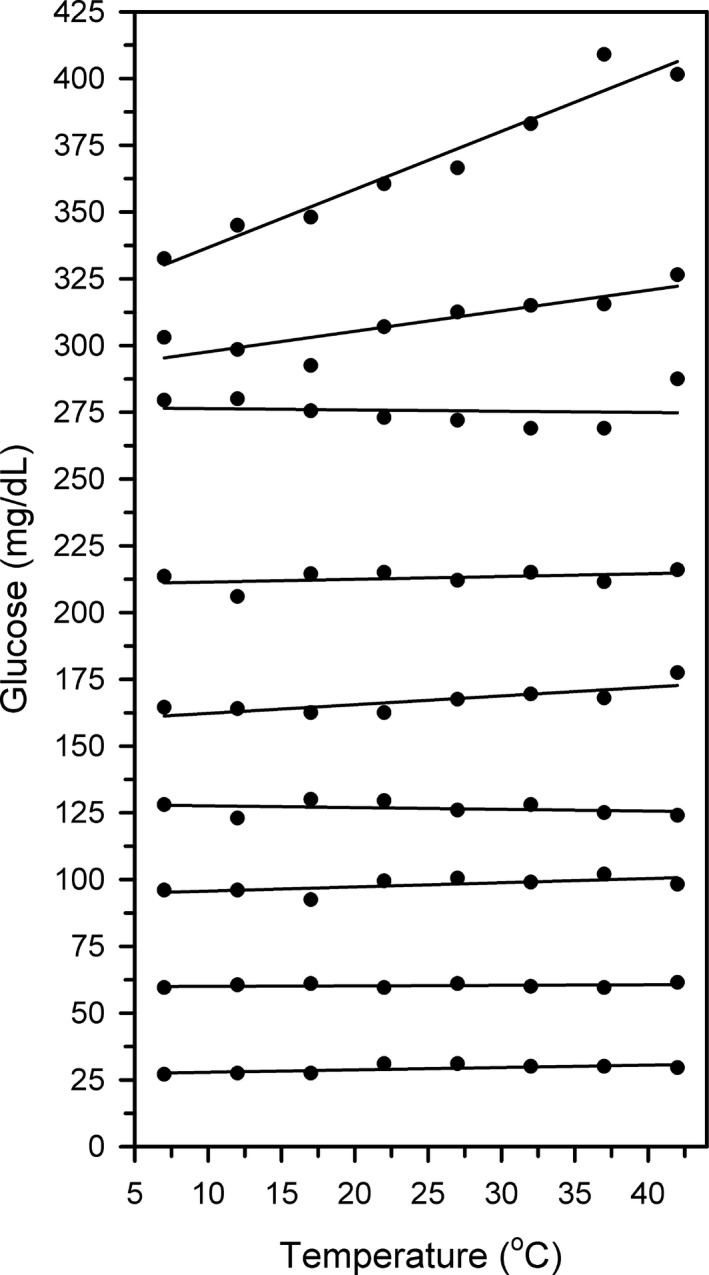
Scatterplots and linear regression line for plasma from 9 Holstein‐Friesian cattle on the day of parturition characterizing the effect of sample temperature (T) ranging from 7–42°C on the plasma glucose concentration ([gluc]) measured by an electrochemical point‐of‐care meter. Note that sample temperature has a significant linear effect on the measured value for [gluc] in 3/5 plasma samples when [gluc] > 160 mg/dL, with animal to animal variability.
Table 1.
Results of linear regression analysis characterizing the effect of sample temperature (T) ranging from 7–42°C on the plasma glucose concentration ([gluc]) measured by an electrochemical point‐of‐care meter, as well as plasma protein concentration ([PP]) and hematocrit (Hct) for blood from nine periparturient Holstein‐Friesian cattle. Note that sample temperature has a significant linear effect on the measured value for plasma [gluc] in 3/5 plasma samples when plasma [gluc] > 160 mg/dL, with animal to animal variability. The bold is to highlight data points with a significant (P < 0.05) P value for the linear regression equation
| [Gluc] at 37°C (mg/dL) | [PP] (g/dL) | Hct (vol %) | P value for linear regression equation | R 2 | Linear regression equation (y = a + bx) |
|---|---|---|---|---|---|
| 30 | 6.5 | 30 | 0.067 | 0.45 | NS |
| 60 | 6.7 | 35 | 0.51 | 0.07 | NS |
| 102 | 6.1 | 42 | 0.09 | 0.41 | NS |
| 125 | 6.0 | 35 | 0.45 | 0.10 | NS |
| 168 | 6.2 | 35 | 0.017 | 0.64 | [gluc] = 159 + 0.33 × T |
| 212 | 7.4 | 32 | 0.33 | 0.16 | NS |
| 269 | 6.7 | 33 | 0.83 | 0.01 | NS |
| 316 | 6.7 | 37 | 0.0050 | 0.76 | [gluc] = 290 + 0.77 × T |
| 409 | 6.8 | 35 | <0.0001 | 0.94 | [gluc] = 315 + 2.18 × T |
NS = not significant.
Method Comparison Study
Median [gluc]hexokinase for 102 cattle on the day of calving was 77 mg/dL, with a range of 33–284 mg/dL. Deming regression for [gluc]b‐meter against [gluc]hexokinase for 102 cattle on the day of parturition indicated proportional bias (1.11; 95% CI, 1.05–1.18) that was significantly higher (P = 0.033) than 1, and constant bias (−17 mg/dL; 95% CI, −22 to −11 mg/dL) that was significantly lower (P < 0.0001) than 0 (Fig. 2A). The meter was linearly related to [gluc]hexokinase but measured 29 mg/dL (1.6 mmol/L) above to 32 mg/dL (1.8 mmol/L) below the true value. Bland–Altman plots indicated that bias increased linearly as mean glucose concentration increased (Fig. 2B).
Figure 2.
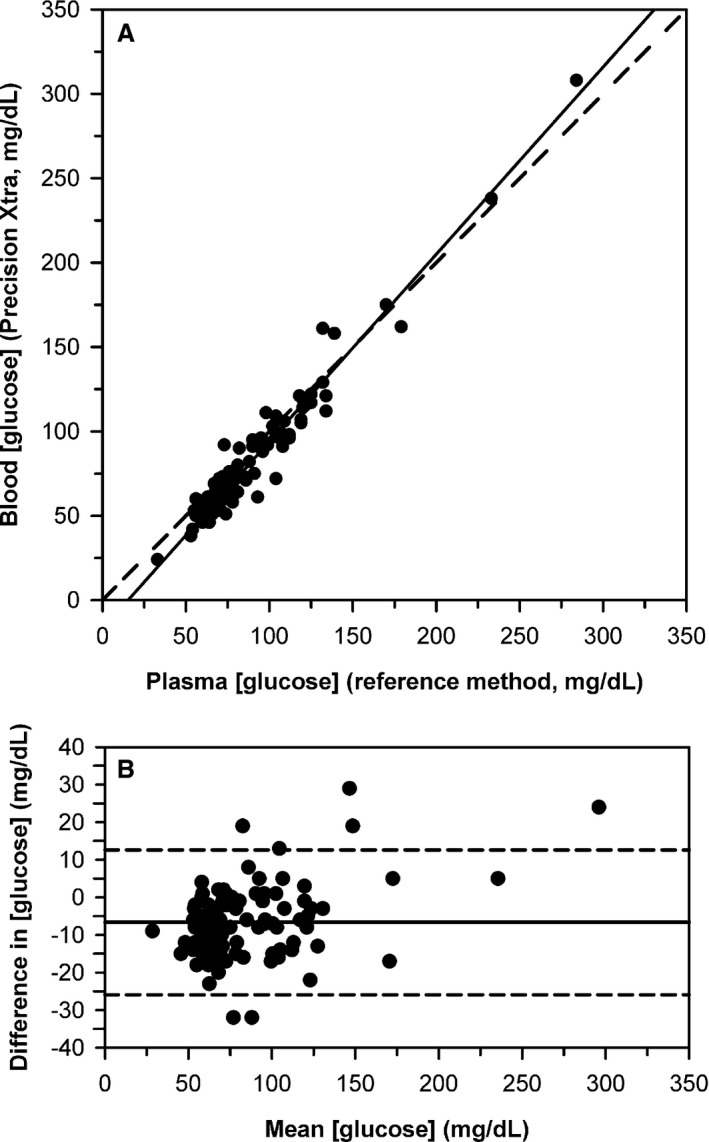
(A) Scatterplot indicating the relationship between blood glucose concentration ([gluc]) measured by an electrochemical point‐of‐care meter versus the plasma [gluc] measured by the reference method for 102 Holstein‐Friesian cattle on the day of parturition. The dashed diagonal line is the line of identity, and the solid line is the line of best fit from Deming regression. (B) Bland–Altman plot of the difference between blood [gluc] measured by the electrochemical meter and the reference [gluc] against the mean glucose concentration for both methods. The solid horizontal line is the mean bias and the two horizontal dashed lines represent the 95% confidence interval for agreement. The plot indicates that bias increased linearly as mean [gluc] increases.
Deming regression for [gluc]p‐meter measured at 37°C against [gluc]hexokinase for the 89 cattle on the day of parturition, where sufficient plasma was available to run both assays indicated proportional bias (1.51; 95% CI, 1.47 to 1.55) that was significantly higher (P < 0.0001) than 1, and constant bias (−22 mg/dL; 95% CI, −25 to −18 mg/dL) that was significantly lower (P < 0.0001) than 0 (Fig. 3A). The meter was linearly related to [gluc]hexokinase but measured 124 mg/dL (6.9 mmol/L) above to 0 mg/dL (0 mmol/L) below the true value. Bland–Altman plots indicated that bias increased linearly as mean [gluc] increased (Fig. 3B).
Figure 3.
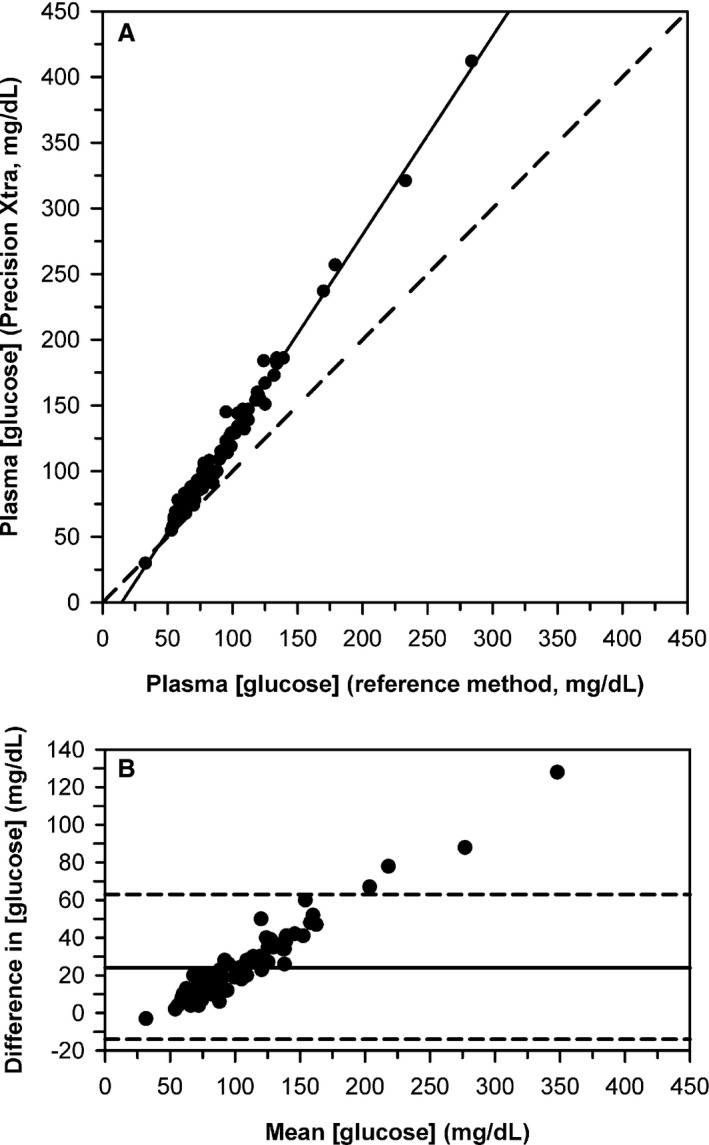
(A) Scatterplot indicating the relationship between plasma glucose concentration ([gluc]) measured by an electrochemical point‐of‐care meter versus the plasma [gluc] measured by the reference method for 89 Holstein‐Friesian cattle on the day of parturition. The dashed diagonal line is the line of identity, and the solid line is the line of best fit from Deming regression. (B) Bland–Altman plot of the difference between [gluc] measured by the electrochemical meter and [gluc] measured by the reference method against the mean [gluc] for both methods. The solid horizontal line is the mean bias and the two horizontal dashed lines represent the 95% confidence interval for agreement. The plot indicates that bias increased linearly as mean [gluc] increases.
Eighteen of 1,127 plasma samples had [gluc]p‐meter≥ 160 mg/dL and were therefore excluded from comparison to [gluc]b‐meter samples obtained at the same time because the temperature of the plasma sample was not measured. Deming regression for [gluc]b‐meter against [gluc]p‐meter for the 1,109 samples from 106 cattle with [gluc]p‐meter < 160 mg/dL indicated a proportional bias of 0.72 (95% CI, 0.69–0.74) that was significantly different (P < 0.0001) from 1 and a constant bias of +1 mg/dL (95% CI, −1 to 2 mg/dL) that was not significantly different (P = 0.33) from 0 (Fig. 4A). This confirmed the implicit assumption in equation (9) that the relationship between [gluc]b‐meter and [gluc]p‐meter was linear with a zero intercept. Bland–Altman plots indicated that bias decreased linearly as mean glucose concentration increased (Fig. 4B).
Figure 4.
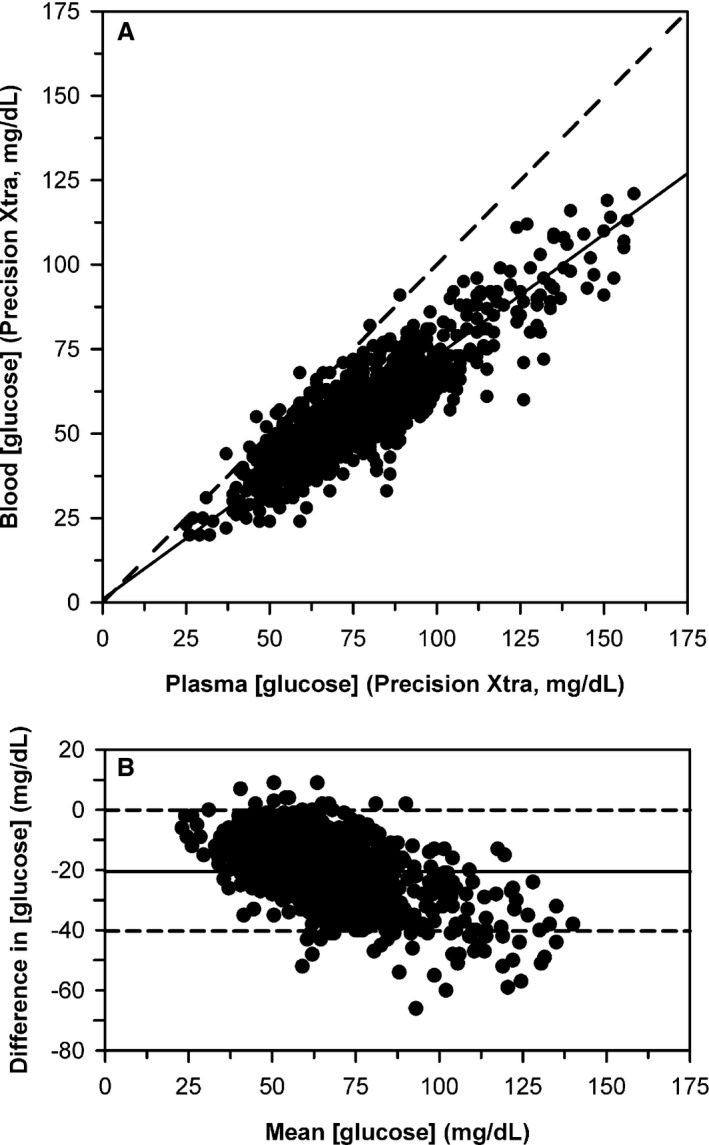
(A) Scatterplot indicating the relationship between plasma glucose concentration ([gluc]) and blood [gluc] measured by an electrochemical point‐of‐care meter for 1,109 blood samples from 106 periparturient Holstein‐Friesian cattle. The dashed diagonal line is the line of identity, and the thick line is the line of best fit from Deming regression. (B) Bland–Altman plot of the difference between plasma [gluc] and blood [gluc] measured by the meter against the mean [gluc] for both methods. The solid horizontal line is the mean bias and the two horizontal dashed lines represent the 95% confidence interval for agreement. The plot indicates that bias decreased linearly as mean [gluc] increases.
Calculation of r and the Ratio of Intra‐Erythrocyte [gluc] to Plasma [gluc]
The median value for r was 0.25 for 1,109 paired blood and plasma samples from 106 periparturient cattle. Hematocrit ranged from 23 to 44% (median, 33%) and [PP] ranged from 30 to 90 g/L (median, 63 g/L). Substituting the median values for r and [PP] and experimentally determined value for f e (0.65) into equation (4) produced the following equivalent expression that should be compared to equations (1) and (3):
| (11) |
where [gluc]b‐meter is the molar value reported by the meter (units of mg/dL) when blood from adult cattle is analyzed.
Application of equation (10) to data from 1,109 paired blood and plasma samples from 106 periparturient cattle indicated that the median intra‐erythrocyte [gluc] was 18% of the plasma [gluc].
Effect of Hematocrit
Multivariable regression of [gluc]blood‐meter against [gluc]hexokinase, Hct, and the interaction between [gluc]hexokinase and Hct indicated that [gluc]hexokinase was the only significant predictor of [gluc]b‐meter (Table 2). This result was consistent with a low intra‐erythrocyte [gluc].
Table 2.
Results of multivariable regression analysis characterizing the effect of plasma glucose concentration determined by the reference method (hexokinase), hematocrit, and the interaction between plasma glucose concentration and hematocrit (not significant; only main effects shown) on the glucose concentration in blood from 106 periparturient Holstein‐Friesian cattle measured by an electrochemical point‐of‐care meter
| Coefficient | Estimated value | 95% confidence interval | P > ¦t¦ |
|---|---|---|---|
| Intercept | 3.2 | −20.3 to 26.8 | 0.79 |
| Plasma [gluc] | 1.00 | 0.90 to 1.10 | <0.0001 |
| Hematocrit | −31 | −98 to 37 | 0.37 |
Sensitivity of Meter Reading to Changes in Hematocrit, Plasma Protein Concentration, and r:
Sensitivity analysis using a spider plot (Fig. 5) indicated that the percent error in the measured value for [gluc]b‐meter was most dependent on the value for r, moderately dependent on the Hct, and only minimally dependent on the [PP].
Figure 5.
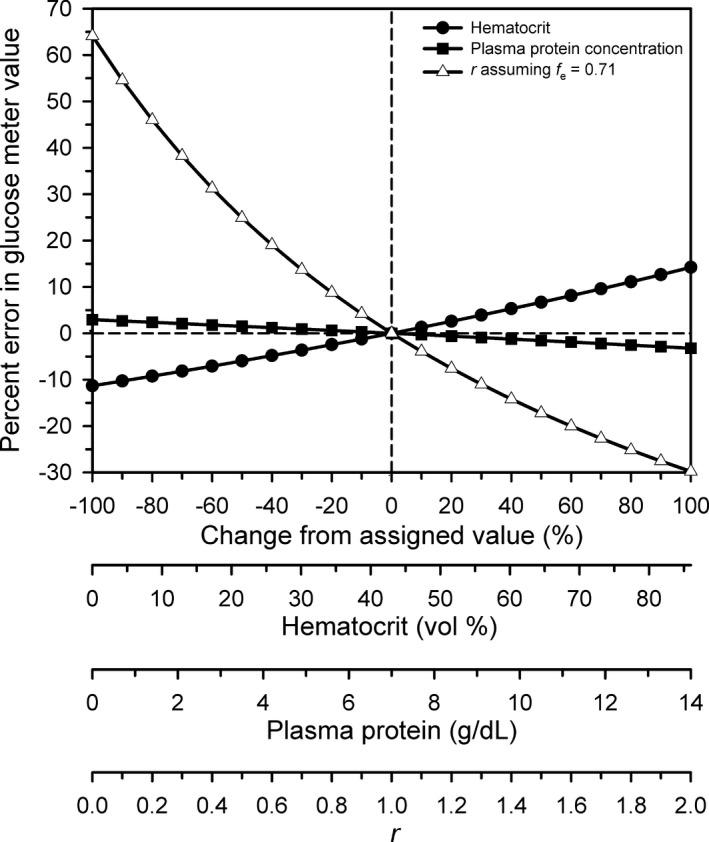
Spider plot revealing the dependence of percent error reading by an electrochemical point‐of‐care glucose meter on changes in the four independent variables (hematocrit, plasma protein concentration, r, and f e). The spider plot was obtained by systematically varying one independent variable while holding the other three independent variables at their assumed values for human blood. Reference values for the four independent variables were as follows: hematocrit = 43% (filled circles), plasma protein concentration = 7.0 g/dL (filled squares); r = 1.0 (open triangles); f e = 0.71 (open triangles, same scale as r on the x axis). The dashed vertical and horizontal lines indicate that the percent error = 0% when hematocrit, plasma protein concentration, r, and f e are at their reference values.
Glucose Meter Performance
The meter failed to meet American Diabetes Association recommended performance requirements in that only 68% (69/102) values for [gluc]b‐meter were within 15% of the reference value (goal is 100%). The meter failed to meet draft recommendations from the Food and Drug Administration published in early 2014 with 68% being within 15% of the reference value (goal 95%) and 82% (84/102) being within 20% of the reference value (goal 99%). The meter did not meet the total allowable error recommended by the American Society for Veterinary Clinical Pathology in hypoglycemic samples (all measurements within 10%) and in hyperglycemic samples (all measurements within 20%; Fig. 2). The meter also failed to meet the 2013 performance requirements recommended by The International Standards Organization in that 88% (65/74) of the individual values for [gluc]b‐meter fell within 15 mg/dL of the reference method value when plasma [gluc] < 100 mg/dL (goal is 95%) and 71% (20/28) fell within 15% of the reference value when plasma [gluc] > 100 mg/dL (goal is 95%).
Discussion
The major finding of the study reported here was that the intra‐erythrocyte [gluc] is low in blood from adult cattle and approximately 18% of the plasma [gluc] when expressed in molar units. This estimate indicates that bovine erythrocytes are not very permeable to glucose and contrasts with human erythrocytes, which are very permeable to glucose.13 Electrochemical point‐of‐care glucose meters calibrated for use in human blood are therefore likely to be inaccurate when applied to domestic animals such as cattle that have erythrocytes that are less permeable to glucose.
Our corpuscle to plasma glucose ratio of 18% was similar to previous estimates in adult cattle blood of 10–45%,13 18%,14 20%,13 22%,13 and 24%.15 Collectively, these results demonstrate that the proprietary algorithm used by the meter cannot be accurately applied to blood from cattle because the algorithm assumes intra‐erythrocyte [gluc] ≈ plasma [gluc] on a molal basis. Interestingly, much higher corpuscle to plasma glucose ratios of 59% and 68% have been reported in calves up to 1 week of age,13, 15 presumably because neonatal erythrocytes express GLUT‐1 receptors.17 It is therefore likely that a method comparison study performed using blood from neonatal calves will provide a different result to that in the study reported here.
This study used a wider range of plasma glucose concentration values (33–284 mg/dL) than that used previously20, 21 to validate the meter in blood from adult cattle. Our study also covered a wider section of the assay range (20–500 mg/dL) described in the meter user's manual than previous studies. A wide analytical range is very helpful in method comparison studies.
The effect of Hct on the accuracy of the meter for monitoring [gluc] in dairy cattle blood does not appear to have been previously investigated. The Hct range is reported to be 30–60% in the user's manual for the meter. The Hct range of cattle blood in this study was 23–44% (median, 33%) and consequently some cattle had Hct values below the recommended range for the meter. At a median Hct of 33%, equation (11) predicts that [gluc]p,molar = 1.38 × [gluc]b,molal; the 1.38 coefficient is markedly different from the 1.11 coefficient in equation (2) that has been recommended for use by point‐of‐care meters designed to analyze human blood.10, 12 In other words, a species specific algorithm will be needed when blood from cattle or other domestic animals is analyzed using point‐of‐care meters that use direct methods to measure glucose content. The ideal algorithm will use equation (11) to adjust for differences in median values of Hct (33% instead of 43%), [PP] (6 g/dL instead of 7 g/dL), r (0.25 instead of 1.00), and f e (0.65 instead of 0.71). Until such algorithms are developed by the manufacturer, we recommend use of the following equations derived in this study from Deming regression to calculate plasma [gluc]; as such these equations assume Hct = 33% and [PP] = 6 g/dL.
| (12) |
| (13) |
The spider plot indicates that these equations will produce minimal error in [gluc] despite large changes in [PP] and moderate changes in Hct. The coefficient of variation for [gluc] was lower for plasma than for blood; consequently the measurement of glucose concentration by the meter was more repeatable in plasma than in blood. Additional support for plasma being the preferred sample for analysis is provided by the value for r having the greatest impact on the accuracy of the meter (Fig. 5), and the estimated value for r revealed large cow to cow variation.
The effect of strip temperature on the accuracy of the Precision Xtra® meter has been evaluated in human blood where use of strips stored at 10°C resulted in under‐reading of the blood [gluc].37 The effect of sample temperature on the accuracy of the Precision Xtra® meter for monitoring [gluc] in dairy cattle has only been investigated in one other study.19 That study expressed their findings as percent change from baseline and as such was not designed to determine whether the effect of sample temperature depended, in part, on the actual plasma [gluc]. Any enzymatic reaction, including that employed by the Precision Xtra® meter, is influenced by temperature and the moles of reactants in the analyzed sample.38 To minimize the effect of temperature on enzyme activity, manufacturers of electrochemical strips provide a marked excess of enzyme so that the temperature dependence of the reaction speed is eliminated. The end result is that electrochemical strips are limited by the rate of diffusion of plasma to the electrodes, which are layered beneath the chemistry layer containing glucose oxidase and potassium ferricyanide. This rate of diffusion is sensitive to temperature with approximately 2% decrease in speed for every 1°C decrease in temperature.38 For comparison, there was a 0.2–0.6% decrease in the measured value for plasma [gluc] for every 1°C decrease in temperature in the three plasma samples that demonstrated an effect of temperature in the study reported here. We suspect that a greater effect of temperature would have been detected if identical temperatures for the plasma sample and meter were investigated.
We investigated the effect of sample temperature on the accuracy of the meter using purposive sampling of a convenience sample. This sampling approach was based on an operational construct that low sample temperature would result in a lower measured value for plasma [gluc], particularly in markedly hyperglycemic samples. We have demonstrated that this is the case for the Precision Xtra® meter when measuring plasma [BHB] (unpublished observations; 9/9 plasma samples with measured [BHB] > 3.1 mmol/L). Purposive sampling is susceptible to bias but appropriate when conducting initial exploratory investigations related to proof of concept, as in the study reported here.39 Based on our preliminary findings, more detailed studies characterizing the effect of temperature on the measured value for plasma [gluc] using stratified random sampling and other appropriate study designs appear indicated.
We believe that measurement of plasma [gluc] is clinically valuable in periparturient dairy cattle, particularly during the period from day 3 to day 28 of lactation. Plasma nonesterified fatty acid concentration provides the best index of energy balance in lactating dairy cows from 1 to 10 weeks postpartum,40 but its current cost (US$9.00/test) prohibits routine use as a monitoring tool. Plasma [gluc] is negatively associated with energy balance in lactating dairy cows to a similar degree as serum [BHB];40 plasma [BHB] also has a marked diurnal change based on time of feeding, whereas plasma [gluc] does not exhibit a diurnal effect.41 Moreover, plasma/serum [gluc] is negatively associated with plasma/serum [BHB] in dairy cattle from 2 months before to 2 months after calving.41, 42, 43, 44, 45 Blood [gluc] can be measured for approximately US$0.30/test whereas measurement of blood or plasma [BHB] costs US$1.30–3.10/test; consequently, four to ten times as many cattle can have their blood [gluc] measured than their blood [BHB] for the same overall cost. This cost differential suggests that blood [gluc] may have clinical utility as a potentially useful index of energy balance in periparturient cattle, either instead of measuring blood [BHB] or as a parallel test with semiquantitative determination of urine acetoacetate concentration using the sodium nitroprusside test. Additional studies are indicated to verify this supposition.
Our results are relevant to the use of electrochemical point‐of‐care meters in all species. In humans and other primates where r = 1.0, the major analytical errors have been attributed to changes in Hct and [PP].25 The derivation of equation (4) and application of the spider plot to graphically depict the relative contributions of four factors to analytical error indicate that changes in Hct play a more important role in these species than do changes in [PP]. It is worth emphasizing that in domestic animals and many other species where r < 1.0, the actual value of r has the greatest effect on the observed analytical error. We attribute the relatively poor performance of the electrochemical meter studied here, when evaluated using performance recommendations by the American Diabetes Association, Food and Drug Administration, American Society for Veterinary Clinical Pathology, and the International Standards Organization, to application of an incorrect algorithm rather than an inherent methodologic error. We anticipate that implementation of a species‐specific algorithm or equations (12) or (13) will improve the performance of the meter when used to measure [gluc] in blood obtained from adult cattle.
Acknowledgments
Conflict of Interest Declaration: Peter Constable is a consulting editor for experimental design and statistics with the Journal of Veterinary Internal Medicine.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This work was performed at Purdue University Dairy Research and Education center in West Lafayette, IN and the College of Veterinary Medicine at the University of Illinois at Urbana‐Champaign, IL. The study was supported, in part, by the Purdue University, College of Veterinary Medicine, Department of Veterinary Clinical Sciences. Results were presented, in part, at the 2015 ACVIM Forum in Indianapolis, IN, USA.
Footnotes
Precision Xtra®; Blood Glucose and Ketone Monitoring System, Abbott Diabetes Care Inc., Alameda, CA
Analog Refractometer, MASTER‐SUR/Nα, Atago Co Ltd, Tokyo 105‐0011, Japan
AU680, Beckman Coulter Inc, Brea, CA
SAS 9.3 software, SAS Inc, Cary, NC
Analyse‐it2.26 software, Analyse‐it Software Ltd., Leeds, UK
Excel spreadsheet, Microsoft Corporation, Redmond, WA
References
- 1. Bell AW. Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. J Anim Sci 1995;73:2804–2819. [DOI] [PubMed] [Google Scholar]
- 2. Drackley KJ, Overton TR, Douglas GN. Adaptation of glucose and long‐chain fatty acid metabolism in liver of dairy cows during the periparturient period. J Dairy Sci 2001;84(E. Suppl.):E100–E112. [Google Scholar]
- 3. Hayirli A, Grummer RR, Nordheim EV, Crump PM. Animal and dietary factors affecting feed intake during the prefresh transition period in Holsteins. J Dairy Sci 2002;85:3430–3443. [DOI] [PubMed] [Google Scholar]
- 4. Rollin F. Tools for a prompt cowside diagnosis: what can be implemented by the bovine practitioner? World Buiatrics Congress 2006;15–19:322–336. [Google Scholar]
- 5. Solnica B, Naskalski JW, Sieradzki J. Analytical performance of glucometers used for routine glucose self‐monitoring of diabetic patients. Clin Chim Acta 2003;331:29–35. [DOI] [PubMed] [Google Scholar]
- 6. Corstjens AM, Ligtenberg JJM, Van der Horst IIC, et al. Accuracy and feasibility of point‐of‐care and continuous blood glucose analyzing in critically ill ICU patients. Crit Care 2006;10:R135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang J. Electrochemical glucose biosensors. Chem Rev 2008;108:814–825. [DOI] [PubMed] [Google Scholar]
- 8. Vanavanan S, Santanirand P, Chaichanajarernkul U. Performance of a new interference‐resistant glucose meter. Clin Biochem 2010;43:186–192. [DOI] [PubMed] [Google Scholar]
- 9. Wang L, Sievenpiper LJ, de Souza JR. Hematocrit correction does not improve glucose monitor accuracy in the assessment of neonatal hypoglycemia. Clin Chem Lab Med 2013;51:1627–1635. [DOI] [PubMed] [Google Scholar]
- 10. Fogh‐Andersen N, D'Orazio P. Proposal for standardizing direct‐reading biosensors for blood glucose. Clin Chem 1998;44:655–659. [PubMed] [Google Scholar]
- 11. Burnett RW, D'Orazio P, Fogh‐Andersen N. IFCC recommendation on reporting results for blood glucose. Clin Chim Acta 2001;307:205–209. [DOI] [PubMed] [Google Scholar]
- 12. Lyon ME, DiBois JA, Fick GH, et al. Estimates of total analytical error in consumer and hospital glucose meters contributed by hematocrit, maltose, and ascorbate. J Diabetes Sci Technol 2010;4:1479–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andreen‐Svedberg A. On the distribution of sugar between plasma and corpuscles in animal and human blood. Skandinav Archlv 1933;66:113–190. [Google Scholar]
- 14. Somogyi M. The distribution of sugar and rate of glycolysis in the blood of some mammals. J Biol Chem 1933;103:665–670. [Google Scholar]
- 15. Goodwin RFW. The distribution of sugar between red cells and plasma: variations associated with age and species. J Physiol 1956;134:88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coldman MF, Good W. The distribution of sodium, potassium and glucose in the blood of some mammals. Comp Biochem Physiol 1967;21:201–206. [DOI] [PubMed] [Google Scholar]
- 17. Montel‐Hagen A, Kinet S, Manel N. Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell 2008;132:1039–1048. [DOI] [PubMed] [Google Scholar]
- 18. Sage JM, Carruthers A. Human erythrocytes transport dehydroascorbic acid and sugars using the same transporter complex. Am J Physiol Cell Physiol 2014;306:C910–C917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iwersen M, Klein‐Jöbst D, Pichler D. Comparison of 2 electronic cowside tests to detect subclinical ketosis in dairy cows and the influence of the temperature and type of blood sample on the test results. J Dairy Sci 2013;96:7719–7730. [DOI] [PubMed] [Google Scholar]
- 20. Voyvoda H, Erdogan H. Use of a hand‐held meter for detecting subclinical ketosis in dairy cows. Res Vet Sci 2010;89:344–351. [DOI] [PubMed] [Google Scholar]
- 21. Wittrock MAJ, Duffield TF, LeBlanc SJ. Short communication: validation of a point‐of‐care glucometer for use in dairy cows. J Dairy Sci 2013;96:4514–4518. [DOI] [PubMed] [Google Scholar]
- 22. Panousis N, Brozos C, Karagiannis I, et al. Evaluation of Precision Xceed® meter for on‐site monitoring of blood β‐hydroxybutyric acid and glucose concentrations in dairy sheep. Res Vet Sci 2012;93:435–439. [DOI] [PubMed] [Google Scholar]
- 23. Hornig KJ, Byers SR, Callan RJ, et al. Evaluation of a point‐of‐care glucose and β‐hydroxybutyrate meter operated in various environmental conditions in prepartum and postpartum sheep. Am J Vet Res 2013;74:1059–1065. [DOI] [PubMed] [Google Scholar]
- 24. Pichler M, Damberger A, Arnholdt T, et al. Evaluation of 2 electronic handheld devices for diagnosis of ketonemia and glycemia in dairy goats. J Dairy Sci 2014;97:7538–7546. [DOI] [PubMed] [Google Scholar]
- 25. Lyon ME, Lyon AW. Patient acuity exacerbates discrepancy between whole blood and plasma methods through error in molality to molarity conversion: “Mind the gap!”. Clin Biochem 2011;44:412–417. [DOI] [PubMed] [Google Scholar]
- 26. National Research Council (NRC) . Nutrient Requirem‐ents of Dairy Cattle, 7th rev ed. 2001; Washington, DC: Natl Acad Sci. [Google Scholar]
- 27. Bogner P, Csutora P, Cameron LI, et al. Augmented water binding and low cellular water content in erythrocytes of camel and camelids. Biophys J 1998;75:3085–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kasahara M, Hinkle CP. Reconstitution and purification of the D‐glucose transporter from human erythrocytes. J Biol Chem 1977;252:7384–7390. [PubMed] [Google Scholar]
- 29. Wheeler TJ, Hinkle CP. The glucose transporter of mammalian cells. A Rev Physiol 1985;47:503–517. [DOI] [PubMed] [Google Scholar]
- 30. Arai T, Washizu T, Sako T, et al. D‐glucose transport activities in erythrocytes and hepatocytes of dogs, cats and cattle. Comp Biochem Physiol 1992;102A:285–287. [DOI] [PubMed] [Google Scholar]
- 31. Maćkowiak P, Nogowski L, Nowak KW. Comparison of erythrocyte insulin receptors in different species of vertebrates. Naturwissenschaften 1992;79:413–415. [DOI] [PubMed] [Google Scholar]
- 32. Constable PD. Total weak acid concentration and effective dissociation constant of nonvolatile buffers in human plasma. J Appl Physiol 2001;91:1364–1371. [DOI] [PubMed] [Google Scholar]
- 33. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;338:307–310. [PubMed] [Google Scholar]
- 34. Tirimacco R, Koumantakis G, Erasmus R, et al. Glucose meters – fit for clinical purpose. Clin Chem Lab Med 2013;51:943–952. [DOI] [PubMed] [Google Scholar]
- 35. Harr KE, Flatland B, Nabity M, et al. ASVCP guidelines: allowable total error guidelines for biochemistry. Vet Clin Path 2013;42:424–436. [DOI] [PubMed] [Google Scholar]
- 36. Tonyushkina K, Nichols JH. Glucose meters: a review of technical challenges to obtaining accurate results. J Diabetes Sci Technol 2009;3:971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nerhus K, Rustad P, Sandberg S. Effect of ambient temperature on analytical performances of self‐monitoring blood glucose systems. Diabetes Technol Ther 2011;13:883–892. [DOI] [PubMed] [Google Scholar]
- 38. Hönes J, Müller P, Surridge N. The technology behind glucose meters: test strips. Diabetes Technol Ther 2008;10:S‐10–S‐26. [Google Scholar]
- 39. Teddlie C, Yu F. Mixed models sampling. A typology with examples. J Mixed Models Res 2007;1:77–100. [Google Scholar]
- 40. Reist M, Erdin D, von Euw D, et al. Estimation of energy balance at the individual and herd level using blood and milk traits in high‐yielding dairy cows. J Dairy Sci 2002;85:3314–3327. [DOI] [PubMed] [Google Scholar]
- 41. Nielsen NI, Ingvartsen KL, Larsen T. Diurnal variation and the effect of feed restriction on plasma and milk metabolites in TMR‐fed dairy cows. J Vet Med A 2003;50:88–97. [DOI] [PubMed] [Google Scholar]
- 42. Kauppinen K. Correlation of whole blood concentrations of acetoacetate, β‐hydroxybutyrate, glucose and milk yield in dairy cows as studies under field conditions. Acta Vet Scand 1983;24:337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Andersson L. Concentrations of blood and milk ketone bodies, blood isopropanol and plasma glucose in dairy cows in relation to the degree of hyperketonemia and clinical signs. Zbl Vet Med A 1984;31:683–693. [DOI] [PubMed] [Google Scholar]
- 44. Sakha M, Ameri M, Rohbakhsh A. Changes in blood β‐hydroxybutyrate and glucose concentrations during dry and lactation periods in Iranian Holstein cows. Comp Clin Pathol 2006;15:221–226. [Google Scholar]
- 45. Tehrani‐Sharif M, Hadadi M, Noughabi HH, et al. Bovine subclinical ketosis in dairy herds in Nishabood, Iran. Comp Clin Pathol 2012;21:1637–1641. [Google Scholar]


