Abstract
Background
Infrared thermography is a painless, noninvasive, nonionizing diagnostic imaging exam used in human medicine as an auxiliary tool for breast cancer diagnosis in women.
Hypothesis/Objectives
Define thermographic mean temperatures of healthy mammary glands and compare these temperatures with those of mammary glands with tumors in dogs.
Animals
Fifty client‐owned female dogs were evaluated, including 20 with histopathologically confirmed mammary tumor and 30 clinically healthy (control).
Methods
A randomized study using infrared thermography analyzed each mammary gland of the animals from the control group and mammary glands with tumors from the tumor group, then the thermographic temperatures obtained were compared. Thermographic exam was performed in a temperature‐controlled room with a cooled thermographic camera—Flir E‐40 (Flir Systems®)
Results
There was significantly a higher temperature in the caudal abdominal and inguinal mammary glands than the other glands in the healthy group (P < .05). Dogs with mammary tumors had significantly higher thermographic temperature compared with unaffected glands regardless of the tumor size and the location (P < .05).
Conclusions and clinical importance
The technique seems to be able to assess for the presence of neoplasia within the mammary tissue in bitches. Further investigation is necessary to determine the impact of this technique when adopted clinically.
Keywords: Cancer, Canine, Diagnostic Tool, Thermography
Abbreviations
- BTU/s
British thermal unit/seconds
- °C
degrees celsius
- M1
cranial thoracic mammary gland
- M2
caudal thoracic mammary gland
- M3
cranial abdominal mammary gland
- M4
caudal abdominal mammary gland
- M5
inguinal mammary gland
- n
number
Infrared thermography is an auxiliary diagnostic imaging tool that measures the surface temperature of an object or body by its heat emission. It is a noninvasive and painless exam that does not use radiation or contrast.1, 2 In humans, medical thermography is used in several medical specialties such as angiology, rheumatology, sports medicine, plastic surgery and in oncology especially for breast cancer diagnosis in women.3, 4, 5, 6, 7
Breast thermograms have been used for the early detection of cancer to improve the diagnostic accuracy of mammography.8, 9 Thermography offers advantages for detecting nonpalpable breast tumors in young women, as the increased density of breast tissue makes the radiographic exam difficult.7, 8, 9, 10 First studies in women with breast cancer showed a mean sensitivity and specificity of 90% of the thermography.11, 12, 13, 14 Thermography has a sensitivity of 83% in detecting breast cancer in women and thermography associated with mammography has a sensitivity of 95%.8, 15, 16
Mammary tumors in dogs are commonly diagnosed by palpation of the mammary gland without complementary exams. To the authors' knowledge, no temperature‐controlled environmental studies have been done to demonstrate the use of infrared thermography as an auxiliary diagnostic tool for mammary tumors in this species. The aim of this study was to define the thermographic temperature of healthy mammary glands in dogs and compare the temperature of glands with mammary tumors with normal glands using infrared thermography.
Materials and Methods
Dog Selection
This was a single‐institution, prospective study including 50 client‐owned dogs admitted to the Veterinary Teaching Hospital at the Federal University of Paraná, Brazil. The inclusion criteria for the control group were absence of mammary nodules, normal physical and skin examination, and normal complete blood count. The infrared thermography was performed first and then the physical exam with palpation of mammary glands, skin examination, and jugular puncture for blood work, if something abnormal was found, the animal was excluded from the study. The tumor group included female dogs with diagnosed mammary tumor, regardless of the number and size of nodules, the criteria was presence of mammary nodules and surgery resection scheduled.
Twenty female dogs with tumors (n = 36 tumors) confirmed by histopathology formed the tumor group and 30 dogs with healthy mammary tissue formed the control group.
To prepare for thermography, careful trichotomy of the ventral thoracic and abdominal area was performed, every mammary gland has been wide clipped around, using a professional hair clipper 2 hours before the exam. A maximum of 5 dogs at a time were allowed inside the temperature‐controlled room maintained at 22°C and 55% relative humidity.15, 17 The dogs were kept in the room for 1 hour before the exam to acclimate. The room was completely closed, with no interference by sunlight or other environmental temperature. The walls and ceiling were made of concrete and the floor was covered with floor tiles. The total size of the room was 12 cubic meters; the power of the air conditioner used was 30,000 BTU/s.1
Using physical restraint and without touching the mammary gland area, animals were put in dorsal recumbency on a metal table for the thermographic exam, which was completed in a mean of 3 minutes. Chemical restraint was not allowed in order to prevent heat loss because of the interference by anesthetic drugs with the blood supply to the skin.18
Thermographic images were obtained with the camera positioned at a distance of 40 cm from the skin surface, forming an angle of 90° between the operator and the dog. Each mammary gland was imaged individually on the right and left sides and referred to as the cranial thoracic (M1), caudal thoracic (M2), cranial abdominal (M3), caudal abdominal (M4), and inguinal mammary glands (M5). The operator of the camera was blind to the physical examination results, and to evaluate the thermographic images in the software, another person also blinded to the previous results was elected.
The thermographic camera used was a Flir E‐402 , which has a thermal sensitivity of 0.07°C, temperature range of 20 negative degrees to 650°C, automatic hot/cold detection, image resolution of 160 × 120 pixels, field of view 25°×19°, spatial resolution of 2,72 mrad, focal plane array detector, uncooled microbolometer, spectral range of 7.5–13 μm, image frequency of 60 Hz, and an integrated color camera of 3.1 megapixels. To obtain the mean temperature of the healthy mammary glands, Flir Systems® software v.1.2 was used. A 60‐mm‐long and 50‐mm‐wide rectangle was drawn around the nipple, using it as the centee point, resulting in an image of 227 × 189 pixels (Fig. 1). For neoplastic mammary glands, a rectangle was drawn around the tumor according to its size (Fig. 2).
Figure 1.
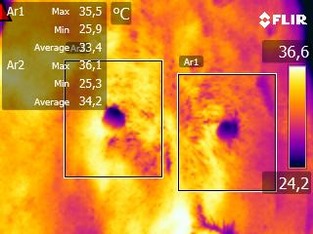
Thermographic image of the abdominal cranial mammary glands of a 7‐year‐old mixed breed bitch. Mean temperature of the left mammary gland was 33.4°C whereas the right mammary gland was 34.2°C. Rectangles delimit the area of the mammary glands, forming an image of 227 × 189 pixels.
Figure 2.
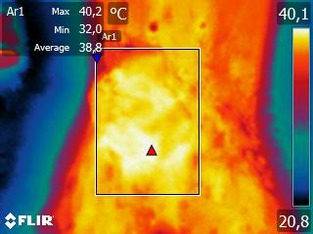
Thermographic image of a tumor involving the caudal abdominal and inguinal mammary glands on the right side. Rectangles delimit the area of the tumor.
After obtaining the images, physical examination was performed to assess respiratory rate, heart rate, capillary refill time, lymph node size, mucous membrane color, rectal temperature, hydration status, and tumor measurement when present, also in every patient mammary palpation was performed carefully to evaluate for tumor presence. Cutaneous examination of the mammary gland region was done to check for the presence of scars, skin diseases, or injuries that could interfere with skin temperature. Relative to the weight of the animals, within each group (control and tumor), we divided them into groups such as small, medium, and large size. To determine the size of the tumors in the tumor group we used a caliper rule to measure and classify them as the TNM staging system modified for mammary tumors in dogs.19
The animals of the tumor group had mammary resection the same day as the thermographic exam was performed. Tumors removed at mastectomy were immersed in 10% formalin and sent for preparation of histological slides, the stain used was hematoxylin–eosin and after preparation an experienced pathologist and a pathologist in training read them.
Statistical Analysis
Grubb's test was performed on both groups (control and tumor) to identify outliers.
Shapiro‐Wilk test was performed to evaluate the normality distribution of the thermographic temperatures from both groups (control and tumor) and to evaluate the age and size of all animals studied.
Two way ANOVA and Bonferroni posttest were performed to assess the temperature difference of the mammary glands between the left and right sides in the control group and to compare M1, M2, M3, M4, and M5 with each other in the healthy mammary gland group.
Afterward, the thermographic results obtained in the control and in the tumor group were compared using the unpaired t‐test. Values were considered significant when P < 0.05. Statistical analysis was performed using Graph Prism® v.5 software.3
This study was approved by the Ethics Committee on Animal Use CEUA/SCA 062/2011.
Results
The control group was comprised of 30 dogs of different breeds with a mean age of 6 years. The tumor group included 20 dogs of different breeds with a mean age of 8 years. The age of both groups was normally distributed. The size of the animals also presented normal distribution. None of the bitches of this study were intact.
None of the animals evaluated (healthy or with tumors) presented scars, injuries, or any skin diseases, which could interfere with the thermographic temperature, hence there was no need to exclude any animal from the study.
Results of the comparison between the thermographic temperature of the right and left side of the mammary glands in the control group and the thermographic temperature between each mammary gland within the control group (P < 0.05) are detailed in Table 1 and the statistical analysis results are depicted graphically in the Fig. 3.
Table 1.
Means and standard deviations of thermographic temperatures of mammary glands in control group (healthy dogs)
| Mammary Glands Group | Left Mammary Glands (T°C) | Right Mammary Glands (T°C) | Means Left and Right Sides |
|---|---|---|---|
| M1—Cranial thoracic | 34.87 ± 1.95 | 35.28 ± 2.05 | 35.078 ± 1.99 |
| M2—Caudal thoracic | 35.05 ± 2.01 | 35.37 ± 1.74 | 35.234 ± 1.86 |
| M3—Cranial abdominal | 35.60 ± 1.88 | 35.91 ± 1.48 | 35.758 ± 1.73 |
| M4—Caudal abdominal | 36.13 ± 1.94 | 36.60 ± 1.38 | 36.370 ± 1.69a |
| M5—Inguinal | 36,30 ± 1.86 | 36.25 ± 2.08 | 36.282 ± 1.96a |
P < 0.05 M4 and M5 mammary glands showed a higher temperature compared with M1 and M2. M3 did not differ from the others. There were no significant differences between the thermographic temperatures of the left and right side.
Figure 3.
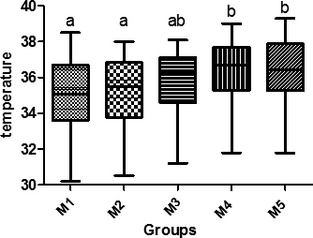
Thermographic temperature comparison between mammary glands in the control group. M1: cranial thoracic mammary glands; M2: caudal thoracic mammary glands; M3: cranial abdominal mammary glands; M4: caudal abdominal mammary glands; M5: inguinal mammary glands. Different letters mean there is a statistically significant difference by Tukey's test (P < .05).
In the tumor group, one value of 35°C was recognized as an outlier by the Grubb's test and excluded from the analysis; this was the only benign tumor (single adenoma) diagnosed by histopathology in this study. The remaining animals had malignant neoplasms, among which simple adenocarcinoma (papillary cystic) was the most common type (n = 20/35 tumors), other types were tubular adenocarcinoma (n = 9/35), mixed tumor carcinoma (n = 3/35), complex carcinoma (n = 2/35), and inflammatory adenocarcinoma (n = 1/35).
The number of tumors, location, prevalence size, means, and standard deviations of the thermographic temperatures from the mammary glands in the tumor group are detailed in Table 2.
Table 2.
Number of tumors, means, and standard deviations of thermographic temperatures from mammary glands in tumor group
| Mammary Glands Tumor Group | Left Mammary Tumors (T°C) | Right Mammary Tumors (T°C) | Means Left and Right Sides | Number of Tumors | Prevalence of Tumor Sizea |
|---|---|---|---|---|---|
| M1 | – | 38.4 | 38.4 | 1 | T2 |
| M2 | 38.5 | 37.9 ± 0.14 | 38.2 ± 0.36 | 4 | T1 |
| M3 | 37.9 ± 0.14 | 38 ± 0.33 | 37.95 ± 0.27 | 6 | T2 |
| M4 | 38.23 ± 0.94 | 38.1 ± 0.34 | 38.16 ± 0.56 | 8 | T3 |
| M5 | 38.22 ± 0.83 | 38.28 ± 0.76 | 38.25 ± 0.75 | 16 | T3 |
No statistical difference between temperatures when comparing tumors with different sizes and locations.
Size: TNM staging system modified for mammary tumors in dogs (Withrow et al.19).
Based on the statistical results, healthy mammary glands were grouped into cranial (M1, M2, and M3) and caudal (M4 and M5). The same groups were organized into the tumor group for further evaluation. There was a thermographic temperature difference between the control and tumor groups (P < 0.05), in M1 and M3 (Fig. 4) as well as between M4 and M5 (Fig. 5).
Figure 4.
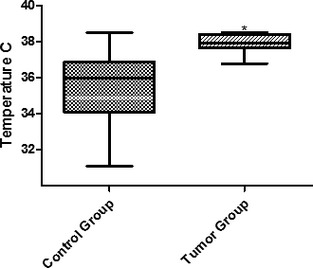
Thermographic temperatures in M1, M2, and M3 in the control and tumor groups. *Statistically significant difference between groups (P < .05).
Figure 5.
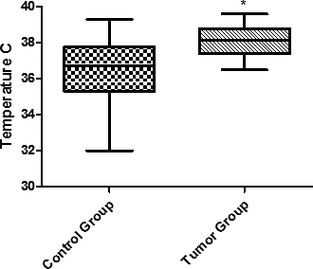
Thermographic temperatures in M4 and M5 in the control and tumor groups. *Statistically significant difference between groups (P < .05).
There was no significant temperature difference between the tumors considering their sizes. The smallest tumor evaluated was 0.5 cm in diameter and 0.5‐cm deep, and presented a temperature of 37.8°C. The largest tumor was 20 cm in diameter and 8‐cm deep and presented a thermographic temperature of 38°C. Regardless of the tumor size, all tumors had higher temperatures than healthy mammary glands (Table 2).
Considering all the mammary glands in this study, the mean thermographic temperature of the control group was 35.07 versus 37.86°C for the tumor group, a variation of 2.79°C. The mammary glands with tumors have higher temperatures than the other glands, besides that, temperature variation between the tumors also has been observed.
Discussion
This study demonstrates that thermography exam can be performed by veterinarians in routine consults to try and diagnose malignant mammary tumors. In dogs, most mammary tumors are first diagnosed by palpation, usually by their owners. About 70% of these tumors are malignant20 and exams to improve clinical diagnosis are essential in order to have a better management and clinical outcome. Other auxiliary tools are used in women to diagnose or follow tumor progression, such as mammography, magnetic resonance imaging, ultrasound, and cytology. Through these exams, diagnoses are based on anatomical changes in the mammary glands; it can take years for a tumor to grow large enough to be detected by these methods. Thermography diagnosis is based on temperature variations related to changes in blood flow and the metabolism of the affected mammary cells.16, 21 Again in women, thermography has been used to improve the sensitivity of tumor diagnoses.
One of the crucial factors in performing thermography exams is the room used for the examination. A previous study22 in horses concluded that thermographic exam must be performed in a room with temperature‐controlled. Another study23 in joints of horses also observed a positive correlation of increase or decrease in temperature of the joints according to the variation of the surrounding temperature, the hotter the ambient temperature, the higher the thermographic temperature of the measured joint.
In this study, the temperature‐controlled room was set up according to the recommendations of the literature about breast thermograms in women.24, 25 These authors described how the dog should stay in the room for a minimum of 15 minutes before the thermographic exam; in our study, a 1 hour acclimatization period was used to ensure thermal balance between the mammary glands and the room temperature in all animals, as there are no references regarding the optimal temperature and acclimatization period for mammary thermography in dogs.
According to the accessed studies, all malignant breast tumors were warmer than healthy breast tissue, regardless of their size and location.18, 21, 26, 27 However, another study observed lower increases in temperature in smaller and deeper tumors.28 In bitches, the anatomy and size of the mammary glands are different from those found in women, providing one possible reason for the lack of difference in temperature between the tumors in our study.
The microcirculatory activity in preneoplastic and neoplastic mammary tissues is described as higher than normal because of the necessity for an abundant supply of nutrients in order to maintain the growth of abnormal cells.2, 16 Tumor angiogenesis is stimulated by the release of several factors, such as endothelial growth factor, integrins, and immature dendritic cells, among others, to promote a rich oxygen supply for tumor growth.29, 30, 31 Other studies have reported that the release of nitric oxide by tumor cells causes vasodilation and increases the temperature in affected mammary glands.32, 33, 34 We believe that a combination of all these factors is responsible for the increase in temperature in neoplastic mammary glands in our study, as the controlled temperature in the room eliminated any influence from the ambient temperature.
Temperature variation between tumors has also been observed. One study observed a positive correlation between thermography and the histological grade of tumors in breast cancer.35 Further studies are needed to assess the correlation between histopathological findings and the thermographic temperature of tumors in bitches.
Our other purpose was to try to define the thermographic temperature of healthy mammary glands; even within the control group, there were different temperatures. It is believed that the temperature variation found between mammary glands in the control group is caused by the differences in vascularization. As the caudal abdominal and inguinal glands are supplied by larger vessels and, consequently, receive more blood, they tend to have higher temperatures, associated with the fact that they have a greater accumulation of adipose tissue and are less exposed than the most cranial mammary glands.36
It is important to remember that not all temperature increases found in mammary glands are associated with cancer, as inflammatory or infectious processes such as mastitis can cause increased blood flow and a consequent increase in temperature.37 However, thermographic temperature changes in rats can discriminate tumors from inflammation and hematomas.38
In women, signs of malignancy in breast thermographic evaluations are considered to include thermal asymmetry between the contralateral glands, localized (>2°C) or generalized (>1°C) hypothermia, and changes in the tissue surrounding the mass.16, 39 It is essential to carry out additional tests and correlate the thermographic images with the patient's history and clinical signs.
In our study, the only benign tumor (found in M2) presented a temperature of 35°C, below the mean temperature of M2 in the control group (35.23°C). According to Hobbins,40 even benign neoplasms will promote a small increase in mammary gland temperature, which is not consistent with our findings. A larger number of animals with benign mammary tumors would be necessary to evaluate this correlation. Bezerra41 stated that an increase in temperature of <1°C may suggest a benign mass.
Most tumors in this study were located in the caudal abdominal and inguinal glands, probably because they have more glandular tissue than the others, in agreement with previous studies.19, 42 In our study, the most prevalent tumor type was a simple adenocarcinoma (papillary cystic), as described in other studies.20, 42
The thermographic camera used in this study has high thermal sensitivity and also we performed the thermographic exams in a controlled temperature room making the design of the study suitable with its objective. However, this study has limitations, one is, we did not have a group with mammary benign tumors to compare with the control and malignant tumor group. Once obtaining thermographic temperatures from benign tumors, we could attach more results, so far additional research is required to confirm the potential of this technology in veterinary oncology. The other limitation is a constraint in using thermographic exams in the clinical routine, it could be the need for an appropriate room with acclimatization and the training to use the camera and the software that comes with the device.
There was some difficulty in delimiting the edges of the healthy mammary tissue during image evaluation; hence the choice of measuring the area by means of a 3000 mm2 rectangle (50 × 60 mm), which proved effective in framing the entire mammary gland for analysis. The standard rectangle used did not interfere regardless of the variety of size of the dogs because of the normally distributed size of the animals in this study.
The development of mathematical calculations and computer programs for use in veterinary medicine, as cited by several studies in humans,41, 43, 44 could increase the accuracy of thermographic examination as an auxiliary tool for mammary tumors in bitches.
Concluding, the infrared mammary thermography seems to be able to assess for the presence of neoplasia within mammary tissue. Regardless of their size and location, tumor presence significantly increases the temperature of the glands. More studies are needed to assess whether there is a positive correlation between the temperature and tumor histopathological grade, and to check the temperature difference between benign and malignant mammary neoplasms in dogs. Thus, these studies are necessary to determine the impact of this technique when adopted clinically.
Acknowledgments
This study was supported by a grant from CAPES (“Coordenação de Aperfeiçoamento de Pessoal de Nível Superior”) of the Brazilian master degree program.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This study was performed at Veterinary Teaching Hospital of Federal University of Paraná, Curitiba, Brazil.
Footnotes
Carrier®, Springer, Canoas, Brazil.
Flir Systems®, Wilsonville, USA
GraphPad software Inc®, La Jolla, CA.
References
- 1. Ng EY‐K. A review of thermography as a promising non‐invasive detection modality for breast tumor. Int J Therm Sci 2009;48:849–859. [Google Scholar]
- 2. Wang J, Chang K‐J, Chen C‐Y, et al. Evaluation of the diagnostic performance of infrared imaging of the breast: A preliminary study. Biomed Eng Online 2010;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prokoski F. History, current status, and future of infrared identification. Proceedings of the IEEE Workshop on Computer Vision Beyond the Visible Spectrum: Methods and Applications; 2000 Jun 16; Hilton Head, SC:5‐14.
- 4. Parisky YR, Sardi A, Hamm R, et al. Efficacy of computerized infrared imaging analysis to evaluate mammographically suspicious lesions. AJR Am J Roentgenol 2003;180:263–269. [DOI] [PubMed] [Google Scholar]
- 5. Fauci MA, Breiter R, Cabanski W, et al. Medical infrared imaging – Differentiating facts from fiction, and the impact of high precision quantum well infrared photodetector camera systems, and other factors, in its reemergence. Infrared Phys Technol 2001;42:337–344. [Google Scholar]
- 6. Vargas JVC, Brioschi ML, Dias FG, et al. Normalized methodology for medical infrared imaging. Infrared Phys Technol 2009;52:42–47. [Google Scholar]
- 7. Borchartt TB, Conci A, Lima RCF, et al. Breast thermography from an image processing viewpoint: A survey. Signal Process 2013;93:2785–2803. [Google Scholar]
- 8. Wishart GC, Campisi M, Boswell M, et al. The accuracy of digital infrared imaging for breast cancer detection in women undergoing breast biopsy. Eur J Surg Oncol 2010;36:535–540. [DOI] [PubMed] [Google Scholar]
- 9. Sree SV, Ng EY‐K, Acharya RU, Faust O. Breast imaging: A survey. World J Clin Oncol 2011;2:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Usuki H, Ikeda T, Igarashi Y, et al. What kinds of non‐palpable breast cancer can be detected by thermography? Biomed Thermogr 1998;18:8–12. [Google Scholar]
- 11. Amalu WC. A review of breast thermography. Breast J 1998;4:245–251.21223443 [Google Scholar]
- 12. Haberman J. The present status of mammary thermography. CA Cancer J Clin 1968;18:314–321. [PubMed] [Google Scholar]
- 13. Hoffman R. Thermography in the detection of breast malignancy. Am J Obstet Gynecol 1967;98:681–686. [DOI] [PubMed] [Google Scholar]
- 14. Stark A, Way S. The screening of well women for the early detection of breast cancer using clinical examination with thermography and mammography. Cancer 1974;33:1671–1679. [DOI] [PubMed] [Google Scholar]
- 15. Brioschi ML, Macedo JF, Macedo Rde AC. Termometria cutânea: novos conceitos. J Vasc Bras 2003;2:151–160. Brazil. [Google Scholar]
- 16. Kennedy DA, Lee T, Seely D. A comparative review of thermography as a breast cancer screening technique. Integr Cancer Ther 2009;8:9–16. [DOI] [PubMed] [Google Scholar]
- 17. Araújo M. Utilização de câmera por infravermelho para avaliação de diferentes patologias em clima tropical e uso conjunto de sistemas de banco de dados para detecção de câncer de mama [dissertation]. Recife, PE, Brazil: Federal University of Pernambuco; 2009. [Google Scholar]
- 18. Kirubha SPA, Anburajan M, Venkataraman B, et al. Evaluation of mammary cancer in 7,12‐dimethylbenz(a)anthracene‐induced Wister rats by asymmetrical temperature distribution analysis using thermography: A comparison with serum CEA levels and histopathology. J Biomed Biotechnol 2012;2012:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Withrow SJ, Lana SE, Rutteman GR. Tumors of the mammary gland In: Withrow SJ, MCEVen EG, eds. Small Animal Clinical Oncology, 4th ed St Louis, MO: Elsevier; 2007:619–636. [Google Scholar]
- 20. Oliveira FilhoJC, Fighera RA, Kommers GD, et al. Estudo retrospectivo de 1647 tumores mamários em cães. Pesq Vet Bras 2010;30:177–185. Brazil. [Google Scholar]
- 21. Keer J. Review of the effectiveness of infrared thermal imaging (thermography) for population screening and diagnostic testing of breast cancer. NZHTA Tech Brief Series 2004; 3.
- 22. Turner TA. Thermography as an aid to the clinical lameness evaluation. Vet Clin North Am Equine Pract 1991;7:311–338. [DOI] [PubMed] [Google Scholar]
- 23. Machado LFS, Dittrich RL, Pavelski M, et al. Padronização do exame termográfico nas articulações do carpo e metacarpofalangeanas de cavalos em treinamento. Arch Vet Sci 2013;18:40–454 Brazil. [Google Scholar]
- 24. Ng EY‐K, Kee EC. Integrative computer‐aided diagnostic with breast thermogram. J Mech Med Biol 2007;7:1–10. [Google Scholar]
- 25. Kontos M, Wilson R, Fentiman I. Digital infrared thermal imaging (DITI) of breast lesions: Sensitivity and specificity of detection of primary breast cancers. Clin Radiol 2011;66:536–539. [DOI] [PubMed] [Google Scholar]
- 26. Gautherie M. Thermopathology of breast cancer: Measurement and analysis of in vivo temperature and blood flow. Ann N Y Acad Sci 1980;335:383–415. [DOI] [PubMed] [Google Scholar]
- 27. Arora N, Martins D, Ruggerio D, et al. Effectiveness of a noninvasive digital infrared thermal imaging system in the detection of breast cancer. Am J Surg 2008;196:523–526. [DOI] [PubMed] [Google Scholar]
- 28. Santos LC, Lima RCF, Bezerra L, et al. Parametric analysis on the influences of tumor position and size in breast temperature profile. Proceedings of 17th International Conference on Systems, Signals and Image Processing; 2010 Jun 17‐19; Rio de Janeiro, RJ, Brazil:478‐481.
- 29. Fainaru O, Almog N, Yung CW, et al. Tumor growth and angiogenesis are dependent on the presence of immature dendritic cells. FASEB J 2010;24:1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weis SM, Cheresh DA. αv Integrins in angiogenesis and cancer. Cold Spring Harb Perspect Med 2011;1:00–00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Modiano JF, Breen M. The etiology of cancer In: Withrow SJ, MCEven EG, eds. Small Animal Clinical Oncology, 4th ed. St Louis, MO: Elsevier; 2007:3–30. [Google Scholar]
- 32. Anbar M. Clinical thermal imaging today. IEEE Eng Med Biol 1998;17:25–33. [DOI] [PubMed] [Google Scholar]
- 33. Wink DA, Vodovotz Y, Laval J, et al. The multifaceted roles of nitric oxide in cancer. Carcinogenesis 1998;19:711–721. [DOI] [PubMed] [Google Scholar]
- 34. Costa MT, Fabeni RdeC, Aptekmann KP, Machado RR. Diferentes papéis do óxido nítrico com ênfase nas neoplasias. Ciência Rural 2003;33:967–974. Brazil. [Google Scholar]
- 35. Ohsumi S, Takashima S, Aogi K, Usuki H. Prognostic value of thermographical findings in patients with primary breast cancer. Breast Cancer Res Treat 2002;74:213–220. [DOI] [PubMed] [Google Scholar]
- 36. Hedlund CS. Surgery of the reproductive and genital system In: Fossum TW, ed. Small Animal Surgery, 3rd ed St. Louis, MO: Elsevier/Mosby; 2007:702–774. [Google Scholar]
- 37. Eckersall PD, Bell R. Acute phase proteins: Biomarkers of infection and inflammation in veterinary medicine. Vet J 2010;185:23–27. [DOI] [PubMed] [Google Scholar]
- 38. Poljak‐Blazi M, Kolaric D, Jaganjac M, et al. Specific thermographic changes during walker 256 carcinoma development: Differential infrared imaging of tumor, inflammation and haematoma. Cancer Detect Prev 2009;32:431–436. [DOI] [PubMed] [Google Scholar]
- 39. Nicandro CR, Efrén MM, María Yaneli AA, et al. Evaluation of the diagnostic power of thermography in breast cancer using Bayesian network classifiers. Comput Math Methods Med 2013;2013:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hobbins WB. Thermography of the breast‐ a skin organ In: Gautherie M, Albert E, Keith L, eds. Thermal Assessment of Breast Health. Lancaster, England, UK: MTP Press; 1983:40–48. [Google Scholar]
- 41. Bezerra LA. Uso de imagens termográficas em tumores mamários para avaliação de simulação computacional [dissertation]. Recife, PE, Brazil: Federal University of Pernambuco; 2007. [Google Scholar]
- 42. Cassali GD, Lavalle GE, De Nardi AB, et al. Consensus for the diagnosis, prognosis and treatment of canine mammary tumors. Braz J Vet Pathol 2011;4:153–180. [Google Scholar]
- 43. Ng EY‐K, Sudharsan NM. Computer simulation in conjunction with medical thermography as an adjunct tool for early detection of breast cancer. BMC Cancer 2004;4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Silva SV. Reconstrução da geometria da mama a partir de imagens termográficas [dissertation]. Niterói (RJ‐Brazil): Fluminense Federal University; 2010. [Google Scholar]


