Abstract
Background
There is a markedly reduced half‐life of transfused RBCs when donor and recipient cats or humans are cross‐match incompatible. Only 10–20% of horses have naturally occurring alloantibodies. Therefore, cross‐match testing before blood transfusion is not always performed.
Hypothesis
Cross‐match incompatibility predicts shortened RBC survival time as compared to that of compatible or autologous blood.
Animals
Twenty healthy adult horses.
Methods
Prospective trial. Blood type, anti‐RBC antibody screen (before and 1 month after transfusion) and major and minor cross‐match determined 10 donor‐recipient pairs. Two pairs were cross‐match compatible, the remainder incompatible. Donor blood (4 L) was collected into citrate phosphate dextrose adenine‐1, labeled with NHS‐biotin, and transfused into recipients. Samples were collected at 1 hour and 1, 2, 3, 5, 7, 14, 21, 28, and 35 days after transfusion, and biotinylated RBCs were detected by flow cytometry. Horses were monitored for transfusion reaction during transfusion and daily for 5 days.
Results
Cross‐match incompatibility was significantly associated with decreased RBC survival time (P < .001). The half‐life of transfused incompatible (cross‐match >1+) allogenic equine RBCs was 4.7 (95% CI, 3.2–6.2) days versus 33.5 (24–43) days for compatible pairings. Cross‐match incompatibility was associated with acute febrile transfusion reaction (P = .0083). At day 30, only 1 horse had developed novel anti‐RBC antibodies.
Conclusions and Clinical Importance
Cross‐match incompatibility was predictive of febrile transfusion reaction and shortened transfused RBC survival, but did not result in production of anti‐RBC antibodies at 30 days. Cross‐match testing before transfusion is recommended.
Keywords: Alloantibodies, Agglutination, Blood type, Hemolysis, Transfusion, Transfusion reaction
Allogenic blood transfusion is an accepted method of resuscitation in horses in cases of anemia, where oxygen delivery to the tissues is below acceptable levels. Ideally blood type and cross‐match compatibility are used to determine an appropriate donor, but this often is not possible in an emergency situation. Horses have 7 known blood groups (A, C, D, K, P, Q, U) and each group can have multiple factors. When groups and factors are combined, there are ~400,000 possible equine blood types, making it nearly impossible to maintain a herd of blood donor horses that will match all potential recipients. Unlike some species, such as cats,1, 2, 3 horses infrequently develop naturally occurring alloantibodies,4 and the majority of these are against blood group antigens such as Ca which have minimal clinical effect.5, 6 However, some horses do develop alloantibodies, and anti‐Aa and Qa antibodies are most commonly associated with transfusion reaction or neonatal isoerythrolysis.7, 8, 9 A point‐of‐care equine blood‐typing method has been developed10 but is not widely used because of lack of a source of anti‐Aa and anti‐Ca antibodies. Therefore, in most emergency situations, blood‐typing is unavailable.
Cross‐matching is often more readily available in equine referral hospitals and is commonly relied upon to detect the presence of antibodies that could cause a transfusion reaction. The entire cross‐match procedure can take a few hours to complete because donor blood must be collected each time a cross‐match is performed11 and the reactions have to incubate, especially to detect hemolysins. For this reason, in emergency situations, a first transfusion is often performed without compatibility testing. Because of the low rate of spontaneous alloantibody production in horses, this transfusion is usually well tolerated, but it means the clinician has no information to predict how long the transfused cells will survive.
In horses, studies of allogenic transfusion using radiolabeling demonstrate transfused RBC survival times of 2–6 days,12 but more recent studies using biotin labeling have shown mean survival times of 89 days for autologous RBC13 and 39 days for allogenic RBC.14 This newer information suggests there could be a clinically relevant difference in response to transfusion for horses that are incompatible if compatibility predicts transfused RBC survival. Cross‐match compatibility has been reported not to predict the survival of allogenic equine RBCs measured by 59Fe‐labeling,12 but the accuracy of radiolabeling methods has been called into question by the recent results with biotin labeling and alloantibody titer has been predictive of shortened transfused RBC survival in another species.15 The predictive value of cross‐match compatibility for transfused RBC survival time has not yet been examined with the biotin label method. We hypothesized that cross‐match incompatibility would predict shortened transfused RBC survival time, when measured by biotin labeling and accounting for blood‐type compatibility. If cross‐reactivity predicts a significantly shortened RBC survival time, then it could be a useful test to estimate duration of effect of the transfusion, even if it cannot be performed pretransfusion.
Methods
Experimental Animals
Prior to initiation, approval was obtained from the University of Pennsylvania's Privately Owned Animal Protocol Review Board and Institutional Animal Care and Use Committee. The study design was a prospective clinical trial. Twenty horses were used for 10 donor‐recipient pairs; horses either acted as a donor or recipient, but not both. Horses were determined to be healthy by physical examination. Neither information was known about prior transfusion in any of these horses, nor whether any had been diagnosed with neonatal isoerythrolysis as foals.
Pretransfusion Testing
Major and minor cross‐matches were performed at the New Bolton Center Clinical Pathology laboratory using saline agglutination and hemolysin testing as previously described and shown to have highly repeatable results (Table 1).11 Donor–recipient pairings were determined based on major cross‐match results such that 2 pairs were compatible and the remaining 8 were not. Horses were blood‐typed (for blood groups A, C, K, P, Q, and U) and screened for anti‐erythrocyte lysin and agglutinin antibodies (against Aa, Ab, Ac, Af, Ca, Ka, Pa, Pb, Qa, Qb, Qc, and Ua) using standard techniques by a commercial veterinary laboratory.1 Briefly, serially diluted serum samples were incubated with equine RBCs of known blood type (and complement for the lysin assay) and the presence of agglutination and hemolysis were assessed based on visual assessment. Screening for Da, Dg, and Dk antibodies was unavailable. Anti‐erythrocyte antibody screening was repeated 28 days post‐transfusion.
Table 1.
Determination of cross‐match incompatibility by agglutination
| Degree of Incompatibility | Microscopic Appearance |
|---|---|
| 0 | No microscopic agglutination |
| 1+ | 3–5 small clumps on the slide |
| 2+ | Small and large clumps, individual cells present |
| 3+ | Many large clumps, some individual cells |
| 4+ | Macroscopic agglutination |
Blood Collection
Each donor horse was manually restrained and sedated with 0.2–0.5 mg/kg xylazine IV if needed for IV catheter placement. A 14 gauge, 2.5 inch catheter was placed retrograde in the jugular vein for blood collection and 4 L of blood was collected by gravity flow into nine 450–mL commercial blood transfer bags containing citrate phosphate dextrose adenine anticoagulant.2 The blood was stored for 0–24 hours before biotinylation and transfusion in a refrigerator with the temperature maintained at 1–4°C.
Biotinylation
Biotinylation of whole blood was performed as previously described.13, 14 Briefly, NHS‐biotin3 was dissolved in DMSO4 at a concentration of 100 mg/mL, and then biotin was diluted to 20 mg/mL in 90% DMSO solution.5 The biotin solution was sterilized by filtering the solution through 0.2‐μm pore nylon syringe filters6 and the solution was diluted with 6 volumes 0.9% saline solution.7 Blood was allowed to warm to room temperature prior to labeling with NHS‐biotin. Biotin‐DMSO‐saline solution was injected into each blood bag and agitated for 30 minutes to achieve a biotin concentration of 0.04 pg of biotin/RBC.13
Transfusion
After biotinylation, 4 L of biotinylated whole blood was transfused into each recipient. The initial rate of transfusion was set such that 60 mL total of blood was delivered over the first 15 minutes and then the transfusion was continued at a rate of 5–10 mL/kg/h.
During transfusion, horses were monitored for signs of a transfusion reaction every 5 minutes for the first 30 minutes and then every 20 minutes until transfusion was complete. Monitoring included heart rate, respiratory rate, rectal temperature, and assessment for urticaria, sweating, colic signs, trembling, or agitation. Any animal in which signs compatible with a transfusion reaction were identified was managed according to the type of identified reaction. After transfusion, complete physical examinations were performed every 24 hours for 5 days to detect delayed transfusion reactions.
Determination of Post‐Transfusion RBC Survival
At 1 hour and 1, 2, 3, 5, 7, 14, 21, 28, and 35 days after transfusion of biotinylated RBCs, 10‐mL samples of peripheral blood were collected from the jugular vein of recipient horses into tubes containing acid‐citrate‐dextrose.7 As a result of rapid decline in surviving labeled RBCs, horses later in the study were sampled up to every 12 hours to improve precision. Sampling was discontinued when the percentage of biotin‐positive cells was <0.5%. Nonbiotinylated blood was used as a negative control and biotinylated blood pooled from the blood bags immediately before transfusion was used as the positive control. Blood was diluted and analyzed by flow cytometry8 in triplicate using streptavidin‐phycoerythrin9 labeling as previously described.13
Statistical Analysis
A power analysis was performed based on prior allogenic red blood cell survival times using the same method of detection.14 An expected sample mean of 21 day lifespan was estimated and the control mean of 39 day lifespan14 was used. The level of significance was set at 5% (P < .05) and the standard deviation was estimated at 25% or 10 days. With these parameters, a sample size of 8 horses gives 100% statistical power. If the sample mean is only reduced to 30 days survival, then the power is 82%.
The percentage of labeled RBCs at 1 hour was used as the baseline or 100% value as sequestration immediately after transfusion could result in a falsely low baseline value at earlier timepoints.13, 14 Tukey's ladder test confirmed continuous data conformed to the normality assumption. The mean ± SD of 24‐hour survival percentage was compared between control and test horses by t‐test. The survival curve of labeled RBCs was determined by dividing the percentage of labeled cells at each time by the mean percentage of biotin‐labeled cells in circulation at the baseline time point. Linear regression was used to determine the RBC lifespan (x‐intercept values of 0% biotinylation) and RBC half‐life (50% biotinylation) for cross‐match compatible horses and incompatible horses. The regression line was forced through the point y = 100%, x = 0. Multivariate linear regression was used to determine whether age, sex, or percent labeling of transfused RBC (positive control) influenced the transfused RBC half‐life. The association of individual transfusion reaction types with cross‐match was investigated by Fisher's exact test. Commercial statistical software was used for all analysis,10 and a P‐value of <.05 was used to denote significance.
Results
Thirty‐three horses were screened for blood type and cross‐match compatibility and 20 horses (10 donor‐recipient pairs) were entered in the study. Seventeen were Thoroughbreds and 3 were other breeds. The final pairings were determined to create 2 completely blood type and cross‐match compatible transfusions (controls) and 8 cross‐match incompatible transfusions. Two horses had unidentified anti‐RBC antibodies with 1+ or 2+ major hemagglutination reaction and no hemolysin reaction. Six horses had anti‐Ca antibodies with 4+ major agglutination and positive hemolysin reactions. Additional incompatibilities in blood type, where the donor had a blood type that the recipient did not, included 5 instances of Ua, 3 of Qabc, 2 of Ka, 1 of Ab, and 1 of Pa (Table S1).
Transfusion reactions were recorded in 7/8 cross‐match incompatible transfusions. The horse with 1+ agglutination on cross‐match showed no transfusion reaction. Neither control horse showed evidence of transfusion reaction during or after transfusion. Mild fever (defined as >1°C increase from baseline, range 38.5–39.5°C; occurred in 6/8) and tachycardia of 48–60 beats/min (7/8) were significantly associated with cross‐match >1+ (P < .033). No other transfusion reactions were statistically associated with cross‐match incompatibility. One horse developed moderate colic signs 25 minutes into the transfusion and was administered phenylbutazone (4.4 mg/kg IV). Although this horse did not meet the criteria for fever, the temperature increased to 38.6°C despite administration of phenylbutazone. Fevers were first noted 45 minutes to 25 hours after completion of the transfusion and lasted up to 2 days. Except for the horse mentioned, no horses were administered antipyretics or anti‐inflammatory medications. Additional signs of transfusion reaction were urticaria or pruritis (3/8, 1 was treated with a single dose of diphenhydramine [1 mg/kg IM]), tachypnea (2/8), sweating and muscle fasciculations (1/8), transient systolic cardiac murmur (1/8), arrhythmia (2/8), and mild icterus (3/8). No horse developed anaphylactic signs nor evidence of severe hemolysis (icterus, pigmenturia) within the first 5 days after transfusion.
The mean standard deviation of proportion RBC labeled for triplicate samples at all time points and for all horses was 0.11% (range 0–0.44%). In 1 control horse, the positive control was confounded because of sampling error. Only 3.38 ± 2.8% of cells from the positive control sample were labeled, however, this horse showed similar percent of labeled cells at 1 hour after transfusion to other horses in the study, demonstrating adequate labeling of the cells had occurred. For the remaining horses, the mean ± SD percent of labeled cells before transfusion (positive control) was 85 ± 15%. The baseline percent RBC labeled 1 hour after transfusion was 3.5 ± 1%. The 24 hour survival was 88.7 ± 10.0% of baseline and there was no difference between control and test horses.
RBC half‐life and survival times were significantly shorter for each increase in cross‐match reaction from 1+ to 4+ (P ≤ .024; Table 2). The age and sex were not confounders. Differences in blood cell survival times in blood stored 24 versus 0 hours were not performed due to small sample size and a previous report that 24 hours of storage did not significantly change transfused RBC survival.13 Higher pretransfusion percent labeling was associated with longer half‐life (P = .012). Cross‐match incompatible horses showed rapid declines in transfused RBC immediately or starting between 3 and 5 days (Fig 1). Conversely, the control horses showed very gradual decline out to the end of the study period. The horse with 1+ major agglutination and unidentified anti‐RBC antibodies appeared to be an outlier, showing a pattern very like the control horses. The predicted elimination curves are presented (Fig 2).
Table 2.
The half‐life of transfused allogenic equine RBC. Presented based on degree of cross‐match compatibility. A single horse with cross‐match = 1+ had a RBC half‐life similar to control horses. This horse was eliminated from analysis in the cross‐match ≥2+ group. Any cross‐match incompatibility resulted in significantly reduced half‐life of transfused RBC (P < .025)
| Major Agglutination Cross‐Match Reaction | Half‐Life Days (95% CI) | n |
|---|---|---|
| 0 | 33.5 (24–43) | 2 |
| ≥1+ | 10.9 (1.1–21) | 8 |
| ≥2+ | 4.7 (3.2–6.2) | 7 |
Figure 1.
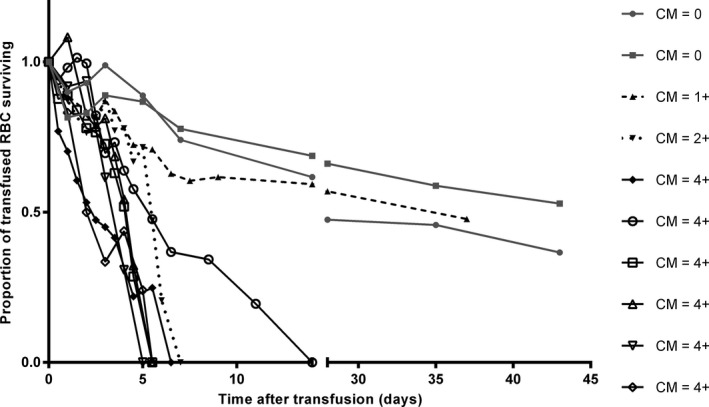
RBC elimination exact data points for each animal after transfusion of allogenic biotinylated RBC that were blood type and cross‐match compatible (CM 0) or incompatible (CM > 0). Data expressed as the proportion of baseline biotinylated RBC remaining at each time. Time 0 was 1 hour after the completion of the infusion.
Figure 2.
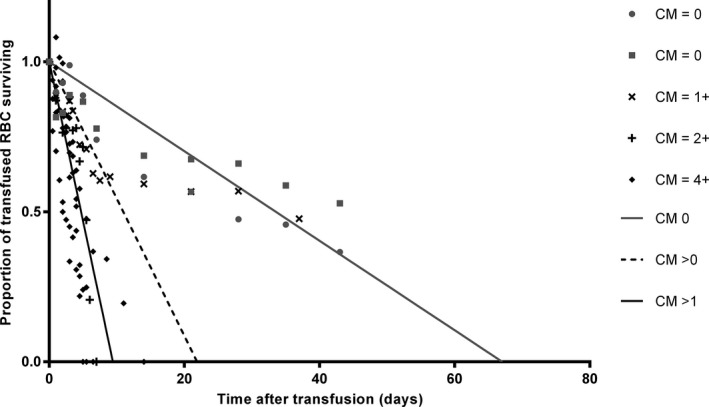
Linear regression predictions of RBC elimination following transfusion of biotinylated allogenic RBC. Predictions were made for horses that were blood type and cross‐match compatible (CM 0), incompatible to any degree (CM > 0), or incompatible with cross‐match major agglutination reaction >1+ (CM > 1). The last group is shown to eliminate the effect of a single horse with cross‐match = 1+ that showed RBC elimination similar to the control horses. Data expressed as the proportion of baseline biotinylated RBC remaining. Time 0 was the completion of the infusion. The line was forced through y = 1, x = 0. The intersection of each curve with the 0.5 line represents the half‐life and the x‐intercept represents the survival time. The elimination rates were significantly different between the compatible and the cross‐match >1+ groups (P = .029).
At 1 month after transfusion, only 1 horse had developed anti‐RBC antibodies to an additional blood type (Table S1). This was a control horse that developed antibodies to an unidentified blood type. In the 6 other recipients having known additional incompatible blood types there was no production of antibodies to these other blood types.
Discussion
This study demonstrates that there is a marked effect of cross‐match incompatibility on survival time of transfused blood in adult horses, resulting in a 7‐fold shorter half‐life for incompatible allogenic RBCs compared to compatible RBCs. In this study, cross‐match incompatibility was always present in the agglutination test whether or not hemolysins were detected, so cross‐match can be useful to predict RBC survival even in clinics that do not have access to hemolysin testing. Although we found a longer RBC half‐life for the compatible transfusions (33.5 days) in this study than has been previously reported for allogenic transfusions in horses (20 days),14 the sample size (n = 2) limits interpretation of this value.
Red blood cell production and recovery from acute hemorrhage can be prolonged, so it would be ideal if a single transfusion could support a horse through the entire recovery period. In a model of a single episode of 15 mL/kg blood loss, the hematocrit remained significantly reduced until 10 days after blood loss. In the same study using a model of severe hemorrhage (15 mL/kg blood loss on 3 consecutive days) the hematocrit remained significantly reduced until 28 days following blood loss, though this was a controlled volume‐based model of hemorrhage rather than an oxygen carrying capacity based model or hematocrit‐target based model.16 Cross‐match and blood‐type compatible allogenic RBC have a reported half‐life of 20 days, which would be expected to be clinically useful in a similar situation.14 The 4.7 day half‐life of agglutination >1+ incompatible transfusions that we found suggests that unmatched blood has limited clinical utility beyond acute patient stabilization.
No horse with known additional instances of blood‐type incompatibility (6 horses, 11 instances) developed new anti‐RBC antibodies after transfusion. This could be because most equine blood types have low antigenicity and require repeated exposure to induce antibody formation.17 A previous study of equine blood transfusion has also demonstrated blood types P and Q to be minimally immunogenic, with most sensitization occurring in the A and D blood groups (groups K and U were not tested).18 Blood group D is the most likely group to be involved in the 3 unidentified anti‐RBC antibodies detected in this study, including the one that developed after transfusion. Unfortunately blood group D testing was not available at the time of this study. The remaining incompatibilities that did not induce antibody production were in the K, P, Q, and U blood groups. The importance of this finding is that it suggests that a previously used donor (or donor with the same allogenic blood type as the original donor) can provide subsequent blood for a recipient with minimal concern that the previous transfusion will have sensitized the recipient to the nonmatching allogenic RBC antigens. However, it is probably prudent to repeat the cross‐match procedure before a second transfusion as the small numbers in this study do not guarantee that these results are generalizable in every case.
This study found that cross‐match incompatibility was predictive of transfusion reaction. The degree of reaction was mild and did not prevent completion of the transfusion, despite 6 recipients showing 4+ agglutination and strongly positive hemolysin testing against the donor blood. There is disagreement in the literature about how reliable cross‐match is to predict transfusion reaction, especially life‐threatening reactions.12, 19, 20 Some reports suggest that cross‐match in horses does not accurately estimate the risk of transfusion reaction.12, 20 In 1 study, 2 of 4 horses that received cross‐match compatible blood had anaphylactic transfusion reactions, while 2 of 2 with incompatible blood transfusions did not have reactions.12 On the other hand, another retrospective study reported that 4 of 6 horses with transfusion reactions had incompatibilities on cross‐match, and they suggested cross‐match is predictive of reaction.21 Reactions can occur secondary to leukocytes, platelets, proteins, or poorly described red blood cell antigens that are not detected in current compatibility testing.21 Although cross‐match results predicted mild reactions in this study, they were not strongly predictive of anaphylaxis or life‐threatening transfusion reaction, although the study was underpowered to detect a small increase in relative risk and lacked cases of Aa or Qa incompatibility.
A major limitation of our study was that the majority of horses tested here were Thoroughbreds and had anti‐Ca antibodies, which are known to be less clinically important than anti‐Aa or anti‐Qa antibodies.5, 22 It would have been ideal to include Aa negative horses (such as many Standardbreds) as they might have been more likely to have anti‐Aa antibodies; the mean RBC survival times and transfusion reaction rate from this group of predominantly Thoroughbred horses may not be fully applicable to other breeds. Our results should not be generalized to other breeds that might be Aa negative and more likely to have anti‐Aa antibodies, nor taken to mean that it is safe to transfuse known cross‐match incompatible blood. Rather, it means that cross‐match cannot distinguish between clinically relevant reactions and those that could be tolerated by a transfusion recipient if required during an emergency. Conversely, one of the experimental horses had a response to transfused RBCs that mirrored the control cases. This horse had 1+ major hemagglutination reaction to the donor blood with no hemolysis, but on antibody testing had unidentified anti‐RBC antibodies. It is possible that the cross‐match was a false positive and this horse received a compatible transfusion, or, if the transfusion was incompatible, then the antibodies present had minimal effect on transfused RBC survival time.
In conclusion, we recommend using cross‐match testing when administering an allogenic blood transfusion to a horse, as it can predict the likelihood of the recipient developing a transfusion reaction. Even if cross‐match must be performed concurrent with or after the actual transfusion because of time constraints, cross‐match testing still has a place to predict the survival of the transfused RBCs and the expected clinical response.
Supporting information
Table S1. Donor‐recipient pairings by blood type.
Acknowledgments
Dave Lorom and Sue Lindborg for technical assistance.
Grant Support: Raymond Firestone Research Trust.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
William R. Pritchard Veterinary Medical Teaching Hospital (UC Davis) Hematology Laboratory, Davis, CA.
CPDA‐1 450 mL blood pack unit, Fenwal Inc, Lake Zurich, IL.
NHS‐biotin, ApexBio, Houston, TX.
Hybri‐max DMSO solution, Sigma Chemical Company, St. Louis, MO.
DMSO solution, Fort Dodge Animal Health, Fort Dodge, IA.
0.2 µm pore nylon syringe filters, Fisher Scientific, Pittsburgh, PA.
ACD evacuated blood tubes, Baxter, Deerfield, IL.
FACScan, Becton, Dickinson and Company, Franklin Lakes, NJ.
Streptavidin phycoerythrin, BioLegend, San Diego, CA.
Stata 12.0, StataCorp, College Station, TX.
References
- 1. Bucheler J, Giger U. Alloantibodies against A and B blood types in cats. Vet Immunol Immunopathol 1993;38:283–295. [DOI] [PubMed] [Google Scholar]
- 2. Gurkan M, Arikan S, Ozaytekin E, Dodurka T. Titres of alloantibodies against A and B blood types in non‐pedigree domestic cats in Turkey: Assessing the transfusion reaction risk. J Feline Med Surg 2005;7:301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arikan S, Akkan HA. Titres of naturally occurring alloantibodies against feline blood group antigens in Turkish van cats. J Small Anim Pract 2004;45:289–292. [DOI] [PubMed] [Google Scholar]
- 4. Gilman MA, Schwarz A, Wallerstein H. Immunohematologic studies of the thoroughbred horse. Am J Vet Res 1960;21:393–396. [PubMed] [Google Scholar]
- 5. Bailey E. Prevalence of anti‐red blood cel antibodies in the serum and colostrum of mares and its relationship to neonatal isoerythrolysis. Am J Vet Res 1982;43:1917–1921. [PubMed] [Google Scholar]
- 6. Suzuki Y, Stormont C, Trommershausen‐Smith A. Alloantibodies: The blood groups they define. Proceedings of 1st International Symposium of Equine Hematology 1975;1:34–41. [Google Scholar]
- 7. Zaruby JF, Hearn P, Colling D. Neonatal isoerythrolysis in a foal, involving anti‐Pa alloantibody. Equine Vet J 1992;24:71–73. [DOI] [PubMed] [Google Scholar]
- 8. Whiting J, David J. Neonatal isoerythrolysis. Compend Contin Educ Vet 2000;22:968–975. [Google Scholar]
- 9. de Graaf‐Roelfsema E, van der Kolk JH, Boerma S, van Haeringen H. Non‐specific haemolytic alloantibody causing equine neonatal isoerythrolysis. Vet Rec 2007;161:202–204. [DOI] [PubMed] [Google Scholar]
- 10. Owens SD, Snipes J, Magdesian KG, Christopher MM. Evaluation of a rapid agglutination method for detection of equine red cell surface antigens (Ca and Aa) as part of pretransfusion testing. Vet Clin Pathol 2008;37:49–56. [DOI] [PubMed] [Google Scholar]
- 11. Harris M, Nolen‐Walston R, Ashton W, et al. Effect of sample storage on blood crossmatching in horses. J Vet Intern Med 2012;26:662–667. [DOI] [PubMed] [Google Scholar]
- 12. Kallfelz FA, Whitlock RH, Schultz RD. Survival of 59Fe‐labeled erythrocytes in cross‐transfused equine blood. Am J Vet Res 1978;39:617–620. [PubMed] [Google Scholar]
- 13. Owens SD, Johns JL, Walker NJ, et al. Use of an in vitro biotinylation technique for determination of posttransfusion survival of fresh and stored autologous red blood cells in thoroughbreds. Am J Vet Res 2010;71:960–966. [DOI] [PubMed] [Google Scholar]
- 14. Mudge MC, Walker NJ, Borjesson DL, et al. Post‐transfusion survival of biotin‐labeled allogeneic RBCs in adult horses. Vet Clin Pathol 2012;41:56–62. [DOI] [PubMed] [Google Scholar]
- 15. Socha WW, Rowe AW, Lenny LL, et al. Transfusion of incompatible blood in rhesus monkeys and baboons. Lab Anim Sci 1982;32:48–56. [PubMed] [Google Scholar]
- 16. Radin MJ, Eubank MC, Weiser MG. Electronic measurement of erythrocyte volume and volume heterogeneity in horses during erythrocyte regeneration associated with experimental anemias. Vet Pathol 1986;23:656–660. [DOI] [PubMed] [Google Scholar]
- 17. Hata R, Sonoda M. Clinical and hematological observations on repeated experimental blood transfusions in horses. Exp Rep Equine Hlth Lab 1974;11:133–151. [Google Scholar]
- 18. Wong PL, Nickel LS, Bowling AT, Steffey EP. Clinical survey of antibodies against red blood cells in horses after homologous blood transfusion. Am J Vet Res 1986;47:2566–2571. [PubMed] [Google Scholar]
- 19. Durham AE. Blood and plasma transfusion in the horse. Equine Vet Educ 1996;8:8–12. [Google Scholar]
- 20. Tocci LJ, Ewing PJ. Increasing patient safety in veterinary transfusion medicine: An overview of pretransfusion testing. J Vet Emerg Crit Care (San Antonio) 2009;19:66–73. [DOI] [PubMed] [Google Scholar]
- 21. Hurcombe SD, Mudge MC, Hinchcliff KW. Clinical and clinicopathologic variables in adult horses receiving blood transfusions: 31 cases (1999–2005). J Am Vet Med Assoc 2007;231:267–274. [DOI] [PubMed] [Google Scholar]
- 22. Bailey E, Albright DG, Henney PJ. Equine neonatal isoerythrolysis: Evidence for prevention by maternal antibodies to the Ca blood group antigen. Am J Vet Res 1988;49:1218–1222. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Donor‐recipient pairings by blood type.


