Abstract
Background
There are no clear treatment guidelines for dogs with clinically well‐regulated hyperadrenocorticism in which serum cortisol concentrations before and after an ACTH stimulation test performed 3–6 hours after trilostane administration are < 2.0 μg/dL.
Objective
To determine if serum cortisol concentrations measured before (Pre1) and after (Post1) ACTH stimulation at 3–6 hours after trilostane administration are significantly lower than cortisol concentrations measured before (Pre2) and after (Post2) ACTH stimulation 9–12 hours after trilostane administration, in a specific population of dogs with clinically well‐regulated hyperadrenocorticism and Pre1 and Post1 <2 μg/dL.
Animals
Thirteen client‐owned dogs with clinically well‐regulated hyperadrenocorticism and Pre1 and Post1 serum cortisol concentrations <2.0 μg/dL 3–6 hours after trilostane administration.
Methods
Prospective study. Dogs had a second ACTH stimulation test performed 9–12 hours after trilostane administration, on the same day of the first ACTH stimulation test. Cortisol concentrations before and after ACTH stimulation were compared using a paired t‐test.
Results
Cortisol concentrations before (1.4 ± 0.3 μg/dL) and after the first stimulation (1.5 ± 0.3 μg/dL, mean ± SD) were significantly lower than cortisol concentration before the second stimulation (3.3 ± 1.6 μg/dL, P = .0012 each). Cortisol concentration before the first stimulation was also significantly lower than cortisol concentration after the second stimulation (5.3 ± 2.4 μg/dL, P = .0001).
Conclusions and clinical importance
In dogs with clinically well‐regulated, trilostane‐treated, hyperadrenocorticism, and cortisol concentrations <2 μg/dL before and after the first stimulation, a second ACTH stimulation test performed 9–12 hours after treatment can result in higher cortisol concentrations that could support continued trilostane treatment.
Keywords: Adrenocorticotropic hormone stimulation test, Cushing's disease, Hyperkalemia, Hypoadrenocorticism, lymphocytosis
Abbreviations
- SD
standard deviation
- Pre1
cortisol concentrations measured before ACTH stimulation at 3–6 hours after trilostane treatment
- Post1
cortisol concentrations measured after ACTH stimulation at 3–6 hours after trilostane treatment
- Pre2
cortisol concentrations measured before ACTH stimulation at 9–12 hours after trilostane treatment
- Post2
cortisol concentrations measured after ACTH stimulation at 9–12 hours after trilostane treatment
It is currently not known how to interpret the results of an ACTH stimulation test performed 3–6 hours after trilostane administration, when serum cortisol concentration after ACTH stimulation is <2 μg/dL and the dog has clinically well‐regulated hyperadrenocorticism. It could be advisable to continue treating the dog with the trilostane dose that achieved clinical control, or serum cortisol concentration after ACTH stimulation of <2 μg/dL could warrant a decrease or discontinuation of the drug because of concern of inducing hypoadrenocorticism.1, 2, 3 Higher serum cortisol concentrations measured before and after an ACTH stimulation test performed 9–12 hours after trilostane administration in this particular population of dogs, could justify continued use of trilostane without a dose change.
Serum cortisol concentrations before and after ACTH stimulation in trilostane‐treated dogs with hyperadrenocorticism are lower at 3 hours after trilostane administration compared to 9 hours after drug administration.4 However, this occurred in a mixed population of dogs including dogs with clinical signs indicative of poorly regulated hyperadrenocorticism and a wide range of serum cortisol concentrations before and after ACTH stimulation. The specific population of dogs with clinically well‐regulated hyperadrenocorticism and serum cortisol concentrations <2 μg/dL before and after ACTH stimulation at 3–6 hours after trilostane administration has not yet been studied.
The goal of this prospective study was to compare results of ACTH stimulation tests performed 3–6 hours after trilostane administration to those obtained 9–12 hours after trilostane administration, in a specific population of dogs with clinically well‐regulated hyperadrenocorticism in which serum cortisol concentrations are <2 μg/dL 3–6 hours after trilostane administration. It was hypothesized that before and after ACTH stimulation serum cortisol concentrations measured 9–12 hours after trilostane administration would be significantly higher than serum cortisol concentrations measured before and after ACTH stimulation 3–6 hours after trilostane administration. The clinical rationale for the study was that higher serum cortisol concentrations at 9–12 hours after trilostane administration could justify continued, unchanged trilostane treatment, in the specific population of dogs with well‐regulated hyperadrenocorticism in which serum cortisol concentrations at 3–6 hours after trilostane administration are low.
Materials and Methods
Dogs were prospectively enrolled into the study from the population of dogs visiting the Mathew J. Ryan Veterinary Hospital of the University of Pennsylvania between April 6, 2012–April 16, 2014. Dogs were defined as having clinically well‐regulated hyperadrenocorticism if they had normal drinking, urination, eating, and activity level, as reported by the owner. All of the hospital's ACTH stimulation test results of dogs treated for hyperadrenocorticism, were reviewed daily during the study period. All ACTH stimulation tests are routinely performed 3–6 hours after trilostane treatment, at this hospital. Owners of dogs with clinically well‐regulated hyperadrenocorticism and serum cortisol concentrations <2 μg/dL before and after ACTH stimulation at 3–6 hours after trilostane administration were contacted while the dog was in the hospital. The study protocol was reviewed with the owner and informed consent was obtained. As part of the study, the dog remained at the hospital for the duration of the day. A second ACTH stimulation test was performed that same day, 9–12 hours after the morning trilostane administration. Only dogs treated with Vetoryl® were included in the study.1 Serum cortisol concentrations were measured before and 1 hour after IV administration of refrigerated 5 μg/kg ACTH.,2,3 Synthetic ACTH was reconstituted with 0.9% sodium chloride solution as per the manufacturer's instructions, and was refrigerated for no longer than 14 days.5 The study and consent form were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Dogs were excluded from the study if they had clinical signs of hypoadrenocorticism or poorly regulated hyperadrenocorticism. Clinical signs suggestive of hypoadrenocorticism were lethargy, vomiting, diarrhea, or anorexia. Clinical signs suggestive of poorly regulated hyperadrenocorticism were polyuria, polydipsia, polyphagia, or excessive panting. Dogs with well‐regulated HAC were excluded if serum cortisol concentrations measured before or after ACTH stimulation 3–6 hours after trilostane administration were ≥ 2 μg/dL. Dogs treated with lysodren or a trilostane product that was not Vetoryl® were also excluded. Finally, dogs in which a diagnosis of HAC could not be confirmed by review of the medical record and dogs belonging to owners who declined participation were excluded.
In addition to the 2 ACTH stimulation tests, each dog had a complete blood count and serum chemistry panel performed on the day of enrollment into the study. 4,5 Historical clinical signs and prior adrenal axis testing were reviewed to confirm the diagnosis of hyperadrenocorticism. Adrenal axis tests were performed and results were interpreted as previously described.6 Presence of concurrent disorders and the type of hyperadrenocorticism did not influence enrollment. However, when data regarding the type of hyperadrenocorticism diagnosed were available, they were recorded. Endogenous ACTH concentration >8.8 pmol/L was considered consistent with pituitary‐dependent hyperadrenocorticism.6,7, 6 A 4 hour serum cortisol concentration <1.4 μg/dL after IV administration of 0.01 mg/kg dexamethasone was also considered consistent with pituitary‐dependent hyperadrenocorticism in dogs with confirmed hyperadrenocorticism.6 Follow‐up data regarding continued trilostane treatment after completion of the study were reviewed when available.
All continuous variables (including the cortisol measurements) were assessed for normality using the Shapiro–Wilks test. The cortisol data were normally distributed and hence mean (±standard deviation [SD]) before and after serum cortisol concentrations from the first and second ACTH stimulation tests were calculated and designated Pre1, Post1 and Pre2, Post2, respectively. Pre1 and Pre2 concentrations were compared to one another and Post1 concentrations were compared to Pre2 and Post2 concentrations using the paired t‐test. Cortisol concentrations <1 μg/dL were assigned a value of 1 μg/dL. All other continuous variables were not normally distributed and are reported as median and range. A P value < .05 was considered significant. All statistical analyses were performed using a statistical software package.8
Results
Thirteen dogs met the inclusion criteria and were enrolled into the study. During the study period 335 ACTH stimulation tests were performed on 80 dogs treated for hyperadrenocorticism. Eighty‐three of these 335 ACTH stimulation tests were performed on the 13 study dogs either during the study (26 ACTH stimulations) or at other times (57 ACTH stimulations). The remaining 252 ACTH stimulation tests were performed on 67 dogs that were excluded from the study for the following reasons: dogs were treated with Vetoryl® but their serum cortisol concentrations before or after ACTH stimulation were ≥2 μg/dL (49 dogs), treatment with lysodren (9 dogs), or treatment with compounded trilostane (7 dogs). Two additional dogs treated with Vetoryl® were excluded despite before and after ACTH stimulation cortisol concentrations <2 μg/dL because they had clinical signs consistent with hypoadrenocorticism. One of the 9 lysodren‐treated dogs also had before and after ACTH stimulation cortisol concentrations <2 μg/dL and clinical signs consistent with hypoadrenocorticism.
Pre1 and Post1 cortisol concentrations (1.4 ± 0.3 μg/dL, range 1–1.9 μg/dL and 1.5 ± 0.3 μg/dL, range 1–1.9 μg/dL, Mean, SD, respectively) were significantly lower than Pre2 cortisol concentration (3.3 ± 1.6 μg/dL, range 1.4–6.3 μg/dL, P = .0012 for each comparison). Post1 cortisol concentration (1.5 ± 0.3 μg/dL) was also significantly lower than Post2 cortisol concentration (5.3 ± 2.4 μg/dL, range 2.8–9.2 μg/dL, P = .0001). When single values of individual dogs were reviewed, it was noted that Post2 cortisol concentration was higher than Post1 cortisol concentration in all dogs. Pre2 cortisol concentration was higher than Pre1 cortisol concentration in 10 of 13 dogs (77%), unchanged in 2 of 13 dogs (15%), and lower than Pre1 cortisol concentration in 1 dog (8%, Fig 1).
Figure 1.
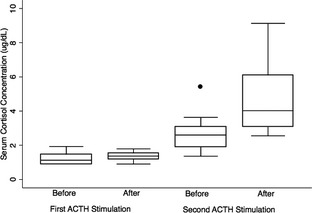
Box and Whisker plot of serum cortisol concentrations before and after two ACTH stimulation tests performed on the same day 3–6 hours after trilostane was administered (first stimulation in red and blue) and 9–12 hours after trilostane was administered (second stimulation in green and orange). The line within the box represents the median, the box represents the interquartile range, whiskers represent the most extreme values within 1.5 of the nearer quartile, and the single point represents an outlier. Cortisol concentrations <1 μg/dL were assigned a value of 1 μg/dL.
Seven of the 13 study dogs were neutered females, 5 were neutered males, and one was an intact male. Age of the 13 study dogs was 9.9 years (median, range 7.5–12.8 years) and body weight was 11 kg (median, range 4–41 kg). Potassium concentration in the 13 study dogs was 5 mmol/L (median, range 4.1–5.9 mmol/L, reference range 4–5.2 mmol/L) and 4 of the 13 dogs had a potassium concentration > 5.2 mmol/L. Sodium to potassium ratio in the 13 study dogs was 29.4 (median, range 24.6–35.6) and 4 of the 13 dogs had a sodium to potassium ratio < 27. The 4 dogs with potassium concentration >5.2 mmol/L were the same 4 dogs that had sodium to potassium ratio < 27. Lymphocyte count in the 13 study dogs was 1.4 cells × 103/μL (median, range 0.7–2.2 cells × 103/μL, reference interval 0.9–5.5 cells × 103/μL) and in the 4 dogs with sodium to potassium ratio <27 the lymphocyte count was 1.8 cells × 103/μL (median, range 1.2–2.2 cells × 103/μL).
The total daily administered trilostane dose was 7.3 mg/kg (median, range 1.6–18.9 mg/kg). This dose was divided and given every 12 hours to all dogs. Dogs had been treated with trilostane for 154 days (median, range 14–631 days) before enrollment into the study. Duration of trilostane treatment with the specific dose given on the day of enrollment into the study was 44 days (range 14–577 days) before enrollment into the study. Follow‐up data were available for review in 12 dogs. These 12 dogs remained on the same trilostane dose given on the study day for 152 days (range 70–786 days) after enrollment into the study, and the subset of 4 dogs with sodium to potassium ratio <27 remained treated with the same dose of trilostane for 93 days (range 88–224 days). Four of the 12 dogs were eventually lost to follow‐up, 3 dogs continue to be treated and monitored on the same dose of trilostane, 1 dog required an increase in the trilostane dose, another required a decrease in the trilostane dose, a different dog was switched to compounded trilostane, another was switched to lysodren, and 1 dog was euthanized for unrelated reasons 6 months after the last trilostane dose follow‐up. Pituitary‐dependent hyperadrenocorticism was diagnosed in 6 dogs based on endogenous ACTH concentration and in two other dogs based on results of the low dose dexamethasone suppression test. In 5 additional dogs the type of hyperadrenocorticism was not known.
Seven of the 13 study dogs received medications other than trilostane. These medications included oral tramadol, levothyroxine, ursodiol, potassium citrate, nitrofurantoin, phenylpropanolamine, firocoxib, cetirizine, brewer's yeast, milk thistle, and fish oil, ophthalmic cyclosporine, and subcutaneous NPH insulin and vitamin B. Potassium concentration in the dog receiving potassium citrate was 4.8 mmol/L.
Discussion
This study focused on a specific population of dogs with clinically well‐regulated, trilostane‐treated hyperadrenocorticism. The specific population of study dogs was further characterized by before and after ACTH stimulation cortisol concentrations <2 μg/dL when the test was performed 3–6 hours after trilostane administration. Results of this study suggest that in the population examined, serum cortisol concentrations measured before and after ACTH stimulation 9–12 hours after trilostane administration are significantly higher than those measured at 3–6 hours after trilostane administration. Although several other studies have documented that serum cortisol concentrations measured before and after ACTH stimulation are lower shortly after trilostane administration compared to several hours later, these studies examined a heterogeneous population of trilostane‐treated dogs with hyperadrenocorticism.2, 4, 7, 8 The present study is unique because it was performed in a particular subset of trilostane‐treated dogs with well‐regulated hyperadrenocorticism and low cortisol concentrations shortly after trilostane administration. Higher cortisol concentrations measured later in the day could help resolve the current ambiguity regarding treatment recommendations and could support continued trilostane treatment at the same dose. The fact that 12 study dogs remained on the same trilostane dose given on the study day for 152 days after enrollment into the study also suggests that continued trilostane treatment at the same dose, could be beneficial for these dogs. However, larger and longer studies might be needed to confirm these findings.
The lack of clear recommendations regarding the adjustment of trilostane dose in dogs with clinically well‐regulated hyperadrenocorticism and low cortisol concentrations is reflected in the literature. The protocol of one study examining trilostane treatment in 47 dogs with hyperadrenocorticism left the decision of whether to decrease the trilostane dose in dogs with clinically well‐regulated hyperadrenocorticism and after ACTH stimulation cortisol concentration <1.5 μg/dL to the discretion of the veterinarian.3 A second study of 14 dogs with trilostane‐treated hyperadrenocorticism called for a decrease in trilostane dose if after ACTH stimulation cortisol concentration was <1 μg/dL, regardless of clinical signs.1 A third study of 32 trilostane‐treated dogs with hyperadrenocorticism endorsed a decrease in trilostane dose if after ACTH stimulation cortisol concentration was <2 μg/dL, regardless of clinical signs.2
This study also raises the question of whether it could be helpful to perform follow‐up monitoring by measuring baseline serum cortisol concentrations later in the day in the specific population of clinically well‐regulated, trilostane‐treated dogs, in which serum cortisol concentrations measured before and after ACTH stimulation at 3–6 hours after medication are known to be low. Future studies monitoring this particular population of dogs over a longer period of time by measuring baseline cortisol concentration 9–12 hours after trilostane administration in lieu of an ACTH stimulation test earlier in the day, are needed. This mode of monitoring would be considered only if the dog continued to be clinically well‐regulated on the same dose of trilostane and only when low cortisol concentrations 3–6 hours after trilostane administration have been documented. It is important to emphasize that in this population of dogs, some form of follow‐up cortisol measurement, such as baseline cortisol concentration or full ACTH stimulation at 9–12 hours after trilostane administration is always necessary, to confirm that the dose of trilostane can be continued safely. It is also important to note that baseline cortisol concentration at 9–12 hours after trilostane administration was higher than baseline cortisol concentration 3–6 hours after trilostane administration in only 10 of 13 dogs (77%). Therefore, a baseline cortisol concentration at 9–12 hours might be helpful for treatment decisions only in a subset of this specific population of dogs, in which baseline cortisol concentration at 9–12 hours after trilostane administration is >2 μg/dL. If baseline cortisol concentration at 9–12 hours after trilostane administration is still <2 μg/dL, a full ACTH stimulation test at 9–12 hours is needed in order to confirm that the dog is able to produce more than baseline cortisol.
Low cortisol concentrations in a trilostane‐treated dog raise the concern for life‐threatening suppression of adrenal function. None of the study dogs, including the 4 dogs with low sodium to potassium ratio and hyperkalemia, developed clinical signs consistent with iatrogenic hypoadrenocorticism during the follow‐up period. Although hyperkalemia does not necessarily imply low aldosterone concentrations in trilostane‐treated dogs, special attention was given to dogs with a sodium to potassium ratio < 27, because this value can help screen for hypoadrenocorticism.1, 9, 10 Lymphocyte counts were also examined because the combination of the lymphocyte count and low sodium to potassium ratio is a better hypoadrenocorticism screening tool than the sodium to potassium ratio alone.10 The lymphocyte count of 4 dogs with low sodium to potassium ratio was higher than the lymphocyte count of all 13 study dogs. This subset of 4 dogs continued unchanged trilostane treatment for 3 months after the documentation of low cortisol and low sodium to potassium ratio with no clinical evidence of iatrogenic hypoadrenocorticism. It is therefore concluded that sodium to potassium ratio, potassium concentration, and lymphocyte count should be interpreted with caution in trilostane‐treated dogs, especially if clinical signs are not consistent with hypoadrenocorticism. Aldosterone concentrations were not measured in this study, but other studies have shown that aldosterone concentration does not correlate with electrolyte concentrations in trilostane‐treated dogs.9 Future studies of aldosterone concentrations in clinically well‐regulated trilostane‐treated dogs with hyperkalemia and cortisol concentrations before and after ACTH stimulation of <2 μg/dL at 3–6 hours would be needed to determine if aldosterone concentrations are helpful in predicting iatrogenic hypoadrenocorticism. It is important to note that the highest potassium concentration documented in this study was 5.9 mmol/L (reference range 4–5.2 mmol/L). If potassium concentration is substantially higher, caution should be used regarding continued trilostane treatment at the same dose.
One of this study's limitations is that a power calculation was not possible, because the specific population and data that this study examined had not yet been reported. However, the study size did allow for significant differences in cortisol concentrations to be documented, and these data will help perform future power calculations for studies which aim to look at this specific population of dogs. Other limitations include small study size, limited follow‐up, and lack of a clear determination of the type of hyperadrenocorticism treated in some of the dogs. In addition, all study dogs were treated with twice daily trilostane. It is not known whether the results of this study would be different for dogs treated with once daily trilostane. Another concern might be that the first ACTH stimulation test influenced the results of the second ACTH stimulation test. Other future studies could investigate the influence of exogenous ACTH on cholesterol stores or other factors that might affect the results of the second ACTH stimulation. However, it seems unlikely that the first ACTH stimulation would influence the results of the second ACTH stimulation test given that the half‐life of exogenously administered ACTH in dogs with hyperadrenocorticism is about 20 minutes.11 Finally, many of the dogs received medications other than trilostane, and these medications might have influenced the results of this study.
It is important that dogs with hyperadrenocorticism are older dogs that have at least one complex metabolic disease but often already have or will develop more than one medical problem. Therefore, treatment and monitoring decisions must be customized to each individual dog and must take into account all concurrent disorders, their respective clinical signs, and clinicopathologic data. Older dogs with hyperadrenocorticism are a heterogeneous group of dogs and it is not possible to make one monitoring and treatment protocol recommendation for all. For example, if a dog has renal insufficiency, and some degree of polyuria and polydipsia is expected, an ACTH stimulation test might have to be performed to determine whether the dog has well or poorly regulated hyperadrenocorticism.
In conclusion, this study suggests that it is safe to continue trilostane treatment without dose reduction, in dogs with clinically well‐regulated hyperadrenocorticism, and low cortisol concentrations 3–6 hours after trilostane administration. As a subset of these dogs will have sodium to potassium ratios and lymphocyte counts consistent with hypoadrenocorticism, a follow‐up ACTH stimulation test at 9–12 hours can support this recommendation. It remains to be investigated whether measuring a baseline cortisol 9–12 hours after trilostane administration can be used to monitor this specific population of dogs, as long as the dogs remain clinically well‐regulated.
Acknowledgments
Funding: Dechra Development, LLC. 7015 College Blvd, Suite 510 Overland Park KS 66211 US.
Conflict of Interest Declaration: Dr. Hess has consulted for Dechra and Dechra, the producer of trilostane, funded the study.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The work was done at the Department of Clinical Studies ‐ Philadelphia, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA 19104.
Presented as an abstract at the 2014 American College of Veterinary Internal Medicine Forum, Nashville, TN, June 2014 and published in the J Vet Intern Med 2014;28:1032–1033.
Footnotes
Vetoryl; Dechra Veterinary Products, Overland Park, KS.
Immulite 2000; Siemens, Washington, DC.
Cortrosyn; Amphastar Pharmaceutical Inc, Rancho Cucamonga, CA.
Cell‐Dyn 3700; AbbottLaboratories, Abbott Park, IL.
Chemistry analyzer, Kodak Ektachem 250; Eastman Kodak Co, Rochester, NY.
Adrenocorticotropic hormone immunoradiometric assay; DiaSorin, Stillwater MN.
ACTH immunoradiometric assay; Scantibodies Laboratory, Inc, Santee, CA.
Stata 11.0 for Windows; Stata Corporation, College Station, TX.
References
- 1. Wenger M, Sieber‐Ruckstuhl NS, Müller C, Reusch CE. Effect of trilostane on serum concentrations of aldosterone, cortisol, and potassium in dogs with pituitary‐dependent hyperadrenocorticism. Am J Vet Res 2004;65:1245–1250. [DOI] [PubMed] [Google Scholar]
- 2. Arenas C, Melian C, Perez‐Alenza MD. Evaluation of 2 trilostane protocols for the treatment of canine pituitary‐dependent hyperadrenocorticism: Twice daily versus once daily. J Vet Intern Med 2013;27:1478–1485. [DOI] [PubMed] [Google Scholar]
- 3. Feldman EC. Evaluation of twice‐daily, lower dose trilostane treatment administered orally in dogs with naturally occurring HAC. J Am Vet Med Assoc 2011;238:1441–1451. [DOI] [PubMed] [Google Scholar]
- 4. Vaughan MA, Feldman EC, Hoar BR, Nelson RW. Evaluation of twice‐daily, low‐dose trilostane treatment administered orally in dogs with naturally occurring HAC. J Am Vet Med Assoc 2008;232:1321–1328. [DOI] [PubMed] [Google Scholar]
- 5. Anantharaman R, Menezes G, Yusuf R, et al. The 1 μg cosyntropin test in normal individuals: A reappraisal. Indian J Endocrinol Metab 2013;17:693–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Melian C, Perez‐Alenza MD, Peterson ME. Hyperadrenocorticism in dogs In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 7th ed St. Louis, MO: Saunders Elsevier; 2010:1816–1840. [Google Scholar]
- 7. Bell R, Neiger R, McGrotty Y, Ramsey IK. Study of the effects of once daily doses of trilostane on cortisol concentrations and responsiveness to adrenocorticotrophic hormone in hyperadrenocorticoid dogs. Vet Rec 2006;159:277–281. [DOI] [PubMed] [Google Scholar]
- 8. Bonadio CM, Feldman EC, Cohen TA, Kass PH. Comparison of adrenocorticotropic hormone stimulation test results started 2 versus 4 hours after trilostane administration in dogs with naturally occurring hyperadrenocorticism. J Vet Intern Med 2014;28:1239–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reid LE, Behrend EN, Martin LG, et al. Effect of trilostane and mitotane on aldosterone secretory reserve in dogs with pituitary‐dependent hyperadrenocorticism. J Vet Intern Med 2014;28:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seth M, Drobatz KJ, Church DB, Hess RS. White blood cell count and the sodium to potassium ratio to screen for hypoadrenocorticism in dogs. J Vet Intern Med 2011;25:1351–1356. [DOI] [PubMed] [Google Scholar]
- 11. Greco DS, Behrend EN, Brown SA, et al. Pharmacokinetics of exogenous corticotropin in normal dogs, hospitalized dogs with non adrenal illness and adrenopathic dogs. J Vet Pharmacol Ther 1998;2:369–374. [DOI] [PubMed] [Google Scholar]


