Abstract
Background
Airway collapse is a common finding in dogs with chronic cough, yet the diagnosis can be difficult to confirm without specialty equipment.
Hypothesis
Bronchoscopic documentation of tracheobronchial collapse will show better agreement with fluoroscopic imaging than with standard radiography.
Animals
Forty‐two dogs prospectively evaluated for chronic cough.
Methods
In this prospective study, three‐view thoracic radiographs were obtained followed by fluoroscopy during tidal respiration and fluoroscopy during induction of cough. Digital images were assessed for the presence or absence of collapse at the trachea and each lobar bronchus. Bronchoscopy was performed under general anesthesia for identification of tracheobronchial collapse at each lung segment. Agreement of imaging tests with bronchoscopy was evaluated along with sensitivity and specificity of imaging modalities as compared to bronchoscopy.
Results
Airway collapse was identified in 41/42 dogs via 1 or more testing modalities. Percent agreement between pairs of tests varied between 49 and 87% with poor–moderate agreement at most bronchial sites. Sensitivity for the detection of bronchoscopically identified collapse was highest for radiography at the trachea, left lobar bronchi, and the right middle bronchus, although specificity was relatively low. Detection of airway collapse was increased when fluoroscopy was performed after induction of cough compared to during tidal respiration.
Conclusions
Radiography and fluoroscopy are complementary imaging techniques useful in the documentation of bronchial collapse in dogs. Confirming the presence or absence of tracheal or bronchial collapse can require multiple imaging modalities as well as bronchoscopy.
Keywords: Airway collapse, Bronchoscopy, Chronic bronchitis, Fluoroscopy
Abbreviations
- FB
flexible bronchoscopy
- FLtidal
fluoroscopy during tidal respiration
- FLcough
fluoroscopy following induced cough
- LB1D1
left cranial lobe bronchus (cranial segment)
- LB1V1
left cranial lobe bronchus (caudal segment)
- LB2
left caudal lobe bronchus
- RB1
right cranial lobe bronchus
- RB2
right middle lobe bronchus
- RB3
accessory lobe bronchus
- RB4
right caudal lobe bronchus
- TBM
tracheobronchomalacia
- VMTH
Veterinary Medical Teaching Hospital
- XR
standard radiography
Airway collapse is a common contributing factor to chronic cough in dogs.1, 2, 3, 4 It can affect the cervical or intrathoracic trachea, large bronchi, or smaller airways. Definitive diagnosis of airway collapse can be challenging given the dynamic nature of disease and small size of some affected airways. Standard radiography is variably helpful in depicting cervical tracheal collapse, with diagnostic success ranging from 60 to over 90%.5, 6 Radiographic documentation of bronchial collapse is more challenging, with diagnostic success rates of 0–50% reported.1, 7
Obtaining inspiratory and expiratory lateral radiographs is often recommended to increase the chance of detecting airway collapse because extrathoracic airways are more likely to collapse on inspiration, whereas intrathoracic airways tend to collapse during expiration. However, in one small study, these additional views did not improve the ability to document airway collapse.5 Also, when compared to fluoroscopy, standard radiography correctly identified the site of tracheal collapse (cervical versus intrathoracic) only 52% of the time,6 indicating superiority of dynamic imaging in identifying airway collapse. Finally, three‐view radiographs are sometimes advised to improve detection of airway collapse because the most common sites of bronchial collapse are at the left cranial and right middle lung lobes,1, 2, 3 which are more easily identified on right and left lateral projections, respectively. Taken together, studies to date suggest that standard radiography is not a highly sensitive or specific means to diagnose airway collapse in dogs. Nonetheless, standard radiography has the advantage over fluoroscopy of higher contrast resolution and it is also essential for identifying pulmonary infiltrates.
A study in dogs in which radiographs were reviewed retrospectively revealed that radiographs were variably accurate in identifying airway collapse at the lobar bronchi when compared to bronchoscopy.1 In people, flexible bronchoscopy (FB) is considered the gold standard for documentation of airway collapse or tracheobronchomalacia (TBM),8, 9, 10 and this technique has proven highly useful in identifying bronchomalacia in dogs.1, 2, 3 Although use of bronchoscopy as a gold standard has not specifically been evaluated in veterinary medicine, it has been referred to as the diagnostic test of choice for identifying airway collapse,11, 12 and in 2 earlier studies, bronchoscopy detected airway collapse more often than fluoroscopy.1, 4 In this prospective study, we hypothesized that the detection of tracheobronchial collapse with bronchoscopy would be more likely to agree with dynamic fluoroscopy than with standard radiography in dogs with spontaneously occurring airway disease, and we anticipated variability in the documentation of collapse at some lobar sites. We aimed to investigate the agreement of noninvasive imaging tests with bronchoscopy in the documentation of the bronchial collapse.
Materials and Methods
All dogs evaluated for cough at the William R. Pritchard Veterinary Medical Teaching Hospital (VMTH) at the University of California, Davis between July 2005 and July 2014 that underwent standard thoracic radiography and tracheal fluoroscopy followed by bronchoscopy performed by one clinician (L.R. Johnson) were included in this prospective study.
Age, breed, sex, neutering status, and pertinent physical examination findings were recorded for all dogs. Left lateral, right lateral and dorsoventral thoracic, and right lateral cervical radiographs were obtained in nonsedated dogs using a commercially available digital radiographic system.1 When patient compliance was possible, attempts were made to obtain thoracic radiographs at full inspiration. Expiratory radiographs were not specifically obtained because fluoroscopy was scheduled to follow radiography. Fluoroscopy2 was performed on the same day with dogs positioned in right lateral recumbency during tidal respiration and after tracheal manipulation to induce a cough. Digital video recordings were obtained of fluoroscopic studies for future review. All radiographs and fluoroscopic studies were re‐evaluated in random order by one board‐certified radiologist (R.E. Pollard) who was masked to bronchoscopic results. For radiographs or fluoroscopy, collapse was defined as a reduction in airway luminal diameter of 25% or more.5 Collapse of right lobar (right cranial, right middle, accessory, and right caudal) bronchi was reported as present or absent based on the examination of radiographs (XR). Collapse of the trachea and left lobar bronchi (left cranial lobe cranial segment, left cranial lobe caudal segment, and left caudal lobe) was recorded as present or absent based on XR, fluoroscopy during tidal respiration (FLtidal), and fluoroscopy during induced cough (FLcough).
Flexible bronchoscopy was performed the day after fluoroscopy to describe the distribution of airway collapse. The endoscopist was not masked to the results of the radiographs or fluoroscopy at the time of the procedure. A balanced anesthetic induction plan was designed for each dog by the Anesthesia Service at the VMTH. Dogs large enough for a size 7 or greater endotracheal tube were induced for anesthesia, and tracheoscopy was performed before intubation. Gaseous anesthesia was maintained using a specialized bronchoscopic adaptor and scavenging system attached to the endotracheal tube. In dogs too small for a size 7 endotracheal tube, anesthesia was maintained using a constant rate infusion of propofol at 0.1–0.4 mg/kg/min. Oxygenation was provided by jet ventilation at a rate of 180 breaths/min. Constant ECG, blood pressure, and pulse oximetry were monitored throughout the procedure. Bronchoscopy was performed in sternal recumbency in all dogs. In dogs that were intubated, endoscopes >4.9 mm outer diameter were used.3 In dogs that received jet ventilation, a 3.8 × 55 cm videoendoscope4 was used.
In each dog, the grade and extent of tracheal collapse was recorded according to a previously defined scheme based on the percent reduction in luminal dimension.5 The canine bronchial map designed by Amis and McKiernan was used for examination of the lower airways.13 Bronchial collapse was identified as >50% loss in luminal diameter of individual bronchi because of the static flattening of the lobar airways, circumferential narrowing, or distortion of the normal round appearance of airway openings.14, 15 Because of the time frame over which this study was completed, distinction was not made between dynamic and static lobar collapse.7
Statistical Analysis
Tracheal and bronchial collapse at each lobar region were recorded as present or absent in all dogs for each diagnostic test. Normality was assessed using D'Agostino & Pearson omnibus test, and normally distributed data are presented as mean ± standard deviation whereas nonparametric data are presented as median with range. The number of tests detecting collapse and the number of bronchial sites at which collapse was detected were calculated. The Kruskal–Wallis test for nonparametric data followed by Dunn's multiple comparison test was used to evaluate differences among detection rates for each diagnostic test. Percent concordance between pairs of diagnostic tests was calculated for the left cranial lobe cranial segment (LB1D1), left cranial lobe caudal segment (LB1V1), left caudal lobe (LB2), right cranial lobe (RB1), right middle lobe (RB2), accessory lobe (RB3), and right caudal lobe (RB4). For each pair of diagnostic tests, the kappa statistic was calculated to evaluate the level of agreement between tests using a scale from 0 to 1, with 0 indicating no agreement and 1 indicating complete agreement. Discordant diagnostic results were statistically evaluated using McNemar's test of proportions with calculation of odds ratios, confidence intervals, and a P value to provide the probability that the difference in test results was because of random chance. Sensitivity, specificity, positive, and negative likelihood ratios were calculated for diagnostic imaging results at each anatomic location using bronchoscopy as the reference test for documentation of airway collapse in comparison with imaging studies. Statistics were performed using commercially available statistical software,5 and P < .05 was considered significant for all analyses.
Results
The study population was comprised of 42 dogs. There were 29 male dogs (27 neutered, 2 intact) and 13 female spayed dogs. Mean ± SD age was 8.0 ± 3.9 years. Median (range) body weight was 5.0 (1.5–23.5) kg; 21 dogs weighed <5 kg, 15 dogs weighed 5.1–10 kg, and 6 dogs weighed >20 kg.
Bronchoscopic and radiographic imaging data were available for all 42 dogs. Fluoroscopy during tidal respiration was performed in all dogs but a complete study was obtained in 36/42 dogs; 6 dogs did not cooperate fully during the study and video recordings could not be evaluated. Fluoroscopic assessment during induction of a cough was achieved in 31/42 dogs; the remaining 11 dogs did not cough in lateral recumbency or were considered too physiologically unstable to undergo induction of cough. Overall, airway collapse was detected at ≥1 location in the tracheobronchial tree by at least one diagnostic test in 41/42 dogs; however, the proportion of dogs in which airway collapse was documented varied by anatomic location and among tests (Table 1).
Table 1.
Airway collapse at one or more sites within the respiratory tract was visualized with at least one diagnostic test in 41/42 dogs
| Site | Radiography (%) | Resting Fluoroscopy (%) | Fluoroscopy During Cough (%) | Bronchoscopy (%) |
|---|---|---|---|---|
| Trachea | 20/39 (51) | 20/36 (56) | 17/31 (55) | 20/42 (48) |
| LB1D1 | 19/39 (49) | 23/36 (64) | 27/31 (87) | 30/42 (71) |
| LB1V1 | 18/39 (46) | 23/36 (64) | 29/31 (94) | 29/42 (69) |
| LB2 | 17/38 (45) | 23/36 (64) | 31/31 (100) | 21/42 (50) |
| RB1 | 12/33 (36) | NA | NA | 9/42 (21) |
| RB2 | 18/33 (55) | NA | NA | 27/42 (64) |
| RB3 | 2/37 (5) | NA | NA | 20/42 (48) |
| RB4 | 15/37 (41) | NA | NA | 9/42 (21) |
See text for abbreviations. Table entries reflect the number and percentage of cases in which airway collapse at specific locations within the tracheobronchial tree was seen with each imaging modality. Fluoroscopic evidence of collapse was available for left lobar bronchi only because dogs were positioned in right lateral recumbency.
Radiography (n = 42) detected collapse at a median of 3/7 locations within the respiratory tract (range 0–7; Fig 1). Fluoroscopy during tidal respiration (n = 36) and after cough (n = 31) detected collapse at a median of 3 of 4 sites that could be evaluated (range 0–4 sites), whereas bronchoscopy (n = 42) revealed airway collapse at a median of 4/7 sites (range 0–7). Overall detection rates did not differ between bronchoscopy and radiography, bronchoscopy and fluoroscopy during tidal respirations, or between fluoroscopy during tidal respirations and fluoroscopy during cough. However, fluoroscopy during tidal respiration or cough (Fig 2, P < .001) detected significantly more sites of collapse than radiography, and fluoroscopy during cough detected significantly more sites of collapse than bronchoscopy (P < .001).
Figure 1.
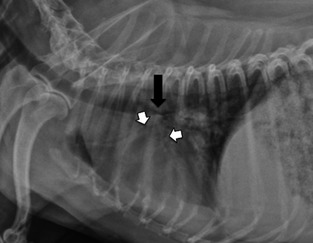
This right lateral radiograph demonstrates collapse of the intrathoracic trachea at the carina (black arrow) as well as collapse of the cranial and caudal segments of the bronchus of the left cranial lung lobe (white arrows).
Figure 2.
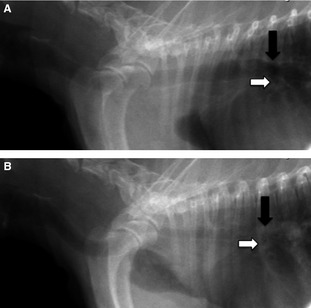
(A) A digitally captured right lateral fluoroscopic image is shown during full inspiration. The tracheal carina (black arrow) has a normal diameter as is the bronchus to the left cranial lung lobe (white arrow). (B) A digitally captured right lateral fluoroscopic image of the same dog as in (A) is shown during cough. The carina has completely collapsed (black arrow) as has the bronchus to the left cranial lung lobe (white arrow). Herniation of the cranial lung lobe through the thoracic inlet is also visible.
Percent concordance between pairs of diagnostic tests ranged from a low of 43% at LB2 to a high of 87% at the trachea for comparison of radiographs and fluoroscopy during cough (Table 2). At 4 anatomic locations, 8 pairs of diagnostic tests yielded significantly different detection rates for airway collapse (Table 2). At LB1D1, dogs were 5 times less likely to have airway collapse identified with standard radiography (n = 2) than with bronchoscopy (n = 10, P = .04, OR 0.2; Fig 3). Also, airway collapse was identified significantly more often by fluoroscopy during cough (n = 12) than by standard radiography (n = 1, P = .006, OR 12) at this site. At both LB1V1 and LB2, significantly more dogs had airway collapse documented with fluoroscopy during cough than with bronchoscopy or radiography (P < .05). In addition, at both LB1V1 and RB3, standard radiography detected airway collapse 5 times less often than did bronchoscopy (P = .026 and .006, OR 0.2) (Fig 4).
Table 2.
For each anatomic location within the tracheobronchial tree, results of imaging tests were tested for agreement in reference to the bronchoscopic findings using the kappa statistic
| A: Flexible Bronchoscopy (FB) Versus Standard Radiography (XR) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Site | Agreement (%) | Both Present | Both Absent | Kappa | FB+/XR− | FB−/XR+ | P Value | Odds Ratio (CI) |
| Trachea | 29/39 (74) | 14/29 | 15/29 | 0.49 | 4/39 | 6/39 | .75 | 1.5 (0.36–7.23) |
| LB1D1 | 27/39 (69) | 17/27 | 10/27 | 0.44 | 10/39 | 2/39 | .043* | 0.2 (0.02–0.94) |
| LB1V1 | 26/39 (67) | 15/26 | 11/26 | 0.16 | 11/39 | 2/39 | .026* | 0.2 (0.02–0.83) |
| LB2 | 26/33 (67) | 13/26 | 13/26 | 0.58 | 3/33 | 4/33 | 1.0 | 1.2 (0.23–9.10) |
| RB1 | 21/33 (64) | 7/21 | 14/21 | 0.25 | 4/33 | 8/33 | .39 | 2 (0.54–9.08) |
| RB2 | 19/33 (58) | 12/19 | 7/19 | 0.16 | 10/33 | 4/33 | .18 | 0.4 (0.09–1.39) |
| RB3 | 18/37 (49) | 1/18 | 17/18 | −0.10 | 16/37 | 3/37 | .0059* | 0.2 (0.04–0.66) |
| RB4 | 19/37 (51) | 1/19 | 18/19 | −0.16 | 5/37 | 13/37 | .099 | 2.6 (0.87–9.32) |
| B: Flexible Bronchoscopy (FB) Versus Fluoroscopy during Tidal Respiration (FLtidal) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Site | Agreement (%) | Both Present | Both Absent | Kappa | FB+/FLtidal | FB−/FLtidal+ | P Value | Odds Ratio (CI) |
| Trachea | 24/36 (67) | 12/24 | 12/24 | 0.34 | 4/36 | 8/36 | .39 | 2 (0.54–9.08) |
| LB1D1 | 23/36 (64) | 17/23 | 6/23 | 0.13 | 7/36 | 6/36 | 1.0 | 0.9 (0.24–2.98) |
| LB1V1 | 24/36 (67) | 18/24 | 6/24 | 0.18 | 7/36 | 5/36 | .77 | 0.7 (0.18–2.61) |
| LB2 | 20/31 (64) | 12/20 | 8/20 | 0.30 | 3/31 | 8/31 | .23 | 2.7 (0.64–15.61) |
| C: Flexible Bronchoscopy (FB) Versus Fluoroscopy During Cough (FLcough) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Site | Agreement (%) | Both Present | Both Absent | Kappa | FB+/FLcough− | FB−/FLcough+ | P Value | Odds Ratio (CI) |
| Trachea | 23/31 (75) | 10/23 | 13/23 | 0.49 | 2/31 | 6/31 | .29 | 3.0 (0.54–30.39) |
| LB1D1 | 23/31 (75) | 20/23 | 3/23 | 0.16 | 1/31 | 7/31 | .077 | 7.0 (0.90–315.5) |
| LB1V1 | 23/31 (75) | 21/23 | 2/23 | 0.04 | 0/31 | 8/31 | .013* | 0 (0–0.54) |
| LB2 | 22/31 (71) | 22/22 | 0/22 | NA | 0/31 | 9/31 | .0077* | 0 (0–0.30) |
| D: Standard Radiography (XR) Versus Fluoroscopy During Tidal Respiration (FLtidal) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Site | Agreement (%) | Both Present | Both Absent | Kappa | XR+/FLtidal− | XR−/FLtidal+ | P Value | Odds Ratio (CI) |
| Trachea | 25/33 (76) | 14/25 | 11/25 | 0.51 | 5/33 | 3/33 | .72 | 0.6 (0.09–3.08) |
| LB1D1 | 26/33 (79) | 16/26 | 10/26 | 0.57 | 1/33 | 6/33 | .13 | 6 (0.73–276) |
| LB1V1 | 25/33 (76) | 15/25 | 10/25 | 0.51 | 5/33 | 3/33 | .72 | 0.6 (0.09–3.08) |
| LB2 | 23/33 (70) | 13/23 | 10/23 | 0.41 | 2/33 | 8/33 | .11 | 4 (0.80–38.67) |
| E: Standard Radiography (XR) Versus Fluoroscopy During Cough (FLcough) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Site | Agreement (%) | Both Present | Both Absent | Kappa | XR+/FLcough− | XR−/FLcough+ | P Value | Odds Ratio (CI) |
| Trachea | 26/30 (87) | 14/26 | 12/26 | 0.73 | 2/30 | 2/30 | .62 | 1 (0.07–13.80) |
| LB1D1 | 17/30 (57) | 15/17 | 2/17 | 0.23 | 1/30 | 12/30 | .013* | 12 (1.77–513) |
| LB1V1 | 16/30 (53) | 14/16 | 2/16 | 0.12 | 0/30 | 14/30 | <.001* | 0 (0–0.30) |
| LB2 | 13/30 (43) | 13/13 | 0/13 | NA | 0/30 | 17/30 | <.001* | 0 (0–0.23) |
See text for abbreviations. Table entries reflect the number and percentage of cases that had concordant results. Fluoroscopic evidence of collapse was available for left lobar bronchi only because dogs were positioned in right lateral recumbency. The kappa statistic reflects the level of agreement between tests on a scale from 0 to 1, with 0 indicating no agreement and 1 indicating complete agreement. A kappa statistic cannot be calculated when one row contains zero. NA, not available. Discordant results of imaging pairs at specific locations within the tracheobronchial tree were evaluated for significant differences between modalities using McNemar's test. P values are presented along with odds ratio and confidence intervals (CI) in reference to bronchoscopic findings. *P < .05. Table entries represent the number of cases related to the total number evaluated.
Figure 3.
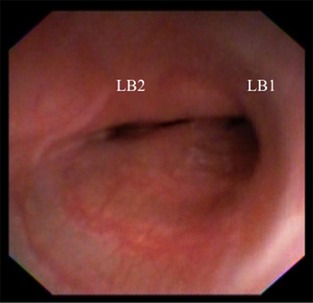
Bronchoscopic image from the bifurcation of the left cranial and caudal lung lobes reveals >75% collapse of each bronchus.
Figure 4.
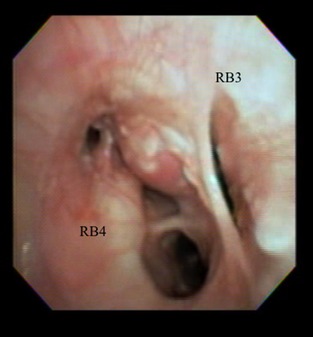
This bronchoscopic image exhibits 100% collapse of RB3 along with nodular and proliferative mucosal changes and airway collapse in RB4.
The level of agreement for pairs of diagnostic tests tended to be higher for the detection of tracheal collapse (67–87%), with the highest kappa value (0.73) for comparison of standard radiography to fluoroscopy during cough. Moderate agreement (kappa 0.4–0.6) between imaging modalities was demonstrated for results of standard radiography and resting fluoroscopy at the trachea, LB1D1, LB1V1, and LB2, for results of standard radiography and bronchoscopy at the trachea, LB1V1, and LB2, and for results of fluoroscopy during cough and bronchoscopy at the trachea. The kappa statistic was negative for the comparison of results of bronchoscopy and standard radiography at RB3 and RB4, indicating disagreement beyond that expected by chance.
Sensitivity and specificity for the detection of airway collapse by radiography and fluoroscopy at distinct sites within the respiratory tract in comparison to bronchoscopy are presented in Table 3. In general, radiographs were more sensitive in the detection of tracheal and airway collapse but specificity was low for radiographs and fluoroscopy during tidal respiration at the left cranial lobar bronchi (cranial and caudal segments). Specificity was higher for fluoroscopy during induction of cough than for fluoroscopy during tidal respiration.
Table 3.
Sensitivity and specificity of imaging modalities for tracheal and airway collapse as defined by bronchoscopy
| Sensitivity (CI), % | Specificity (CI), % | Positive Likelihood Ratio | Negative Likelihood Ratio | |
|---|---|---|---|---|
| Trachea | ||||
| Radiography | 70 (46–88) | 79 (54–94) | 3.32 | 0.38 |
| FLtidal | 60 (36–81) | 75 (48–93) | 2.40 | 0.53 |
| FLcough | 62 (35–85) | 87 (60–98) | 4.69 | 0.44 |
| LB1D1 | ||||
| Radiography | 89 (67–99) | 50 (27–83) | 1.79 | 0.22 |
| FLtidal | 74 (52–90) | 46 (19–75) | 1.37 | 0.56 |
| FLcough | 74 (54–89) | 75 (19–99) | 2.96 | 0.35 |
| LB1V1 | ||||
| Radiography | 88 (64–99) | 50 (28–72) | 1.77 | 0.24 |
| FLtidal | 78 (56–93) | 46 (19–75) | 1.45 | 0.48 |
| FLcough | 72 (52–87) | 100 (16–100) | NA | 0.28 |
| LB2 | ||||
| Radiography | 76 (50–94) | 81 (54–96) | 4.08 | 0.30 |
| FLtidal | 60 (36–81) | 73 (39–94) | 2.20 | 0.63 |
| FLcough | NA | NA | NA | NA |
| RB1 | ||||
| Radiography | 47 (21–73) | 78 (52–94) | 2.10 | 0.68 |
| RB2 | ||||
| Radiography | 75 (47–93) | 41 (18–67) | 1.28 | 0.59 |
| RB3 | ||||
| Radiography | 25 (1–81) | 52 (34–69) | 0.52 | 1.44 |
| RB4 | ||||
| Radiography | 7 (0–34) | 78 (56–93) | 0.33 | 1.19 |
CI, confidence intervals.
Discussion
Results of this study indicate that radiography, resting fluoroscopy, fluoroscopy during cough, and FB yield similar results when determining the presence of tracheal but not bronchial collapse in dogs. All tests detected tracheal collapse in ~50% of cases, and agreement between tests at the level of the trachea was good, with kappa values ranging from 67 to 87%. Although this level of agreement is reasonable, it is important to realize that there were still many dogs where tracheal collapse was diagnosed with 1 test and not with another. Sensitivity at the level of the trachea was highest for radiography (70%) but specificity was highest for fluoroscopy during cough (87%) as compared to bronchoscopy. In practice, thoracic and cervical radiography is often the first test performed in the pursuit of a diagnosis of tracheal collapse. Results of this study, in conjunction with the high number of false positive radiographic findings of tracheal collapse in comparison to fluoroscopy reported in a previous study6, highlight the importance of additional diagnostic tests in assessment of tracheal collapse.
Radiographs were slightly more sensitive in detecting collapse at the level of the trachea and left sided bronchi than fluoroscopy for bronchoscopically identified collapse. This is likely because of the poor contrast resolution of the latter imaging modality because of the low mA used during fluoroscopy to limit radiation exposure.16 Curiously, sensitivity of radiographs in documenting airway collapse at the right middle lobar bronchus was relatively high (75%). This might be related to the commonality of collapse at this region.1, 2, 3 With the exception of collapse at the right middle lung lobe, radiography had a much lower sensitivity in detecting airway collapse at other right lobar bronchi (right cranial, accessory, and right caudal bronchi) in comparison to the left, but reasons for this are unclear.
In this study, fluoroscopy during an induced cough was more specific in ruling out airway collapse than radiographs or resting fluoroscopy at all lobar bronchi evaluated in the left lung. In a prospective study of pediatric patients with TBM, fluoroscopy was much less sensitive in detecting airway collapse (24%) than in this study in dogs (60–89%) but was 100% specific.17 In dogs examined here, only fluoroscopic imaging at LB1V1 after induction of a cough was 100% specific, however, bronchi on the right side of the thorax were not assessed with fluoroscopy, which is a limitation of the study. Standard protocol for fluoroscopy at our institution utilizes right lateral recumbency but this raises the question of whether fluoroscopy should be performed in left lateral recumbency as well as in right lateral to aid in the detection of dynamic airway collapse in the right lobar bronchi. Dog positioning is rarely described for fluoroscopic studies, and the primary protocol available for obtaining a complete fluoroscopic assessment of the airways only recommends performing the study over several respiratory cycles and after the induction of cough.18 Therefore, clinical judgment must be employed to determine the need for additional studies in varied positions, taking care to avoid excessive exposure of technical staff to radiation and to limit undue stress to the dog.
Dog positioning might have also played a role in the interpretation of results from bronchoscopy, which is performed in sternal recumbency at our institution compared to the lateral positioning for radiographs and fluoroscopy. In people, fluoroscopy is performed with the patient in a supine position and the fluoroscopy unit is oriented horizontal to the subject.19 A restraint system that allows positioning in an upright position might be useful to develop for use in airway studies similar to what has been designed for videofluoroscopic assessment of swallowing in dogs.20 This would likely be more physiologic, although perhaps more challenging to induce a cough and capture images.
As indicated by our results, radiography, fluoroscopy during tidal respiration, fluoroscopy during cough, and bronchoscopy are not necessarily comparable tests in the assessment of bronchial collapse. Bronchoscopy usually allows the operator to observe changes in airway dimensions during inspiration and expiration but evaluation of airway changes during cough is limited. Conversely, radiography provides assessment during a single instant so that changes in airway diameter might not be imaged at the time of exposure. The likelihood of missing bronchial collapse is further increased because thoracic radiographs are often obtained at peak inspiration. This is done to maximize lung inflation and subject contrast, but selects for the phase of respiration where intrathoracic airway collapse is least likely to occur. Although fluoroscopy is also a dynamic test that can detect airway collapse during different respiratory phases,21, 22 contrast resolution is lower than radiography.16 The trachea is large enough to see clearly with fluoroscopy, however, the bronchi are substantially smaller, compromising the ability to define bronchi clearly and assess changes in luminal diameter. This feature is worsened in small or obese subjects,17 which could be a limitation in some subjects examined here.
A factor that supports the use of bronchoscopy to document airway collapse is the 3‐dimensional view of bronchi provided by this diagnostic test. Lateral radiographs and fluoroscopy provide a 2‐dimensional assessment of the airways, which can only identify collapse in the dorsoventral dimension. Collapse of an airway in the axial or circumferential dimension, which is common with bronchial collapse, would go undetected. Although the dorsoventral radiograph might be used to evaluate axial dimension of some airways, and would specifically allow better evaluation of the accessory lobar bronchus, it is the authors' opinion that assessment of airway dimensions is more difficult on this projection. Computed tomography is increasingly utilized in the evaluation of the respiratory tract, and in normal dogs and humans has documented 25–35% changes in tracheal dimensions between inspiration and expiration.19, 23, 24 However, alignment of the CT with the bronchi and coordination with respiration make assessment of static or dynamic changes in bronchial conformation challenging to obtain.
It is important to recognize that the change in airway diameter generated during cough in a normal dog has not been established, partly because normal dogs do not cough with stimulation.25 In people, an abnormal degree of airway collapse is defined by >50% reduction in lumenal cross‐sectional area during cough.26, 27 In this study, airway collapse was considered radiographically or fluoroscopically present when the luminal diameter was reduced by 25% or more. This percentage was chosen based on the grading scheme for tracheal collapse in the dog developed by Tangner and Hobson.5 Given the knowledge that normal dogs experience up to 25% reduction in luminal diameter during respiration,24 this percentage might have been too low. Therefore, it is possible that airway collapse might have been overdiagnosed by fluoroscopy with cough in the subset of dogs where >25% but <50% of the airway lumen was attenuated. A fluoroscopic study in coughing dogs that lack airway collapse would be necessary to establish normal values for diseased but not malacic airways.
Results of this study demonstrate that fluoroscopy during cough identifies airway collapse at certain left lobar sites more commonly than other modalities, including bronchoscopy. Coughing results in a dramatic increase in intrathoracic pressure. This results in a reduction in airway diameter to increase the speed of airflow thereby making the cough more effective.26, 27 In people with chronic cough, respiratory muscles receive regular exercise so that abnormally high intrathoracic and intraluminal pressures can be generated as the glottis closes.26 Presuming that this is true in dogs with TBM and chronic cough, it makes sense that airway collapse would be exacerbated during cough. Because fluoroscopy is the only method available to evaluate airways during cough, it is likely that some cases with airway collapse and some bronchial sites that develop collapse would only be identified with this diagnostic test. This theory would argue in favor of performing fluoroscopy in both right and left lateral recumbency.
One limitation of this study is that a relatively small number of dogs with diverse diseases were evaluated here. Fluoroscopy was generally recommended for dogs in which a clinical suspicion of airway collapse was present based on the character of cough, signalment, and physical examination findings. Therefore, the study was biased toward the detection of collapse at some site within the respiratory tract, and the commonality of collapse was not unexpected. Bronchoscopy has long been considered the gold standard for documenting airway collapse in human medicine8, 9, 10 but has only recently been confirmed as such28 In people, forced respiratory maneuvers enhance the detection of collapse,29 however, in animals, bronchoscopy is performed under general anesthesia and voluntary maneuvers are not possible. Cough is not specifically induced during bronchoscopy as this obscures airway visualization and can result in airway damage. Also, anesthesia likely alters airway tone, which could impact results.
Detection of airway collapse on radiography and fluoroscopy was compared to that identified at bronchoscopy to determine the agreement of these noninvasive imaging modalities with bronchoscopically documented airway collapse in a group of dogs. Contrary to our hypothesis, bronchoscopy and fluoroscopy during cough showed poor agreement on the identification of collapse in left sided bronchi. Use of left lateral recumbency during fluoroscopy to evaluate airways on the right side of the thorax would have provided additional sites for the assessment of agreement and should be considered in certain clinical situations when bronchoscopy is not available or the patient is not a suitable candidate for anesthesia. Suppression of both cough and dynamic airway collapse during anesthesia might have confounded our estimation of agreement. Concordance might have been improved if the anesthetic plane could have been lightened, if cough had been induced, or if specific record had been made of dynamic versus static collapse. Although radiography and fluoroscopy were supportive of airway collapse in many dogs, discordant results were relatively common. For example, bronchoscopy detected collapse at the accessory lung lobe in almost half the dogs examined here, whereas radiographs documented collapse in only 5%. Fluoroscopy during cough reported collapse of left cranial and caudal lobar bronchi more frequently than other testing modalities. Our study is limited by the fact that the right lobar bronchi were not assessed fluoroscopically, however, results indicate that complete documentation of the airway collapse in dogs and the definitive exclusion of airway collapse requires completion of a number of imaging tests as well as bronchoscopy.
Acknowledgments
The authors thank Dr Phil Kass for statistical assistance.
Funding: Supported in part by the Bailey Wrigley Fund, University of California, Davis.
Conflict of Interest Declaration: Dr Johnson: Feline Advisory Board, speaker honoraria.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Sound Eklin, Carlsbad, CA
EasyDiagnost Eleva, Philips Medical Systems, N.A., Bothell, WA
Olympus P180 or GIF N180, Olympus Corporation, Melville, NY
Olympus BF3C160, Olympus Corporation
GraphPad Prism Version 5.0d, San Diego CA
References
- 1. Johnson LR, Pollard RE. Tracheal collapse and bronchomalacia in dogs: 58 cases (2001–2008). J Vet Intern Med 2010;24:298–305. [DOI] [PubMed] [Google Scholar]
- 2. Adamama‐Moraitou KK, Pardali D, Day MJ, et al. Canine bronchomalacia: A clinicopathological study of 18 cases diagnosed by endoscopy. Vet J 2012;191:261–266. [DOI] [PubMed] [Google Scholar]
- 3. Singh MK, Johnson LR, Kittleson MD, Pollard RE. Bronchomalacia in dogs with myxomatous mitral valve degeneration. J Vet Intern Med 2012;26:312–319. [DOI] [PubMed] [Google Scholar]
- 4. Nafe LA, Robertson ID, Hawkins EC. Cervical lung lobe herniation in dogs identified by fluoroscopy. Can Vet J 2013;54:955–959. [PMC free article] [PubMed] [Google Scholar]
- 5. Tangner CH, Hobson HP. A retrospective study of 20 surgically managed cases of tracheal collapse. Vet Surg 1982;11:146–149. [Google Scholar]
- 6. Macready DM, Johnson LR, Pollard RE. Fluoroscopic and radiographic evaluation of tracheal collapse in 62 dogs. J Am Vet Med Assoc 2007;230:1870–1876. [DOI] [PubMed] [Google Scholar]
- 7. Bottero E, Bellino C, De Lorenzi D, et al. Clinical evaluation and endoscopic classification of bronchomalacia in dogs. J Vet Intern Med 2013;27:840–846. [DOI] [PubMed] [Google Scholar]
- 8. Rudman DT, Elmaraghy CA, Shiels WE, Wiet GJ. The role of airway fluoroscopy in the evaluation of stridor in children. Arch Otolaryngol Head Neck Surg 2003;129:305–309. [DOI] [PubMed] [Google Scholar]
- 9. Doshi J, Krawiec ME. Clinical manifestations of airway malacia in young children. J Allergy Clin Immunol 2007;120:1276–1278. [DOI] [PubMed] [Google Scholar]
- 10. Heyer CM, Nuesslein TG, Jung D, et al. Tracheobronchial anomalies and stenoses: Detection with low‐dose multidetector CT with virtual tracheobronchoscopy—comparison with flexible tracheobronchoscopy. Radiology 2007;242:542–549. [DOI] [PubMed] [Google Scholar]
- 11. Hedlund CS. Surgery of the upper respiratory system In: Fossum TW, ed. Small Animal Surgery, 3rd ed St. Louis, MO: Mosby Elsevier; 2007:817–866. [Google Scholar]
- 12. Sura PA, Durant AM. Trachea and bronchi In: Tobias KM, Johnston SA, eds Veterinary Surgery Small Animal. St. Louis, MO: Elsevier Saunders; 2012:1734–1751. [Google Scholar]
- 13. Amis TC, McKiernan BC. Systematic identification of endobronchial anatomy during bronchoscopy in the dog. Am J Vet Res 1986;47:2649–2657. [PubMed] [Google Scholar]
- 14. Jokinen K, Palva T, Sutinen S, et al. Acquired tracheobronchomalacia. Ann Clin Res 1977;9:52–57. [PubMed] [Google Scholar]
- 15. Boogard R, Huijsmans SH, Pijmeburg MWH, et al. Tracheomalacia and bronchomalacia in children: Incidence and patient characteristics. Chest 2005;128:3391–3397. [DOI] [PubMed] [Google Scholar]
- 16. Bushberg JT, Seibert JA, Leidholdt EM Jr, Boone JM, editors. The Essential Physics of Medical Imaging, 2nd ed Philadelphia, PA: Lippincott Williams and Wilkins; 2002:231–255. [Google Scholar]
- 17. Sanchez MO, Greer MC, Masters IB, Chang AB. A comparison of fluoroscopic airway screening with flexible bronchoscopy for diagnosing tracheomalacia. Pediatr Pulmonol 2012;47:63–67. [DOI] [PubMed] [Google Scholar]
- 18. Alexander K. The pharynx, larynx and trachea In: Thrall DE, ed. Textbook of Veterinary Diagnostic Radiology, 6th ed St. Louis, MO: Elsevier Saunders Inc; 2013:489–499. [Google Scholar]
- 19. Pepin JL, Ferretti G, Veale D, et al. Somnofluoroscopy, computed tomography, and cephalometry in the assessment of the airway in obstructive sleep apnoea. Thorax 1992;47:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bonadio CM, Pollard RE, Dayton PA, et al. Effects of body positioning on swallowing and esophageal transit in healthy dogs. J Vet Intern Med 2009;23:801–805. [DOI] [PubMed] [Google Scholar]
- 21. Juan L, Sotomayor MD, Rudolfo I, et al. Large‐airway collapse due to acquired tracheobronchomalacia in infancy. Am J Dis Child 1986;140:367–371. [DOI] [PubMed] [Google Scholar]
- 22. ltman KW, Wetmore RF, Mahboubi S. Comparison of endoscopy and radiographic fluoroscopy in the evaluation of pediatric congenital airway abnormalities. Int J Pediatr Otorhinolaryngol 1998;44:43–46. [DOI] [PubMed] [Google Scholar]
- 23. Murgu SD, Colt HG. Tracheobronchomalacia and excessive dynamic airway collapse. Respirology 2006;11:388–406. [DOI] [PubMed] [Google Scholar]
- 24. Leonard CD, Johnson LR, Bonadio CM, Pollard RE. Changes in tracheal dimensions during inspiration and expiration in the healthy dogs as detected by computed tomography. Am J Vet Res 2009;70:986–991. [DOI] [PubMed] [Google Scholar]
- 25. Boyle TE, Hawkins EC, Davis JL, Robertson ID. Failure of nebulized irritant, acidic, or hypotonic solutions or external mechanical stimulation of the trachea to consistently induce coughing in healthy, awake dogs. Can J Vet Res 2011;75:228–232. [PMC free article] [PubMed] [Google Scholar]
- 26. Rayl JE. Tracheobronchial collapse during cough. Radiology 1965;85:87–92. [DOI] [PubMed] [Google Scholar]
- 27. West JB. Pulmonary Physiology, the Essentials, 9th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 28. Majid A, Gaurav K, Sanchez JM, Berger RL. Evaluation of tracheobronchomalacia by dynamic flexible bronchoscopy. A pilot study. Ann Am Thorac Soc 2014;11:951–955. [DOI] [PubMed] [Google Scholar]
- 29. Aquino SL, Shepard JA, Ginns LC, et al. Acquired tracheomalacia: Detection by expiratory CT scan. J Comput Assist Tomogr 2001;25:394–399. [DOI] [PubMed] [Google Scholar]


