Abstract
Background
Thoracic ultrasonography (US) and bronchoalveolar lavage fluid (BALF) analysis are antemortem methods used to identify the lung lesions associated with bovine respiratory disease (BRD). Accuracy of US and the cell distributions in BALF have not been characterized in calves with subclinical disease.
Objectives
To evaluate the accuracy of US and BALF and describe BALF characteristics in calves with subclinical lung lesions.
Animals
Twenty‐five Holstein calves, 1–12 weeks old.
Methods
In this prospective study, calves with low respiratory scores underwent US, BALF and postmortem examination (normal US, n = 5; comet‐tails, n = 5; consolidation, n = 15). Bronchoalveolar lavage fluid was collected and analyzed for total and differential cell counts. Lung lesions were assessed by gross and histopathologic examination. Data were analyzed using nonparametric methods and relative risk analysis. The accuracy of US and BALF were estimated relative to postmortem examination.
Results
The sensitivity and specificity of US for detecting lung lesions was 94% (95% CI, 69–100%) and 100% (95% CI, 64–100%), respectively. A cut‐point of ≥4% BALF neutrophils was associated with the highest BALF sensitivity and specificity, 81% (95% CI, 56–94%) and 75% (95% CI, 36–95%). The presence of consolidation on US increased the risk of having a BALF neutrophil proportion ≥4% (RR, 3.9; 95% CI, 1.13–13.45; P = .003).
Conclusions and Clinical Importance
Ultrasonography accurately detects lung lesions in calves with subclinical disease. Clinicians should use a cut‐point of ≥4% BALF neutrophils to diagnose subclinical respiratory disease.
Keywords: Bovine, Pneumonia, Respiratory, Validation
Abbreviations
- BAL
bronchoalveolar lavage
- BALF
bronchoalveolar lavage fluid
- BRD
bovine respiratory disease
- ICS
intercostal space
- LS
ultrasonographic lesion score
- RS
respiratory score
- sBRD
subclinical bovine respiratory disease
- Se
sensitivity
- Sp
specificity
- TNCC
total nucleated cell count
- US
thoracic ultrasonography
Clinical bovine respiratory disease (BRD) is a common cause of morbidity and mortality in young dairy heifers.1 Within‐herd prevalence is highly variable, ranging from 0 to 90% of calves affected.2, 3 This within‐herd variation could result from differences in disease frequency, recording method, or scoring system. The constituents of scoring systems vary, but in general they are based on the combined severity of several clinical signs associated with respiratory disease.4, 5 Pitfalls of scoring systems include the subjective nature of ranking the severity of clinical signs5 as well as the inherent failure to identify calves with subclinical bovine respiratory disease (sBRD).
The association between postmortem lung lesions and decreased average daily gain in the absence of clinical signs is evidence of sBRD in beef and veal calves.6, 7 Others have documented increased neutrophil proportions8, 9 or pathogens in bronchoalveolar lavage fluid (BALF) from clinically normal calves.10 Although little work has been done to define and measure sBRD in dairy calves, the association between greater body weight gains after implementation of metaphylaxis suggests the presence of sBRD.11 Accurate antemortem methods of detecting sBRD by identification of lung lesions or detection of low‐grade lung inflammation will improve classification of disease in both individuals and herds.
Direct methods of identifying lung inflammation have relied on sampling the airways by means of transtracheal aspiration or bronchoalveolar lavage (BAL). Both methods are invasive, seldom used on the farm, and cut‐points for differential cell counts have not been well defined. Indirect methods of detecting lung inflammation identify the downstream effects of inflammation, such as fever and acute phase proteins,12 but these tests lack specificity (Sp).
Recently, interest in using thoracic ultrasonography (US) to diagnose the lung lesions associated with sBRD in dairy calves has grown.13, 14, 15 Bacterial and occasionally viral respiratory diseases result in non‐aerated superficial lung lobules. This changes lung density, altering the US image from that of a strong reflector with reverberation artifact to a homogenous hypoechoic structure similar to liver.16
The accuracy of US has been documented in clinical cases of bronchopneumonia,17, 18 but few of these reports were prospective or case‐controlled19, 20 and none examined sBRD. Therefore, the first objective of this study was to determine the sensitivity (Se) and Sp of a widely available, portable US unit1 in detecting the lung lesions associated with sBRD in apparently healthy calves. The second objective was to evaluate BALF characteristics and develop a BALF neutrophil proportion cut‐point for determining Se and Sp for detecting sBRD. We hypothesized that US would accurately detect lung lesions and that these lesions would be associated with inflammatory BALF.
Materials and Methods
General
This prospective study was completed between January 1, 2012 and December 15, 2012 at the Elora and Ponsonby Dairy Research Centres of the University of Guelph in southwestern Ontario, Canada. Sixty‐two 3–6‐day‐old Holstein bull calves were enrolled into a separate study evaluating the effect of an intranasal respiratory vaccine over a 12‐week follow‐up period. From this population, 25 calves were selected for the current intensive study. Calves were raised in individual stalls, fed whole milk until 6 weeks of age, and moved to groups by 8 weeks. The Animal Care Committee of the University of Guelph approved this study (AUP #11R110).
Respiratory Scoring and Thoracic Ultrasonography
As part of the other study, one author (TO) and a research technician visited the research centers twice weekly to determine respiratory scores (RSs) according to a standardized respiratory scoring system.4 Calves with RS > 4 were considered sick4 and were excluded from the current intensive study.
Once per week, immediately after the RS, US was performed using a portable linear rectal ultrasound unit1, set at a depth of 9 cm, frequency of 6.2 MHz, and gain of 16 dB (near 13 dB far 36 dB). Approximately 300 mL of 70% isopropyl alcohol was applied to the hair as the transducing agent. The hair was not shaved. Systematic scanning started at the level of the epaxial muscles in the right/left 10th intercostal space (ICS). Within each ICS, the probe was positioned parallel to the ribs and moved ventrally toward the costal arch or the sternum until specified ultrasonographic landmarks were visualized (Tables 1, 2). The probe was moved cranially to examine each ICS up to the right 1st or left 2nd ICS (Fig 1). The scapula marked the dorsal margin of the examination cranial to the 7th ICS bilaterally. The lung adjacent to the right 4th through 1st ICS and left 4th through 2nd ICS was examined with the transducer between the forelimb and the cranial ventral thoracic body wall. Peripheral lung tissue was considered normal when a hyperechoic line with reverberation artifact was present signifying the interface between the high impedance tissue of the thorax and the low impedance tissue of the lung.21 Pleural roughening, or comet‐tailing artifact, was noted when a vertical hyperechoic line emanated from the pleural surface.21 Lung lesions (also referred to as consolidated lung or nonaerated lung) appeared hypoechoic and lacked both the bright white band at the pleural interface and reverberation artifact.16 Lung lesions were documented according to location and size as measured by the ventral‐dorsal distance. All observations were dictated, digitally recorded, and later transcribed to a database.
Table 1.
Landmarks for the right lung during ultrasonographic examination
| Lung Lobe | ||||
|---|---|---|---|---|
| Caudal | Middle | Caudal Aspect of Cranial Lobe | Cranial Aspect of Cranial Lobe | |
| R‐ICS | 6–10 | 5 | 3–4 | 1–2 |
| Ventral landmark(s) | Diaphragm | CCJ & pleural deviation | Heart | Internal thoracic artery & vein |
R‐ICS, right intercostal space; CCJ, costochondral junction.
Table 2.
Landmarks for the left lung during ultrasonographic examination
| Lung Lobe | |||
|---|---|---|---|
| Caudal | Caudal Aspect of Cranial Lobe | Cranial Aspect of Cranial Lobe | |
| L – ICS | 6–10 | 4–5 | 2–3 |
| Ventral landmark(s) | Diaphragm | CCJ & pleural deviation | Heart |
L – ICS, left intercostal space; CCJ, costochondral junction.
Figure 1.
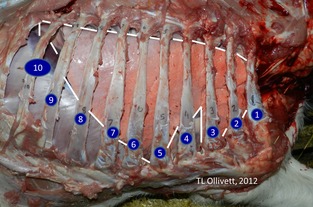
Right lung in situ. The lung is outlined in white. Ribs are labelled by number. Cranial aspect of the right cranial lobe is situated in the 1st and 2nd intercostal spaces.
Animal Selection for Intensive Study
Selection criteria for this intensive study included: RS < 5, previously normal US, and no antimicrobial treatment within the prior 2 weeks or since birth if calves were <2 weeks old. Based on sample size calculations, 4 calves were needed per comparison group to detect a difference of 10% in the BALF neutrophil proportion (standard deviation, 5%; power, 80%; alpha = 0.05). Therefore, for this study, the goal was to obtain 5 calves for each category of a 5‐point ultrasonographic lesion score (LS) for a total of 25 calves. The categorizations were based on one author's clinical experience (TO) regarding normal (LS = 0), very mild (LS = 1), mild (LS = 2), moderate (LS = 3), and severe (LS = 4) levels of ultrasonographic lung lesions in large animals at a University hospital. Definitions for each were as follows: LS0: normal aerated lung imaged as a smooth hyperechoic line adjacent to the body wall with reverberation artifact and no comet‐tail artifact or hypoechoic consolidations; LS1: comet‐tail artifacts imaged as vertical hyperechoic lines emanating from the pleural surface without hypoechoic consolidations; LS2: <1 cm of hypoechoic consolidation lacking the hyperechoic line of the pleura and reverberation artifact in the area of the lesion; LS3: 1–3 cm of hypoechoic consolidation; and LS4: >3 cm of hypoechoic consolidation. Consolidations were measured in the dorsal‐ventral plane.
Approximately once a week, 1–2 calves that met the above criteria were selected for BALF collection, euthanasia, and postmortem examination until all 25 calves were obtained.
Collection and Analysis of Bronchoalveolar Lavage Fluid
Calves were sedated with xylazine hydrochloride2 (0.05 mg/kg IV) and butorphanol3 (0.1 mg/kg IV) and restrained in sternal recumbency. The external naris was cleaned with gauze and a 9 mm (outer diameter), 1.5 m flexible fibreoptic bronchoscope4 previously sterilized with a 2% glutaraldehyde solution, was introduced into the ventral nasal meatus of the clean naris. The bronchoscope then was passed through the nasopharynx into the trachea, extending into a distal airway within the lung containing the ultrasonographic evidence of consolidation or, if normal, the right lung. Once wedged into the bronchus, 2 aliquots of 120 mL sterile saline4 were sequentially dispensed into and retrieved from the airway via the biopsy channel. Once 50% of the aliquot was retrieved, the scope was removed. Diagnostic samples were taken from the last aliquot retrieved, placed in Ca‐EDTA commercial blood collection tubes, and held in an ice water bath until submission to the Animal Health Laboratory at the University of Guelph. Samples were evaluated within 4 hours of collection. Wright‐stained sediment and cytocentrifuge preparations of BALF were evaluated to determine the 200 cell differential cell count. Automated methods5 were used to determine total nucleated cell count (TNCC).
Euthanasia and Postmortem Examination
Immediately after BALF collection, calves were euthanized using captive bolt according to the AVMA's Guidelines for Euthanasia. A respiratory system‐based postmortem examination was performed immediately after euthanasia by one author (TO). During the postmortem examination, the carcass was in lateral recumbency with the affected lung up. The skin, subcutaneous tissues, and rib caudal to the lesion were reflected dorsally to confirm location of the lesion with respect to external landmarks (Fig 2). Once confirmed, all of the ribs were reflected dorsally to expose the whole affected lung. The heart and lung were removed en bloc, placed on ice, and transferred back to the University pathology suite for further evaluation.
Figure 2.
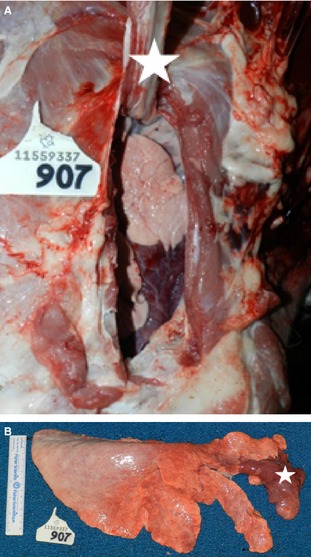
Right lung with lobar consolidation (LS4) of the cranial aspect of the right cranial lobe. (A) In situ specimen. Carcass is in left lateral recumbency. The right 2nd rib (white star) is reflected dorsally exposing the combined 1st and 2nd intercostal space, revealing the cranial aspect of the right cranial lobe. (B) Right lung removed, showing consolidation of cranial aspect of cranial lobe (white star).
Once in the pathology suite, all lung lesions were recorded on a standard diagram after thorough palpation of the tissues to confirm consolidation and identify any pulmonary changes within the parenchyma. Tissue samples for histopathology were taken from the border zone between normal and consolidated lung. Additional samples for histopathology were taken from the right and left cranial lobes, the right middle lung lobe, and the right and left caudal lobes in unaffected portions of affected lungs. In unaffected lungs, samples for histopathology were taken from the right and left cranial lobes, the right middle lung lobe, and the right and left caudal lobes. Routine hematoxylin and eosin stains were made of prepared histopathology sections after at least 24 hours of fixation in 10% buffered formalin. The histologic appearance of the lung was categorized as either normal or consistent with bacterial pneumonia (ie, the presence of neutrophils within the bronchiolar or alveolar lumens) or viral pneumonia (ie, the presence of mononuclear peribronchial or peribronchiolar infiltrates, with or without epithelial necrosis and lobular atelectasis).
Statistical Analyses
Medians and interquartile ranges were calculated for the following variables: age, RS, US lesion size, TNCC, and differential cell counts including neutrophil proportion, macrophage proportion and lymphocyte proportion. For the purposes of analyses, in addition to describing each US category individually, all calves with US consolidation (LS2–4) were combined into 1 group (consolidated, n = 15) for comparison to completely normal (LS0, n = 4) calves and calves with comet‐tailing (LS1, n = 5). The TNCC, neutrophil proportion, macrophage proportion and lymphocyte proportions were compared using Wilcoxon rank sum test.6
Sensitivity and Sp were estimated for US using gross postmortem examination as the gold standard. Ultrasonographic lung lesions (positive = any nonaerated lung imaged; negative = only aerated lung imaged) and gross postmortem lesions (positive = presence of dark red, firm lung tissue; negative = absence of dark red, firm lung tissue) were coded as dichotomous results. Sensitivity was calculated by dividing the number of calves affected with both US and gross lesions (true positives) by the total number of calves with gross lesions (true positive + false negatives). Specificity was calculated by dividing the number of calves without either US or gross lesions (true negatives) by the total number of calves without gross lesion (true negatives + false positives). Exact confidence intervals (95%) were calculated for both Se and Sp.
A receiver operator characteristic (ROC) curve was developed to determine which BALF neutrophil proportion provided the highest combined Se and Sp for predicting lung lesions using gross postmortem examination as the gold standard. Exact confidence intervals (95%) were calculated for both Se and Sp. The relative risk (95% CI) was calculated based on a contingency table for the outcome, BALF neutrophil proportion ≥4%, and the predictor, US lung consolidation (LS2–4). Alpha was set at ≤.05 except during multiple comparisons in which Bonferroni's correction for 5 comparisons set alpha at ≤.01). Unless otherwise noted, commercially available software was used for analysis.7
Results
One BALF sample was damaged in transport, resulting in 24 calves for analysis (LS0, n = 4; LS1, n = 5; LS2, n = 5; LS3, n = 5; LS4, n = 5). Descriptive statistics for age, RS, US lesion size, and BALF for each US category are summarized in Table 3.
Table 3.
Description of respiratory score (RS) and bronchoalveolar lavage fluid (BALF) findings grouped by ultrasonographic (US) lesion score (LS) in Holstein dairy calves. All values represent medians (interquartile ranges)
| Variable | Normal | Comet‐Tails | Varying Levels of US Consolidation | All US Consolidation | ||
|---|---|---|---|---|---|---|
| LS0, n = 4 | LS1, n = 5 | LS2, n = 5 | LS3, n = 5 | LS4, n = 5 | n = 15 | |
| Age (days) | 28 (25–41) | 32 (25–65) | 68 (54–76) | 62 (24–64) | 71 (71–84) | 65 (52–74) |
| RS | 2 (2–2) | 2 (2–3) | 2 (2–4) | 3 (3–3) | 2 (2–3) | 3 (2–4) |
| US lesion (cm) | 0 | 0 | 0.5 (0.25–0.50) | 1.0 (1.0–1.5)b | 6 (4–8)b | 1.5 (0.63–4)a , b |
| TNCC (×109 cells/L) | 0.59 (0.45–0.80) | 0.34 (0.30–0.90) | 0.51 (0.25–0.73) | 0.46 (0.33–0.56) | 0.72 (0.52–0.82) | 0.52 (0.29–0.73) |
| Neut (%) | 1 (1–2) | 2 (2–11) | 25 (9–28) | 8 (3–14) | 17 (12–20) | 14 (7–22)a |
| Mac (%) | 97 (96–99) | 91 (78–93) | 69 (67–79) | 82 (80–92) | 79 (74–85) | 79 (74–89)a |
| Lymph (%) | 1 (1–2) | 7 (5–8) | 6 (3–11) | 5 (4–8) | 3 (3–3) | 4 (3–8) |
LS0, completely normal ultrasonographic exam; LS1, comet‐tailing artifacts on ultrasonographic exam; LS2, <1 cm consolidation on ultrasonographic exam; LS3, 1–3 cm consolidation on ultrasonographic exam; LS4, >3 cm consolidation on ultrasonographic exam; TNCC, total nucleated cell count; Neut, BALF neutrophil proportion; Mac, BALF macrophage proportion; Lymph, BALF lymphocyte proportion.
aDiffers significantly from LS0. bDiffers significantly from LS1. Bonferroni's correction for multiple comparisons P ≤ .01.
The Se and Sp of US for detecting the lung lesions associated with sBRD was 94% (95% CI, 69–100%) and 100% (95% CI, 64–100%), respectively. Ultrasonographic consolidation was associated with firm, red lung lesions on gross examination in all cases (n = 15). All lung lesions were located within the cranial or right middle lung lobes. The cranial aspect of the right cranial lung lobe was completely consolidated in 4/5 LS4 calves (Figs 2, 3). Ultrasound examination failed to detect a 1 cm area of atypical consolidation located in the dorsomedial aspect of the right lung of 1 calf, but severe diffuse comet‐tailing artifacts were observed within the right 5th ICS of this particular calf. Histopathology results are summarized in Figure 4. Briefly, 4/4 LS0 calves had normal histopathology results, whereas 5/5 LS4 calves had evidence of only bacterial lung lesions. Both viral and bacterial lesions were present histologically in calves in the remaining categories (LS1–3).
Figure 3.
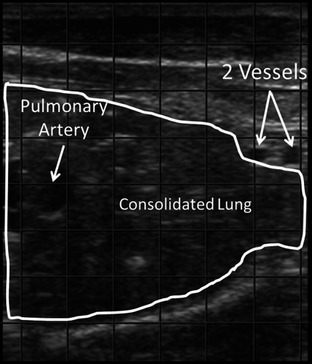
Ultrasonographic image of consolidated lung imaged from the right 1st intercostal space. Image orientation: left = dorsal, right = ventral, top = superficial, bottom = deep. The 2 blood vessels are the internal thoracic artery and vein.
Figure 4.
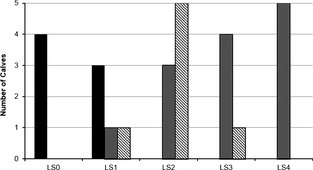
Lung tissue histopathology results (n = 24). Black = no lesions. Gray = bacterial lesion. Striped = viral lesion. LS0: no ultrasound (US) consolidation; LS1: comet‐tail artifact; LS2: <1 cm US consolidation; LS3: 1–3 cm US consolidation; LS4: >3 cm US consolidation.
The TNCC was 0.52 × 109 cells/L in BALF from consolidated lungs (LS2–4, n = 15) versus 0.59 × 109 cells/L from completely normal lungs (LS0, n = 4; P = .65). The neutrophil proportion was 14% in BALF from consolidated lungs versus 1.25% from completely normal lungs (P = .005). The neutrophil proportion in calves with comet‐tailing (LS1, n = 5) was 2% versus 14% from consolidated lungs (P = .05) and 1.25% from completely normal lungs (P = .22). The BALF macrophage proportion was 79% from consolidated and 97% from completely normal lungs (P = .006). The macrophage proportion in calves with comet‐tailing was 91% compared to 97% from that of completely normal lungs (P = .06) and 79% from consolidated lungs (P = .24).
The highest BALF Se (81%, 95% CI, 56–94%) and Sp (75%, 95% CI, 36–95%) was associated with a cut‐off of ≥4% neutrophils based on the receiver operator characteristics (AUC, 0.85; 95% CI, 0.67–1.0; Fig 5). The presence of US lung lesions increased the risk of having a BALF neutrophil proportion ≥4% (RR, 3.9; 95% CI, 1.13–13.45; P = .003).
Figure 5.
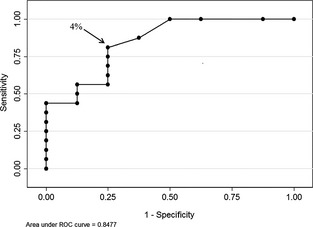
Receiver operator characteristic curve demonstrating the neutrophil proportion ≥4% in bronchoalveolar lavage fluid provides the highest combined sensitivity and specificity for detecting subclinical lung lesions in Holstein dairy calves (n = 24).
Discussion
To the authors' knowledge, this is the first study evaluating a defined US technique and BALF characteristics in dairy calves with sBRD. Identification of US lung lesions accurately predicted the presence of gross and histopathologic lung lesions and was associated with an increased proportion of BALF neutrophils.
Other work has supported the general accuracy of US,17, 19, 20 but direct comparisons among studies should be made with caution considering differences in technique, equipment, and study design. Previously, researchers did not routinely and systematically extend their examinations cranially beyond the 3rd ICS.17, 18, 19, 20, 22, 23, 24 In the current study, 4 of 5 of the most severely affected calves would have been misclassified as normal had these previously mentioned techniques been implemented because the lesions were visible only from the right 1st and 2nd ICS. Because BRD often starts in the cranial aspect of the right cranial lung lobe, it is logical that this lobe must be imaged during examination.25, 26
Although older studies often used linear probes, none report using a linear rectal transducer. The streamlined design of the rectal transducer is conducive to a better examination of the cranial thorax, specifically the right 1st and 2nd ICS. The handle and cord extend from the midsection of traditional probes instead of the end, preventing it from easily sliding between the forelimb and the cranial thoracic body wall for evaluation of the cranial aspect of the cranial lobes. This area previously was thought to be unreachable.18
In the current study, 1 case of atypical consolidation was not detected because it was surrounded by aerated lung. Another study17 observed a similar situation in which a 10 cm abscess was obscured by aerated lung. Ultrasound waves cannot penetrate aerated lung tissue, which serves to hide lesions deep within the parenchyma. Fortunately, most lung lesions associated with BRD extend to the pleural surface.25, 26
Analysis of BALF is used to detect the pulmonary inflammation associated with BRD and has been used to control the confounding effect of sBRD.8 Suggested cut‐points for the proportion of BALF neutrophils are variable, ranging from 10 to 40%.4, 8 Normal calves in the current study had neutrophil proportions similar to those in another study27 which sampled normal lungs at necropsy. Neutrophil proportions also were similar to a previous report8, but 18 of 30 clinically normal calves were excluded from that study8 for having neutrophil proportions >10%. Others have reported much higher BALF neutrophil proportions in clinically normal control animals,9, 10 likely reflecting the presence of sBRD in the control population. Results from the current study suggest that BALF from truly normal calves has very few neutrophils and that clinicians should consider using a cut‐off of 4% when classifying respiratory disease status.
Points to consider in this study include blinding of the examiner and the age of calves. One author (TO) performed all of the US and postmortem examinations. The decision to forego blinding was made a priori based on the need for the individual conducting the postmortem examination to know where to focus the examination of the lung to assess the accuracy of the ultrasound examination in localizing the lesion(s). Future studies incorporating blinding would provide more information regarding test characteristics of ultrasound in these different settings. Furthermore, the Se and Sp estimated in this study are applicable to dairy calves <12 weeks of age. As calves mature, the forelimb musculature increases, limiting access to the first and second ICS. Therefore, the Se of US in older cattle might be lower because of the inability to examine the cranial aspect of the cranial lung lobes.
Conclusions
Thoracic US, when used as described in this study, can provide a rapid, objective assessment of lung health, improve classification of BRD status, and should be considered a primary method of detecting lung lesions for both clinical and research purposes. A neutrophil proportion cut‐off of 4% should be used when using BAL.
Acknowledgments
Financial support was provided by the Ontario Ministry of Agriculture, Food, and Rural Affairs and Zoetis Canada. We thank the following research technicians: Jolene Cyples, Jessica Cyples, Melissa Wagner, Vivianne Bielmann, Sam Deelen, Brittany Stinson, Patrick Chung, and Sarah Stanger‐Guy as well as the Elora and Ponsonby Dairy Research Centre and Animal Health Laboratory staff.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This work was performed at the Elora and Ponsonby Dairy Research Centres, University of Guelph, Ontario, Canada.
This study was presented as a research abstract at the 2013 American Association of Bovine Practitioners Annual Conference, Milwaukee, WI (by Ollivett) and 2014 American College of Veterinary Internal Medicine Annual Forum, Nashville, TN (by Hewson). This manuscript was part of the PhD thesis submitted by Dr. Theresa Ollivett to the University of Guelph.
Footnotes
Ibex Pro, E. I. Medical, Loveland, CO
Rompun, 20 mg/mL, Bayer Inc., Mississauga, ON, Canada
Torbugesic, 10 mg/mL, Zoetis, Kirkland, QC, Canada
E‐22VGS99x17 Veterinary Fiberscope, LSVP International, Los Altos, CA
Coulter ZBI, Hialeah, FL
Kaleidograph 4.1.1, Synergy Software, Reading, PA
Stata 12.1, Stata Corp LP, College Station, TX
References
- 1. USDA . Dairy 2007, Heifer calf health and management practices on U.S. dairy operations, 2007. USDA:APHIS:VS, CEAH Fort Collins, CO #550 0110 2010; 2010.
- 2. Lago A, McGuirk S, Bennett T, et al. Calf respiratory disease and pen microenvironments in naturally ventilated calf barns in winter. J Dairy Sci 2006;89:4014–4025. [DOI] [PubMed] [Google Scholar]
- 3. Heins B, Nydam D, Woolums A, et al. Comparative efficacy of enrofloxacin and tulathromycin for treatment of preweaning respiratory disease in dairy heifers. J Dairy Sci 2014;97:372–382. [DOI] [PubMed] [Google Scholar]
- 4. McGuirk SM. Disease management of dairy calves and heifers. Vet Clin North Am Food Anim Pract 2008;24:139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amrine DE, White BJ, Larson R, et al. Precision and accuracy of clinical illness scores, compared with pulmonary consolidation scores, in Holstein calves with experimentally induced Mycoplasma bovis pneumonia. Am J Vet Res 2013;74:310–315. [DOI] [PubMed] [Google Scholar]
- 6. Wittum T, Woollen N, Perino L, Littledike E. Relationships among treatment for respiratory tract disease, pulmonary lesions evident at slaughter, and rate of weight gain in feedlot cattle. J Am Vet Med Assoc 1996;209:814–818. [PubMed] [Google Scholar]
- 7. Leruste H, Brscic M, Heutinck LFM, et al. The relationship between clinical signs of respiratory system disorders and lung lesions at slaughter in veal calves. Prev Vet Med 2012;105:93–100. [DOI] [PubMed] [Google Scholar]
- 8. Pringle JK, Viel L, Shewen PE, et al. Bronchoalveolar lavage of cranial and caudal lung regions in selected normal calves: Cellular, microbiological, immunoglobulin, serological and histological variables. Can J Vet Res 1988;52:239–248. [PMC free article] [PubMed] [Google Scholar]
- 9. Allen J, Viel L, Bateman K, et al. Cytological findings in bronchoalveolar lavage fluid from feedlot calves: Associations with pulmonary microbial flora. Can J Vet Res 1992;56:122–126. [PMC free article] [PubMed] [Google Scholar]
- 10. Allen J, Viel L, Bateman K, Rosendal S. Changes in the bacterial flora of the upper and lower respiratory tracts and bronchoalveolar lavage differential cell counts in feedlot calves treated for respiratory diseases. Can J Vet Res 1992;56:177–183. [PMC free article] [PubMed] [Google Scholar]
- 11. Stanton A, Kelton D, LeBlanc S, et al. The effect of respiratory disease and a preventative antibiotic treatment on growth, survival, age at first calving, and milk production of dairy heifers. J Dairy Sci 2012;95:4950–4960. [DOI] [PubMed] [Google Scholar]
- 12. Angen Ø, Thomsen J, Larsen LE, et al. Respiratory disease in calves: Microbiological investigations on trans‐tracheally aspirated bronchoalveolar fluid and acute phase protein response. Vet Microbiol 2009;137:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buczinski S, Forte G, Francoz D, Bélanger AM. Comparison of thoracic auscultation, clinical score, and ultrasonography as indicators of bovine respiratory disease in preweaned dairy calves. J Vet Intern Med 2014;28:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buczinski S, Forté G, Bélanger A. Short communication: Ultrasonographic assessment of the thorax as a fast technique to assess pulmonary lesions in dairy calves with bovine respiratory disease. J Dairy Sci 2013;96:1–6. [DOI] [PubMed] [Google Scholar]
- 15. Ollivett TL, Burton AJ, Bicalho RC, Nydam DV. Use of rapid US for detection of subclinical and clinical pneumonia in dairy calves In: Proceedings American Association of Bovine Practitioner, Vol. 44. Stillwater, OA: VM Publishing Company; 2011:148. [Google Scholar]
- 16. Reef VB, Whittier M, Griswold Allam L. Thoracic ultrsonography. Clin Tech Equine Pract 2004;3:284–293. [Google Scholar]
- 17. Rabeling B, Rehage J, Döpfer D, Scholz H. Ultrasonographic findings in calves with respiratory disease. Vet Rec 1998;143:468–471. [DOI] [PubMed] [Google Scholar]
- 18. Reinhold P, Rabeling B, Günther H, Schimmel D. Comparative evaluation of ultrasonography and lung function testing with the clinical signs and pathology of calves inoculated experimentally with Pasteurella multocida . Vet Rec 2002;150:109–114. [DOI] [PubMed] [Google Scholar]
- 19. Flöck M. Diagnostic ultrasonography in cattle with thoracic disease. Vet J 2004;167:272–280. [DOI] [PubMed] [Google Scholar]
- 20. Jung C, Bostedt H. Thoracic ultrasonography technique in newborn calves and description of normal and pathological findings. Vet Radiol Ultrasound 2004;45:331–335. [DOI] [PubMed] [Google Scholar]
- 21. Blond L, Buczinski S. Basis of ultrasound imaging and the main artifacts in bovine medicine. Vet Clin North Am Food Anim Pract 2009;25:553–565. [DOI] [PubMed] [Google Scholar]
- 22. Braun U, Pusterla N, Flückiger M. Ultrasonographic findings in cattle with pleuropneumonia. Vet Rec 1997;141:12–17. [DOI] [PubMed] [Google Scholar]
- 23. Babkine M, Blond L. Ultrasonography of the bovine respiratory system and its practical application. Vet Clin North Am Food Anim Pract 2009;25:633–649. [DOI] [PubMed] [Google Scholar]
- 24. Abutarbush SM, Pollock CM, Wildman BK, et al. Evaluation of the diagnostic and prognostic utility of ultrasonography at first diagnosis of presumptive bovine respiratory disease. Can J Vet Res 2012;76:23–32. [PMC free article] [PubMed] [Google Scholar]
- 25. Allan EM, Gibbs HA, Wiseman A, Selman IE. Sequential lesions of experimental bovine pneumonic pasteurellosis. Vet Rec 1985;117:438–442. [DOI] [PubMed] [Google Scholar]
- 26. Dagleish MP, Finlayson J, Bayne C, et al. Characterization and time course of pulmonary lesions in calves after intratracheal infection with Pasteurella multocida A:3. J Comp Pathol 2010;142:157–169. [DOI] [PubMed] [Google Scholar]
- 27. Fogarty U, Quinn P, Hannan J. Bronchopulmonary lavage in the calf – A new technique. Ir Vet J 1983;37:35–38. [Google Scholar]


