Abstract
Background
Canine cognitive dysfunction (CCD) is an age‐dependent neurodegenerative condition dominated by changes in behavioral patterns. Cohort studies investigating cognitive status in dogs are lacking.
Objectives
To investigate cognitive function, progression of age‐related behavioral changes, survival, and possible biomarkers of CCD in aged dogs.
Animals
Fifty‐one dogs >8 years old; 21 with no cognitive deficits, 17 with mild cognitive impairments (MCI) and 13 with CCD.
Methods
Longitudinal study. Recruitment period of 12 months and an observational period of 24 months including a baseline and 3 planned subsequent assessments. Cognitive status was determined using validated questionnaires. Plasma Aβ‐peptides were quantified using commercial ELISA assays and cytokines by a validated immunoassay.
Results
Signs characterizing dogs with CCD were aimless wandering, staring into space, avoid getting patted, difficulty finding dropped food and anxiety. Thirty‐three percent of dogs with a normal cognitive status progressed to MCI and 22% classified as MCI progressed to CCD during the study period. For 6 dogs diagnosed with CCD, signs of cognitive dysfunction increased with time. A diagnosis of CCD did not affect survival. The level of plasma Aβ42 was significantly increased (P < .05) in the CCD group (92.8 ± 24.0 pg/mL) compared to the MCI (77.0 ± 12.3 pg/mL) and normal group (74.9 ± 10.0 pg/mL), but no significant differences in concentrations of systemic inflammatory markers were detected.
Conclusions
Canine cognitive dysfunction is a progressive disorder with an individual variability in the rate of cognitive decline and clinical signs. Plasma Aβ42 seems to be an interesting plasma biomarker of CCD.
Keywords: Amyloid‐beta, Canine, Dementia, Geriatric, Longitudinal
Abbreviations
- AD
Alzheimer's disease
- Aβ
amyloid‐beta
- CCD
canine cognitive dysfunction
- CCDR
canine cognitive dysfunction rating scale
- CSF
cerebrospinal fluid analysis
- CT
computed tomography
- HRP
horse‐radish peroxidase
- MCI
mild cognitive impairment
- MRI
magnetic resonance imaging
- MSD
Meso Scale Discovery®
- OP
optical density
- SD
standard deviations
Senior dogs (those aged > 8 years) spontaneously can develop neurodegenerative cerebral changes and associated impairment of cognitive functions. A specific clinical syndrome characterized by cognitive changes which are not normal for age and cannot be explained by other medical conditions occurs in dogs more than 8 years of age1, 2, 3 and shares multiple similarities to human dementia of the Alzheimer's type.4, 5, 6, 7, 8 The syndrome is referred to as canine cognitive dysfunction (CCD),2, 9 cognitive dysfunction syndrome,1 and canine counterpart of senile dementia of the Alzheimer's type.10 The term CCD shall be used in this article.
The prevalence of CCD ranges from 14 to 35% in companion dogs more than 8 years of age, and the risk of developing CCD increases exponentially with increasing age.11, 12, 13, 14 Changes in behavior and daily routines are considered the most important clinical markers of cognitive dysfunction in aged dogs. Therefore, the diagnosis of CCD is primarily driven by owner‐based questionnaires and clinical rating scales addressing behavioral alterations within the categories disorientation, social interaction, sleep‐wake cycle disturbances, house‐soiling, and changes in activity.1, 3, 9, 15, 16 Furthermore, signs of fear and anxiety that have not been present at a younger age are common in dogs with CCD.1, 2 Although developed from different designs and strategies, the dementia score from 3 of the existing CCD screening questionnaires correlated well.10 The result obtained from CCD questionnaires must, however, always be supported by a thorough clinical evaluation in order to exclude systemic or primary behavioral conditions that could possibly be causing the signs displayed by the affected dog.1
The natural history of cognitive dysfunction in senior dogs is sparsely documented.2, 17 It is therefore desirable to gain more information regarding the clinical phenotype, progression, and prognosis of CCD. For this purpose, epidemiological longitudinal studies are needed. Another area of interest is to search for potential biomarkers, which in the future might help clinicians to identify dogs suffering from CCD and prompt early supportive initiatives.
The aim of the present study was to provide longitudinal information of cognitive function, progression of age‐related behavioral changes and survival in a cohort of dogs more than 8 years old with and without signs of cognitive dysfunction at study inclusion. As easily accessible biomarkers for CCD are needed in veterinary medicine, we also investigated if systemic levels of Aβ‐peptides, cytokines, or inflammatory markers were significantly higher in dogs with CCD compared to dogs with no or mild cognitive impairments (MCI).
Materials and Methods
Study Design and Procedure
The study was designed as a prospective longitudinal cohort study and was carried out at the Department of Veterinary Clinical and Animal Sciences, University of Copenhagen from February 2012 to April 2015. Client‐owned dogs were recruited consecutively through the community practice and neurology referral clinic at the University Hospital for Companion animals.
The animals were treated according to the EU directive on handling and protection of animals used for scientific purposes (2010/63/EU) and the study was approved by the Ethical Committee of the Department of Veterinary Clinical and Animal Science, University of Copenhagen, Denmark. Informed and written consent was obtained from all owners.
The inclusion criteria was that dogs should be more than 8 years old and exclusion criteria were brain diseases other than CCD or concurrent medical problems that could possibly mimic signs of cognitive impairment. The inclusion period was open from February 2012 to March 2013 and the study period ended in May 2015.
The study design implicated a baseline assessment at inclusion (E0), and 3 planned subsequent evaluations which served to monitor cognitive status over time: Six months after inclusion (E1), 12 months after inclusion (E2), and 24 months after inclusion (E3). All investigations were performed by the principal investigator (TS) to secure a structured and consistent evaluation of all dogs.
The baseline assessment E0 served to recruit dogs and furthermore to categorize the study population into 3 groups; a cognitively normal non‐CCD group, a group with MCI, and a cognitively impaired CCD group. All dogs had a medical and cognitive evaluation including a clinical and neurological examination and assessment of body condition score. The auditory and visual system was evaluated as is standard in a full neurological examination. Hearing was tested by evaluating if the animal responded to sudden and unexpected sounds. Vision was evaluated by observing how the dog navigated in an unfamiliar environment and by testing the menace response which includes evaluation of all visual pathways. Additionally, the owner was instructed to report any changes of behavior in the home environment which could indicate a hearing or vision problem. The clinical evaluation also included collection of blood samples for complete blood count, serum biochemistry (inclusive of C‐reactive protein and fibrinogen), thyroid profile and quantification of plasma Aβ‐peptides and circulating cytokines. Further diagnostic work‐up such as urinalysis, abdominal sonography, echocardiography, computed tomography (CT), magnetic resonance imaging (MRI), or cerebrospinal fluid analysis (CSF) were performed as indicated at the discretion of the principal investigator. Cognitive evaluation was based on face‐to face interviews with the owners using the Canine Cognitive Rating Scale (CCDR)9 (Table S1) and a supplementary CCD screening questionnaire10 (Table S2). To address signs of fear and anxiety, 2 additional standardized questions were given to the owners. An interview would last for a minimum of 30 minutes. Answers were recorded for each dog in a separate file. The owners were encouraged to record their dog's behavior on video and the video observations were included in the assessment of cognitive status when available. Dogs were distributed into the non‐CCD group, the MCI group and the CCD group based on the scoring results from the CCDR. According to the CCDR, a total score below 39 classifies a dog as having a normal cognitive status (non‐CCD), a score of 40–49 classifies a dog as being at risk of developing CCD (MCI), and a total score above 50 classifies a dog as having CCD. The owners were asked to consent to donate the dog for postmortem examination if the dog was euthanized by the principal investigator during the study period.
Evaluations at 6 months and 24 months (E1 and E3) were conducted as structured telephone interviews using the CCDR questionnaire9 and a supplementary CCD screening questionnaire.10
Evaluation at 12 months (E2) was performed as a clinical control visit and included the same investigations as performed at E0 except for quantification of thyroid hormones, plasma Aβ‐peptides and circulating cytokines. For dogs that were euthanized or died spontaneously during the study period, the last investigator‐client contact was at time of death or at the first coming prescheduled contact.
Blood Sampling and Analysis
Blood samples were collected from the cephalic or jugular vein. The hematologic and biochemical profiles (including C‐reactive protein, fibrinogen, and thyroid hormones) were analyzed at the Central Laboratory, Department of Veterinary Clinical and Animal Sciences, University of Copenhagen.
Blood samples for Aβ and cytokine quantification were collected into vials containing EDTA, centrifuged (2,500 × g, 15 minutes, 4°C), and plasma was immediately separated, snapfrozen on dry ice and stored at −80°C until batch analysis. Aβ40 and Aβ42 was measured using commercially available ELISA sandwich kits; Human βAmyloid(1–40) II and Human/Rat βAmyloid(42) High‐Sensitive.1 The applied capture antibodies for the N‐terminal portion of human Aβ40 and Aβ42 are monoclonal anti‐Aβ1–16 (BAN50) and monoclonal Aβ11–28 (BNT77), the C‐terminal detection antibodies are HRP‐conjugated anti‐Aβ1–40 (BA27) and anti‐Aβ35–43 (BC05), respectively. All samples were initially diluted 1:1 in urea (8 M) in order to monomerize the Aβ fibrils and reveal more epitopes to the detection antibody. Addition of urea to the samples did not have any influence on the performance of the ELISA kits from Wako.
N‐terminal pyroglutamate‐modified Aβ was quantified using the specific ELISA kit; Amyloid‐β N3pE‐42.2 Capture antibody for this assay is antihuman Aβ(38–42) and the detection antibody is HRP‐conjugated anti‐Aβ N3pE (8E1). All samples were run in duplicates and optical density (OD) values were measured at 450 nm using an ELISA plate reader.3
Plasma concentration of IL‐2, IL‐6, IL‐8, and TNFα were simultaneously measured with a commercially available canine‐specific multiplex immunoassay which employs an electrochemiluminescence detection technology.4 The provided protocol for custom assay was used with no major modifications. The lowest detectable limit specified in the data sheet for IL‐2, IL‐6, IL‐8, and TNFα were 7.6, 2.4, 1.3, and 0.17 pg/mL, respectively.
Statistical Analysis
Descriptive analyses of the results obtained from the questionnaires and the additional questions regarding anxiety were carried out stratified by cognitive status. One‐way analysis of variance (ANOVA) with post‐tests (Tukey's multiple comparison test) was used for comparisons of parametric data from the 3 groups and Kruskal‐Wallis test with post‐tests (Dunn's multiple comparison test) was used for ordinal variables. Because of low sample sizes Fischer's exact test was applied for analysis of contingency tables containing 2 or 3 categorical variables. Correlations were assessed graphically as well as by Pearson's or Spearman Rank correlation coefficient where appropriate.
Survival curves, median survival time, and 95% confidence intervals were obtained by the Kaplan‐Meier method and differences in survival were tested by the log‐rank test. Survival time was counted from the day of birth to the day of death or right censored at end of the study. Outcome registration ended first of May 2015, outcome was defined as euthanasia primarily because of behavioral changes as a consequence of CCD or euthanasia/death because of other reasons.
Statistical significance was defined as P < .05. All statistical analyses were conducted using commercial statistical software.5
Results
Descriptive Data
A total of 57 privately owned dogs, 32 females and 25 males of various breeds, and ranging from 8 to 15 years (108–197 months) were enrolled in the study during the inclusion period. A total of 6 dogs were excluded, 3 dogs because of systemic disease, and 3 dogs because of inadequate owner interviews and thereby insufficient data, leaving a study population of 51 dogs. All dogs had a normal clinical and neurologic examination. No deficits of vision or hearing that could account for the cognitive changes were detected. Based on the scoring results from the CCDR, 21 dogs were categorized in the non‐CCD group, 17 dogs in the MCI group, and 13 dogs in the CCD group at E0.
The non‐CCD group represented 14 different breeds and 4 mixed breeds with an age range of 106–197 months. The MCI group represented 10 different breeds and 4 mixed breeds with an age range of 108–192 months and the CCD group represented 7 different breeds and 4 mixed breeds with an age range of 127–192 months.
There were no significant differences with respect to sex distribution, weight, and body condition score between the cognitive groups at inclusion (Table 1). A significantly higher age was found for the dogs in the CCD group compared to dogs in the non‐CCD and MCI groups (P = .04).
Table 1.
Association between sex, weight, body condition score, age, and cognitive status
| Non‐CCD (n = 21) | MCI (n = 17) | CCD (n = 13) | P‐value | |
|---|---|---|---|---|
| Sex | ||||
| Female | 12 | 11 | 5 | .25a |
| Male | 9 | 6 | 8 | |
| Weight (kg) | ||||
| Mean ± SD | 18.9 ± 9.2 | 19.6 ± 9.0 | 13.9 ± 8.7 | .20b |
| Body condition score (1–9) | ||||
| Median | 6 | 4 | 7 | .11c |
| Lower quantile | 5 | 4 | 4.5 | |
| Upper quantile | 7 | 6 | 8 | |
| Age (months) | ||||
| Mean ± SD | 148 ± 22 | 149 ± 22 | 167 ± 19 | .039b |
CCD, canine cognitive dysfunction; MCI, mild cognitive impairment. Categorization in cognitive groups is based on the total canine cognitive ratings scale score.
P‐values is calculated by: aFischer's exact test, bANOVA, cKruskal‐Wallis test.
Fifteen dogs (10 dogs with CCD, 2 dogs with MCI and 3 dogs with no signs of CCD) had a postmortem examination of the brain using the trimming protocol for evaluation of large‐sized brains.18 Aside from cortical thinning and ventricular enlargement which were present in a number of CCD brains, no macroscopic lesions were detected.
Clinical Phenotype of CCD
Table 2 shows the distribution of impaired behavioral items for the 3 groups. The most frequent signs displayed by dogs in the CCD group were “pacing/wandering with no direction or aim” and “staring blankly at the walls or floor” as 91.7% of dogs displayed this behavior once a week or more frequently. “Avoiding being patted” and “difficulty finding dropped food” was observed in 75% of dogs in the CCD dogs. Supplementary questions revealed that separation anxiety or irrational fear to well‐known objects/situations was observed in 33 and 58.3% of dogs in the CCD group, respectively.
Table 2.
Frequency distribution of number of dogs (and percentages) with affected behavioral items in the non‐CCD, MCI, and CCD group
| Category | Items | Non‐CCD (n = 21) n (%) | MCI (n = 16) n (%) | CCD (n = 12) n (%) | P‐value$ |
|---|---|---|---|---|---|
|
Spatial orientation Disorientation Awareness |
Gets stuck behind objects and is unable to get around (happens once a week or more frequently) | 1 (4.8) | 7 (43.8) | 6 (50) | 1.0 |
| Walks into doors or walls (happens once a week or more frequently) | 1 (4.8) | 1 (6.3) | 6 (50) | * | |
| Stares blankly at walls or floor (happens once a week or more frequently) | 4 (19) | 9 (56.3) | 11 (91.7) | .09 | |
| Disoriented at home | 0 | 2 (12.5) | 4 (33) | .43 | |
| Memory | Fails to recognize familiar people or pets (happens once a week or more frequently) | 0 | 0 | 1 (8.3) | .43 |
| Indoor urination/defecation in areas previously kept clean happens much more compared to 6 months ago | 0 | 1 (6.3) | 5 (41.7) | .057 | |
| Activity—apathy | Avoids contact or being patted by the owner (happens once a week or more frequently) | 3 (14.3) | 1 (6.3) | 9 (75) | *** |
| Much less active compared to 6 months ago | 1 (4.8) | 1 (6.3) | 6 (50) | * | |
| Impaired olfaction | Difficulty finding dropped food in more than 31% of times | 0 | 4 (25) | 9 (75) | * |
| Locomotion | Paces up and down or wanders with no direction/purpose (happens more than once a week) | 2 (9.5) | 13 (81.3) | 11 (91.7) | .61 |
| Anxiety | Separation anxiety arisen after 8 years of age | 2 (9.5) | 0 | 4 (33.3) | *** |
| Irrational fear to well‐known objects/situations | 5 (23.8) | 2 (12.5) | 7 (58.3) | * | |
| Learning and memory | Decreased ability/slow to learn new tasks | 4 (19) | 6 (37.5) | 9 (75) | ** |
| Decreased ability to perform known tasks | 0 | 6 (37.5) | 7 (58.3) | .45 | |
| Sleep‐wake cycle | Sleeps at day and restless at night | 0 | 5 (33) | 5 (41.7) | .70 |
CCD, canine cognitive dysfunction; MCI, mild cognitive impairment. Categorization in cognitive groups is based on the total canine cognitive ratings scale score.
Data were derived from the inclusion visit (C0) as we wished to include answers from the first time the owner were given the questionnaires. Note that for 2 dogs (1 dog in the MCI group and 1 dog in the CCD group), specific data from the canine cognitive dysfunction rating scale are missing at inclusion, therefore these dogs are excluded in this analysis.
$ P‐values corresponding to statistical significant differences between the MCI and the CCD group.
*P < .05, **P < .01, ***P < .001.
The below signs were significantly more prevalent for dogs in the CCD group than for dogs in the MCI group: “avoids contact or being patted by the owner” (P < .001), “much less active compared to 6 months ago” (P < .05), “difficulty finding dropped food” (P < .05), and “walks into doors or walls” (P < .05).
The proportion of dogs with affected behavioral items in the CCD group was significantly higher than for the proportion of dogs with affected items in the non‐CCD group for all behavioral items except for “separation anxiety” (P = .16), “irrational fear to well‐known object or situations” (P = .052), and “fails to recognize family members” (P = .36).
Changes in Cognitive Status over Time
When analyzing changes in the CCDR score over time for the individual dogs, we found that in the non‐CCD group 7 dogs (33%) progressed to the MCI group from baseline assessment to death or end of study. Two of these dogs progressed further from MCI to CCD. For the MCI group, 4 dogs (22%) progressed into a status of CCD from baseline assessment to death or end of study. No dogs evaluated as CCD during the study changed to a non‐CCD or MCI status during the study. Four dogs with CCD at E0 and the 2 dogs which progressed from non‐CCD to CCD displayed an increasing CCDR score with time. In 8 dogs (61.5%) in the CCD group had died or were euthanized within four months after the date of inclusion therefore no follow‐up evaluation was possible. The CCDR score and time of death for the individual dogs from inclusion to end of study period is illustrated in Fig 1.
Figure 1.
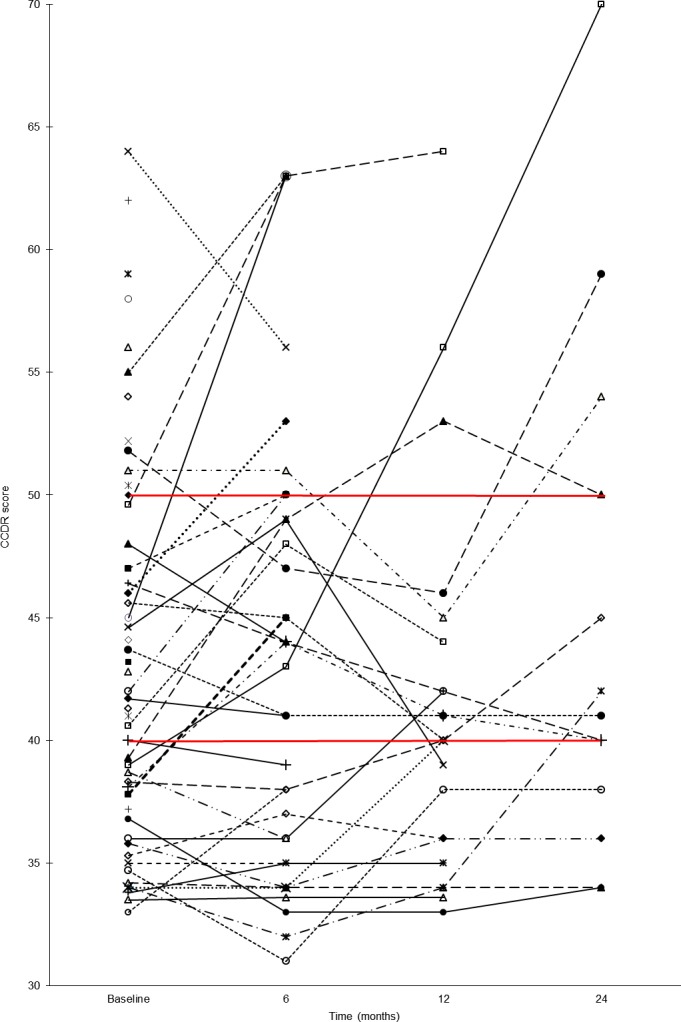
The canine cognitive dysfunction rating scale score and time of death for the individual dogs from inclusion to end of study period. The red lines indicate the cut‐off values between cognitive groups. Note that for dogs which died between 2 preplanned assessments, time of death will appear in the figure at the next preplanned evaluation. At 24‐month evaluation, 5 dogs had died between the 12 months and the 24 months evaluation and 8 dogs were still alive, thus evaluation was accomplished for 13 dogs in total.
Survival Analysis
At the last contact (C3), 41 dogs (82.4%) were dead and 8 dogs (15.7%) were still alive. Two dogs (3.9%) were lost for follow‐up between E0 and E1. For the survival analysis, the dogs were divided into 2 groups, a control group which consisted of 33 dogs that had non‐CCD or MCI at baseline evaluation and no progression of cognitive status from non‐CCD/MCI to CCD from baseline to death/end of study. The survival of this group was compared to the CCD group which consisted of 19 dogs that was diagnosed with CCD at baseline evaluation or had progressed to develop CCD during the study. Median survival time was 4,844 days for the control group and 5,367 days for CCD dogs (log‐rank test P‐value of .62). The mean follow‐up time from inclusion to death for this study was 406 days (range from 0 to 1,113 days).
Plasma Biomarkers
There were no significant differences in concentrations of biochemical and hematological parameters between the non‐CCD, MCI, and CCD groups (data not shown). The mean Aβ concentrations and Aβ42/Aβ40 ratio for each group are shown in Table 3. Concentration of plasma AβpN3‐42 was nonquantifiable as all samples were below lower limit of detection (7.75 pg/mL). Levels of Aβ40 correlated well with Aβ42 level across the study groups (r = 0.59, P < .0001).
Table 3.
Concentration of plasma Aβ40 and Aβ42 (pg/mL) and Aβ42/40 ratios
| Group | Aβ40 (pg/mL) | Aβ42 (pg/mL) | Aβ42/Aβ40 |
|---|---|---|---|
| Non‐CCD (n = 21) | 276.1 ± 63.0 | 74.9 ± 10.0 | 0.28 |
| MCI (n = 17) | 314.8 ± 89.4 | 77.0 ± 12.3 | 0.26 |
| CCD (n = 13) | 369.3 ± 100.9* non‐CCD | 92.8 ± 24.0** non‐CCD, MCI | 0.26 |
CCD, canine cognitive dysfunction; MCI, mild cognitive impairment. Categorization in cognitive groups is based on the total canine cognitive ratings scale score.
*P < .01 to the non‐CCD group; **P < .01 to the non‐CCD group, and P < .05 to the MCI group.
Plasma concentration of both Aβ40 and Aβ42 varied considerably between dogs in all 3 groups, as denoted by the high standard deviations (SD). The CCD group revealed significantly higher levels of plasma Aβ42 levels (P < .05) than the MCI and the non‐CCD group (Fig 2).
Figure 2.
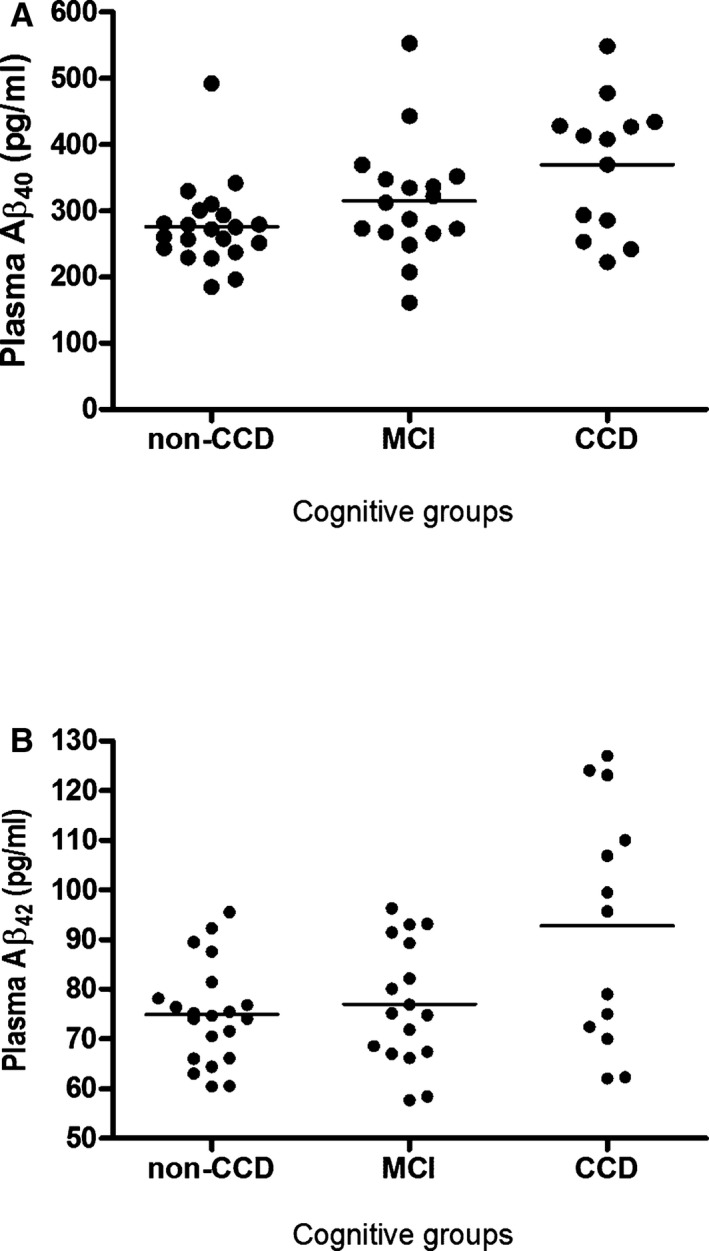
Plasma Aβ40 and Aβ42 measurements for individual dogs stratified by cognitive status. MCI, mild cognitive impairment; CCD, canine cognitive dysfunction.
Nevertheless, individual values of Aβ42 measurements showed considerable overlap between the 3 groups. Within the CCD group, a wide dispersion of the Aβ42 measurements was present resulting in an apparent distribution into 2 clusters. One cluster of 7 CCD dogs had Aβ42 levels above 95 pg/mL and for the other cluster consisting of 6 dogs, the Aβ42 levels were less than 80 pg/mL. No significant difference in CCDR score was evident between the 2 clusters of CCD dogs. An increased plasma level of Aβ40 (r = 0.39, P < .005) and Aβ42 (r = 0.31, P < .05) were positively correlated with the CCDR score. Aβ40 levels or the Aβ42/Aβ40 ratio was not significantly different between groups.
Concentrations of CRP, fibrinogen, and IL‐8 were quantified in all dogs. IL‐2 and IL‐6 could not be quantified in plasma from 56 and 33% of the dogs, respectively, as measurements were below the lower limit of detection. Measurements of plasma TNFα could not be quantified or were just above lower detection limit in all dogs. Dogs with non‐quantifiable concentrations of IL‐2 and IL‐6, and all TNFα measurements were excluded from the statistical analysis. There were no significant differences in concentrations of any of the systemic cytokines, C‐reactive protein, or fibrinogen between dogs with CCD and dogs with no or MCI (data not shown).
Discussion
This study investigated cognitive function in a cohort of senescent dogs in a prospective longitudinal study design. The clinical phenotype associated with cognitive dysfunction in older dogs has been compared to humans suffering from Alzheimer's disease (AD),4, 5, 6, 7, 8, 19, 20 and our study supported that striking similarities exists when evaluating the clinical appearance of affected dogs. The study documented that some dogs will develop signs of cognitive dysfunction with increasing age, whereas others remain cognitively healthy. Clinical signs of cognitive decline in dogs with CCD are cumulative and will worsen over time. This is also the case for humans developing (AD).21, 22 Based on the scoring results from the CCDR, the categories which were most affected in dogs with CCD were social interaction, activity, spatial orientation, and sleep‐wake cycle, consistent with other studies.2, 11, 13 With respect to specific clinical signs, “aimless wandering,” “staring blankly into space,” “avoiding being patted,” and “difficulty with finding dropped food” were the most common signs displayed by dogs with CCD (Table 2). Decreased recognition of familiar people was an uncommon sign in CCD dogs in the present study. This is in accordance with that has been reported in 2 previous studies,2, 16 but in contrast to another study which reported that decreased recognition of familiar people was present in 60% of dogs with severe behavioral changes and thus a dominant sign of CCD.9 The discrepancy between studies is most possibly explained by variations in the age and disease severity displayed by the animals included in the study populations. Furthermore, a factor which hampers comparisons between studies is that different questionnaires which do not necessarily address the same behavioral categories have been used to assess cognitive status.
Except for the CDS checklist,1 the CCDR is the only CCD screening questionnaire which has also incorporated “difficulty finding dropped food” as an indicator of decreased olfaction. This sign was prevalent in CCD dogs in this study population. Decreased olfaction has previously been associated with CCD9 and our results support that this sign could be a possible clinical indicator for CCD. Olfactory dysfunction and more specific impaired odor identification have also been reported for patients with AD and is presently investigated as an early marker of preclinical AD.23, 24, 25
Only few studies exploring CCD have investigated anxiety and fear as signs of significant interest.1, 2 This is quite surprising given that behavioral changes related to fear, phobias and anxiety have previously been reported to be prevalent signs by owners of senior dogs.26 Such signs are presumably comparable to agitation and anxiety which is well known in humans with MCI and AD.27, 28 Our research group has previously shown that anxiety and fear are common in dogs with CCD,2 which was why we also found it of importance to investigate such signs in this study. We found that separation anxiety and irrational fear to well‐known objects/situations were present in 33% and 58% of dogs with CCD, respectively. This is even higher than the one previously reported2, and emphasize both the importance of including questions addressing anxiety in future CCD screening questionnaires and the need to treat dogs with such troubling problems.
We used the CCDR for assessment of cognitive status as it is very useful for monitoring the progression of cognitive dysfunction.9 A progression of the CCDR score over time was documented to occur in dogs from all groups (non‐CCD, MCI, and CCD) and some dogs progressed from having mild cognitive disturbances (MCI) to displaying convincing signs of CCD. Our results supports that a preclinical stage of CCD is present in a subset of aged dogs. As previously reported, some dogs with MCI will develop CCD, whereas others will never progress.2 In humans, such a symptomatic pre‐dementia phase is recognized as MCI due to AD.29 Only a proportion of human patients identified with mild cognitive deficits experience successive worsening of cognitive impairment, which eventually develops into fulminant AD.29, 30 For 2 dogs with a normal cognitive status at baseline assessment, a significant progression of the CCDR score was reported at E1 and the CCDR score was further increased at E2, now categorizing the dogs as CCD. Both dogs were evaluated at E2, where no other medical causes than CCD could explain the rapid progression of cognitive impairment. This study demonstrates how the rate of progression of cognitive impairment can be variable between individuals and compares to the progressive phases of dementia severity reported for human AD.29, 31
In this study, median survival time for dogs with CCD was not significantly different from dogs which experienced healthy aging. We have previously investigated survival with CCD2 where CCD dogs had a longer survival time than non‐CCD dogs. We speculated that this was possibly because of a close investigator‐client contact which motivated the owners to keep dogs with CCD despite their cognitive deterioration. Because of the longitudinal design of this study, the investigator‐client contact was even more consistent and in theory this might influence survival time for dogs with CCD even more positively. However, it might simply be that dogs with CCD do not experience a reduced life span because of the fact that the disease debut is late in life and because the human‐animal bond is strong.
It is of great importance to search for diagnostic tests which can support a clinical suspicion of CCD and detect cases of MCI. We investigated selected biomarkers and found that plasma Aβ42 was significantly higher in the groups of CCD dogs compared to the MCI and non‐CCD groups. There was more variation in the level of Aβ42 in the group of CCD dogs compared to the MCI and non‐CCD group. This might imply that some CCD dogs have an increased level because of some underlying causes or that there is simply more variation in the CCD group. However, we do not have full explanation for this variation and further investigations including a larger study population is needed in order to examine this finding in more detail. The present study was a small‐scale study and studies with more statistic power including serial plasma and possibly CSF measures would be beneficial to investigate if Aβ42 is truly a relevant blood biomarker for CCD. Our results are supported by a previous study which reported significantly higher plasma Aβ42 levels in cognitive impaired companion dogs compared to age‐matched controls.16 However, they also reported that dogs with severe cognitive impairments had lower Aβ42 level than dogs with MCI, which was not the case in this study. There are several restrictions when comparing the results from the 2 studies. One striking difference is the substantially higher levels of both Aβ40 and Aβ42 and the lower variation in measurements recorded in our study. This difference might be explained by Aβ peptides binding to carrier proteins in plasma thus masking the epitopes and underestimating true Aβ values.32 To minimize the risk of epitope masking, we pretreated the plasma samples with urea. Another factor adding to discrepancy between ELISA measurements from different studies could be because of diversity of the applied antibodies, exhibiting varying sensitivities and sometimes poorly defined specificities.33
A meta‐analytic review concluded that it is not possible to establish a clear relationship between plasma Aβ peptide levels and clinical measures of AD severity.33 Thus, a model of differential longitudinal changes in plasma Aβ42 levels in cognitively stable human subjects versus those who go on to develop AD dementia has been proposed.33 Individuals that will develop AD have a higher baseline Aβ42 than cognitively stable individuals and the plasma levels will increase gradually during the MCI phase. At the conversion point from MCI to AD plasma Aβ42 levels will diminish. Aβ levels in cognitively stable individuals will increase slightly with age.33
We speculate that the finding of higher plasma Aβ42 levels in the CCD dogs compared to the cognitively unimpaired dogs in this study reflects that dogs with CCD are not as cognitively impaired as people with AD and thus may correspond to early AD or the MCI phase. To gain a better understanding of plasma Aβ across the cognitive spectrum, longitudinal studies designed to include multiple time points for Aβ quantifications and where the enrolled dogs have the same age at inclusion would be beneficial. Validation against other biomarkers and histopathology would also be interesting.
The role of neuro‐inflammation in disease progression of AD has been extensively studied. Fibrils of Aβ are believed to induce microglia activation with subsequent release of both pro‐ and anti‐inflammatory mediators.34, 35, 36 Complement factors, acute phase proteins, reactive oxygen species and cytokines such as IL‐6 and TNFα are considered among the most prominent neurotoxic factors.35 Circulating cytokine concentrations in dogs with CCD have not been examined previously. Several of the cytokines investigated in this study could not be quantified although internal controls (spike recovery) were within acceptable limits and calibrations were valid. Although our results do not show a significant difference in circulating cytokine concentrations in dogs with CCD, a role for cytokines in the pathogenesis of CCD cannot be excluded. Local changes in the cerebral cortex of cytokine expression might possibly be more pronounced than reflected in the systemic concentrations.
Conclusions
This study documented that CCD is a progressive condition where the course of disease varies between individuals and thus compares to the progression through successive phases in human AD. Dogs with CCD commonly display specific clinical signs such as aimless wandering, staring blankly into space, avoiding being patted, and difficulty with finding dropped food. Furthermore, signs related to anxiety and unexplained fear is also common and should be addressed in future CCD screening questionnaires. Plasma Aβ42 was found to be highest in dogs from the CCD group and thus may represent an interesting plasma biomarker which, however, needs further investigation.
Supporting information
Table S1. Questions and scores included in the Canine Cognitive Dysfunction Rating Scale developed by Salvin and co‐workers.9
Table S2. Questions and scoring method included in the questionnaire developed by Rofina and co‐workers.10
Acknowledgment
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Wako Pure Chemical Industries, Ltd., Japan. Cat. no. 298‐64601 and 292‐64501
Immuno‐Biological Laboratories Co., Ltd., Gunma, Japan. Cat. no. JP27716
Multiscan FC, Fisher Scientific UK Ltd., Leicestershire, England
Canine Proinflammatory Panel 3 Assay, Meso Scale Discovery®, Rockville, MD
GraphPad Prism 4.03 for Windows, GraphPad Software, La Jolla, CA
References
- 1. Landsberg GM, Nichol J, Araujo JA. Cognitive dysfunction syndrome: A disease of canine and feline brain aging. Vet Clin North Am Small Anim Pract 2012;42:749–768. [DOI] [PubMed] [Google Scholar]
- 2. Fast R, Schutt T, Toft N, et al. An observational study with long‐term follow‐up of canine cognitive dysfunction: Clinical characteristics, survival, and risk factors. J Vet Intern Med 2013;27:822–829. [DOI] [PubMed] [Google Scholar]
- 3. Pugliese M, Carrasco JL, Andrade C, et al. Severe cognitive impairment correlates with higher cerebrospinal fluid levels of lactate and pyruvate in a canine model of senile dementia. Prog Neuropsychopharmacol Biol Psychiatry 2005;29:603–610. [DOI] [PubMed] [Google Scholar]
- 4. Cummings BJ, Head E, Ruehl W, et al. The canine as an animal model of human aging and dementia. Neurobiol Aging 1996;17:259–268. [DOI] [PubMed] [Google Scholar]
- 5. Studzinski CM, Araujo JA, Milgram NW. The canine model of human cognitive aging and dementia: Pharmacological validity of the model for assessment of human cognitive‐enhancing drugs. Prog Neuropsychopharmacol Biol Psychiatry 2005;29:489–498. [DOI] [PubMed] [Google Scholar]
- 6. Ruehl WW, Bruyette DS, DePaoli A, et al. Canine cognitive dysfunction as a model for human age‐related cognitive decline, dementia and Alzheimer's disease: Clinical presentation, cognitive testing, pathology and response to 1‐deprenyl therapy. Prog Brain Res 1995;106:217–225. [DOI] [PubMed] [Google Scholar]
- 7. Cotman CW, Head E. The canine (dog) model of human aging and disease: Dietary, environmental and immunotherapy approaches. J Alzheimers Dis 2008;15:685–707. [DOI] [PubMed] [Google Scholar]
- 8. Bosch MN, Pugliese M, Gimeno‐Bayon J, et al. Dogs with cognitive dysfunction syndrome: A natural model of Alzheimer's disease. Curr Alzheimer Res 2012;9:298–314. [DOI] [PubMed] [Google Scholar]
- 9. Salvin HE, McGreevy PD, Sachdev PS, Valenzuela MJ. The canine cognitive dysfunction rating scale (CCDR): A data‐driven and ecologically relevant assessment tool. Vet J 2011;188:331–336. [DOI] [PubMed] [Google Scholar]
- 10. Rofina JE, Van Ederen AM, Toussaint MJM, et al. Cognitive disturbances in old dogs suffering from the canine counterpart of Alzheimer's disease. Brain Res 2006;1069:216–226. [DOI] [PubMed] [Google Scholar]
- 11. Azkona G, Garcia‐Belenguer S, Chacon G, et al. Prevalence and risk factors of behavioural changes associated with age‐related cognitive impairment in geriatric dogs. J Small Anim Pract 2009;50:87–91. [DOI] [PubMed] [Google Scholar]
- 12. Osella MC, Re G, Odore R, et al. Canine cognitive dysfunction syndrome: Prevalence, clinical signs and treatment with a neuroprotective nutraceutical. Appl Anim Behav Sci 2007;105:297–310. [Google Scholar]
- 13. Neilson JC, Hart BL, Cliff KD, Ruehl WW. Prevalence of behavioral changes associated with age‐related cognitive impairment in dogs. J Am Vet Med Assoc 2001;218:1787–1791. [DOI] [PubMed] [Google Scholar]
- 14. Salvin HE, McGreevy PD, Sachdev PS, Valenzuela MJ. Under diagnosis of canine cognitive dysfunction: A cross‐sectional survey of older companion dogs. Vet J 2010;184:277–281. [DOI] [PubMed] [Google Scholar]
- 15. Colle M‐A, Hauw J‐J, Crespeau F, et al. Vascular and parenchymal abeta deposition in the aging dog: Correlation with behavior. Neurobiol Aging 2000;21:695–704. [DOI] [PubMed] [Google Scholar]
- 16. Gonzalez‐Martinez A, Rosado B, Pesini P, et al. Plasma beta‐amyloid peptides in canine aging and cognitive dysfunction as a model of Alzheimer's disease. Exp Gerontol 2011;46:590–596. [DOI] [PubMed] [Google Scholar]
- 17. Bain MJ, Hart BL, Cliff KD, Ruehl WW. Predicting behavioral changes associated with age‐related cognitive impairment in dogs. J Am Vet Med Assoc 2001;218:1792–1795. [DOI] [PubMed] [Google Scholar]
- 18. Garman RH. Evaluation of large‐sized brains for neurotoxic endpoints. Toxicol Pathol 2003;31(Suppl):32–43. [DOI] [PubMed] [Google Scholar]
- 19. Cummings JL. Cognitive and behavioral heterogeneity in Alzheimer's disease: Seeking the neurobiological basis. Neurobiol Aging 2000;21:845–861. [DOI] [PubMed] [Google Scholar]
- 20. Harwood DG, Barker WW, Ownby RL, Duara R. Relationship of behavioral and psychological symptoms to cognitive impairment and functional status in Alzheimer's disease. Int J Geriatr Psychiatry 2000;15:393–400. [DOI] [PubMed] [Google Scholar]
- 21. Jack CR Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013;12:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging‐Alzheimer's Association Workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Growdon ME, Schultz AP, Dagley AS, et al. Odor identification and Alzheimer disease biomarkers in clinically normal elderly. Neurology 2015;84:2153–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kjelvik G, Saltvedt I, White LR, et al. The brain structural and cognitive basis of odor identification deficits in mild cognitive impairment and Alzheimer's disease. BMC Neurol 2014;14:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Velayudhan L, Gasper A, Pritchard M, et al. Pattern of smell identification impairment in Alzheimer's disease. J Alzheimers Dis 2015;46:381–387. [DOI] [PubMed] [Google Scholar]
- 26. Horwitz D. Dealing with common behavior problems in senior dogs. Vet Med 2001;96:869. [Google Scholar]
- 27. McCurry SM, Gibbons LE, Logsdon RG, Teri L. Anxiety and nighttime behavioral disturbances. Awakenings in patients with Alzheimer's disease. J Gerontol Nurs 2004;30:12–20. [DOI] [PubMed] [Google Scholar]
- 28. Porter VR, Buxton WG, Fairbanks LA, et al. Frequency and characteristics of anxiety among patients with Alzheimer's disease and related dementias. J Neuropsychiatry Clin Neurosci 2003;15:180–186. [DOI] [PubMed] [Google Scholar]
- 29. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging‐Alzheimer's Association Workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alzheimer's Association . Alzheimer's disease facts and figures. Alzheimers Dement 2014;10:e47–e92. [DOI] [PubMed] [Google Scholar]
- 31. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the alzheimer's pathological cascade. Lancet Neurol 2010;9:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mehta PD, Pirttila T, Mehta SP, et al. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1–40 and 1–42 in Alzheimer disease. Arch Neurol 2000;57:100–105. [DOI] [PubMed] [Google Scholar]
- 33. Song F, Poljak A, Valenzuela M, et al. Meta‐analysis of plasma amyloid‐beta levels in Alzheimer's disease. J Alzheimers Dis 2011;26:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eikelenboom P, Veerhuis R, Scheper W, et al. The significance of neuroinflammation in understanding Alzheimer's disease. J Neural Transm 2006;113:1685–1695. [DOI] [PubMed] [Google Scholar]
- 35. Schwab C, McGeer PL. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J Alzheimers Dis 2008;13:359–369. [DOI] [PubMed] [Google Scholar]
- 36. Schlachetzki JC, Hull M. Microglial activation in Alzheimer's disease. Curr Alzheimer Res 2009;6:554–563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Questions and scores included in the Canine Cognitive Dysfunction Rating Scale developed by Salvin and co‐workers.9
Table S2. Questions and scoring method included in the questionnaire developed by Rofina and co‐workers.10


