Abstract
Background
The efficacy and benefits of telmisartan in cats with chronic kidney disease (CKD) have not previously been reported.
Hypothesis
Long‐term treatment of cats with CKD using telmisartan decreases urine protein‐to‐creatinine ratio (UP/C) similar to benazepril.
Animals
Two‐hundred and twenty‐four client‐owned adult cats with CKD.
Methods
Prospective, multicenter, controlled, randomized, parallel group, blinded clinical trial with noninferiority design. Cats were allocated in a 1 : 1 ratio to either telmisartan (1 mg/kg; n = 112) or benazepril (0.5–1.0 mg/kg; n = 112) PO q24 h. The primary endpoint was prospectively defined as the change in proteinuria (benazepril:telmisartan) based on a log transformed weighted average of UP/C change from baseline (AUC 0→t/t) as a percentage compared using a confidence interval (CI) approach. Changes of UP/C from baseline were assessed on all study days and corrected for multiple comparisons.
Results
Telmisartan proved noninferior to benazepril in controlling proteinuria (CI, −0.035 to 0.268). At Day 180, UP/C compared to baseline in the telmisartan group was significantly lower (−0.05 ± 0.31; P = .016), whereas in the benazepril group the change (−0.02 ± 0.48) was not statistically significant (P = .136). Similar results were obtained at all assessment points with significant decrease in UP/C occurring with telmisartan but not benazepril.
Conclusion and Clinical Importance
Both telmisartan and benazepril were well tolerated and safe. Telmisartan proved to be noninferior to benazepril and significantly decreased proteinuria relative to baseline at all assessment points whereas benazepril did not.
Keywords: ACE inhibitor, Angiotensin II receptor blocker, Benazepril, Proteinuria
Abbreviations
- ACE
angiotensin converting enzyme
- AE
adverse event
- AT‐II
angiotensin‐II
- ARB
angiotensin receptor blocker
- AT1
angiotensin II type 1 receptor
- AT2
angiotensin II type 2 receptor
- AUC
area under curve
- CFU
colony forming units
- CI
confidence interval
- CKD
chronic kidney disease
- CP
control product (benazepril)
- GCP
good clinical practice
- GFR
glomerular filtration rate
- ITT
intention‐to‐treat population
- IVP
investigational product (telmisartan)
- IRIS
International Renal Interest Society
- NYHA
New York Heart Association
- UP/C
urine protein‐to‐creatinine ratio
- PPAR‐γ
peroxisome proliferator‐activated receptor gamma
- PPS
per‐protocol‐population dataset
- RAAS
renin angiotensin aldosterone system
- SBP
systolic blood pressure
Chronic kidney disease (CKD) is irreversible, progressive, and one of the most common medical reasons for medical evaluation of older cats. The prevalence increases with age and up to 35% of the geriatric cat population is affected.1, 2 Clinical signs such as polyuria and polydipsia, lethargy, decreased appetite and weight loss often are described, and a considerable effect of CKD on the quality of life can be assumed.3 Furthermore, CKD negatively impacts survival, with an average life expectancy of 1–3 years once clinical signs become apparent.4, 5 Early management can improve quality of life and prognosis.1, 6 Compensatory chronic activation of the renin–angiotensin–aldosterone system (RAAS) in CKD to maintain glomerular filtration rate (GFR) increases angiotensin‐II (AT‐II) production with renal concentrations exceeding plasma concentrations.7, 8 Angiotensin‐II is a central mediator of renal injury because of its ability to produce glomerular hypertension that results in glomerular damage, proteinuria, and activation of pro‐inflammatory and profibrotic pathways.9, 10 Furthermore, even mild proteinuria is strongly associated with and predictive of onset of azotemia,11 progression of CKD,12 and survival in both humans and cats.4, 13 Several AT‐II receptors have been identified.14 The major detrimental renal effects of AT‐II described above are mediated by AT1 receptors. The AT2 receptors modulate actions of AT‐II that are renoprotective, namely vasodilation, natriuresis, inhibition of renin secretion, and anti‐inflammatory, anti‐ischemic and antifibrotic effects. The expression of the AT2 receptor is increased in pathologic circumstances.15, 16
Angiotensin converting enzyme (ACE)‐inhibitors prevent enzymatic conversion of angiotensin‐I, decreasing AT‐II concentrations. However, in mammals, including the cat, alternative pathways (ACE‐escape) exist for AT‐II generation.17, 18, 19 Although increased plasma renin activity has been reported during treatment with angiotensin receptor blockers (ARBs) in humans, a phenomenon that also could partially overcome competitive receptor blockade, this phenomenon seems to be without clinical relevance.20
The ARBs, such as telmisartan,1 selectively block the AT1 receptor with high affinity and displace AT‐II, while leaving beneficial effects of the AT2 receptor activation unaffected. Furthermore, the efficacy of ARBs is unaffected by ACE‐escape mechanisms. The renoprotective properties of telmisartan have been demonstrated in ex vivo models and in vivo studies in human patients with diabetic and nondiabetic nephropathies.21, 22, 23, 24 In contrast to other ARBs, telmisartan also is a partial agonist of the peroxisome proliferator‐activated receptor gamma (PPAR‐γ) receptor and its renoprotective properties are increased by this dual action.22, 23, 25
There are no published studies on telmisartan or other ARBs in cats with naturally occurring CKD, whereas benazepril has been shown to decrease the time‐averaged urine protein‐to‐creatinine ratio (UP/C) relative to that of placebo‐treated cats.28 The primary objective of this study was to compare the antiproteinuric effect of telmisartan with that of benazepril in cats with naturally occurring CKD. Additional variables were evaluated to assess effect on quality of life.
Materials and Methods
Animals
Client‐owned adult cats suspected of having CKD were screened for CKD at 48 centers across Europe including: Germany (15), France (12), United Kingdom (11), Netherlands (4) Belgium (4), and Italy (2).
Inclusion Criteria
Cats of either sex were eligible for inclusion only if owner informed consent was given and they were >2.0 kg body weight and diagnosed with clinically stable CKD International Renal Interest Society (IRIS)27 stage 2–3. Diagnosis of CKD was based on history, physical examination and laboratory findings. The following criteria had to be met: plasma or serum creatinine concentration ≥1.6 and <5.0 mg/dL, urine specific gravity <1.035, UP/C ≥ 0.2 and <2.0, plasma or serum T4 concentrations ≤3.1 μg/dL, and systolic blood pressure (SBP) ≤170 mmHg. Cats with SBP > 170–<180 mmHg were eligible if they had been stable on amlodipine treatment for ≥4 weeks.
Cats receiving a renal diet were eligible if they had received the diet for ≥4 weeks.
Exclusion Criteria
Cats were ineligible for inclusion if they had received ACEinhibitors, ARBs or other vasodilating agents, or diuretics (eg, furosemide) <14 days before screening or had their diet changed <4 weeks before screening. Cats with urinary tract infection identified by urine cytology and culture (>1,000 colony forming units [CFU]/mL), palpably enlarged kidneys, renal neoplasia, pre‐ or postrenal azotemia, heart failure (New York Hear Association [NYHA] class II, III, IV), acute kidney injury, pregnancy, or lactation were excluded.
Study Design
A multicenter prospective, randomized, investigator‐blinded, positive‐controlled, parallel group design was adopted. At each study site, the investigator was blinded by use of a dispenser, who was responsible for allocation and dispensing of medication as well as its return only. The outer package of both medications was visually identical, and the weight of each package was adjusted to prevent differentiation between medications to ensure that all persons involved in the evaluation were not aware of the medication given.
Client‐owned cats were allocated to receive telmisartan1 or benazepril2 (1 : 1 ratio). The protocol was prepared in consultation with independent experts in CKD of cats and approved by European regulatory agencies and by an ethical review committee at all sites where required. The study was powered to assess noninferiority regarding the primary endpoint UP/C change from baseline of telmisartan against benazepril, and was conducted according to good clinical practice (GCP)3 guidelines (VICH GL9).
The randomization sequence was generated as a single list, which was held by a single center, not located at a study site.
Procedures were available to allow unblinding in the event of medical emergency.
An solution of telmisartan1 was administered PO by the cat owner at a dosage of 1 mg/kg (0.25 mL/kg) q24 h. Benazepril2 was administered at 0.5–1.0 mg/kg PO q24 h as a flavored 2.5 mg tablet in accordance with the product label. Cats >2 and <5 kg in weight received 1 tablet, and cats >5 and <10 kg body weight received 2 tablets.
Routine treatments with no impact on CKD (eg, vaccinations, antiparasitic drugs) were allowed. Any concomitant treatment was documented. Amlodipine (0.625–1.25 mg PO q24 h) was allowed if cats had been stabilized for ≥4 weeks before inclusion, and treatment was continuous. Antimicrobial treatment was not permitted in the 7 or 21 days before inclusion for short‐acting and long‐acting agents, respectively. If sedation of a cat was required for a diagnostic procedure during the study, a standard sedation protocol was used. Anti‐inflammatory or anti‐infective treatment was only permitted as acute treatment.
Schedule of Events
Cats with a history typical of CKD or those >7 years and suspected of having CKD at a routine screening underwent further examination to diagnose and stage CKD based on IRIS guidelines.27 Fasted serum creatinine concentrations were determined in well‐hydrated cats at least twice (screening and Day 0). Cystocentesis was performed for urine collection whenever possible. Urinalysis was performed on site. Laboratory examinations were conducted at IDEXX Laboratory Germany, except for those from the United Kingdom, which were performed at the IDEXX Laboratory United Kingdom. A cross‐validation of the 2 IDEXX Laboratories confirmed their comparability.
Systolic blood pressure was measured, if indicated, by an indirect method for safety purposes only, using a consistent method for an individual cat (usually Doppler ultrasound examination).
The inclusion date (Day 0) was the day on which the cat first received telmisartan or benazepril. Detailed follow‐up physical examinations and urinalyses were scheduled on Days 7, 30, 60, 90, 120, and 180. Routine hematology and blood biochemistry were repeated on Days 30, 90, and 180. Additional diagnostic tests could be undertaken whenever considered indicated by the investigator. All adverse events (AEs) were reported in accordance with local regulations.
Outcome Measures
The primary efficacy outcome of the study was prospectively defined as the decrease in proteinuria, measured by UP/C, compared to baseline. Telmisartan was hypothesized to be similarly efficacious to benazepril. Representing secondary efficacy variables, the changes in general demeanor and appetite in comparison to baseline were recorded as surrogates for quality of life variables. These variables were assessed by the owners who scored appetite as normal, increased, decreased, or no eating and demeanor as normal, alert, listless, or lethargic.
The number of treatment failures, defined as death or euthanasia of any cause, owner noncompliance or deterioration of clinical signs requiring hospitalization, was recorded. Furthermore, according to GCP any AE, defined as “any observation in animals that is unfavorable and unintended and occurs after the use of a veterinary product or a investigational veterinary product, whether or not considered to be product related”, was recorded.
Statistical Methods
Cats included in the efficacy analysis were required to be followed to Day 90 (per‐protocol‐population dataset [PPS], Fig 1) whereas all treated cats (intention‐to‐treat [ITT] population) were included in the safety analysis. Baseline characteristics of the groups were compared for homogeneity.
Figure 1.
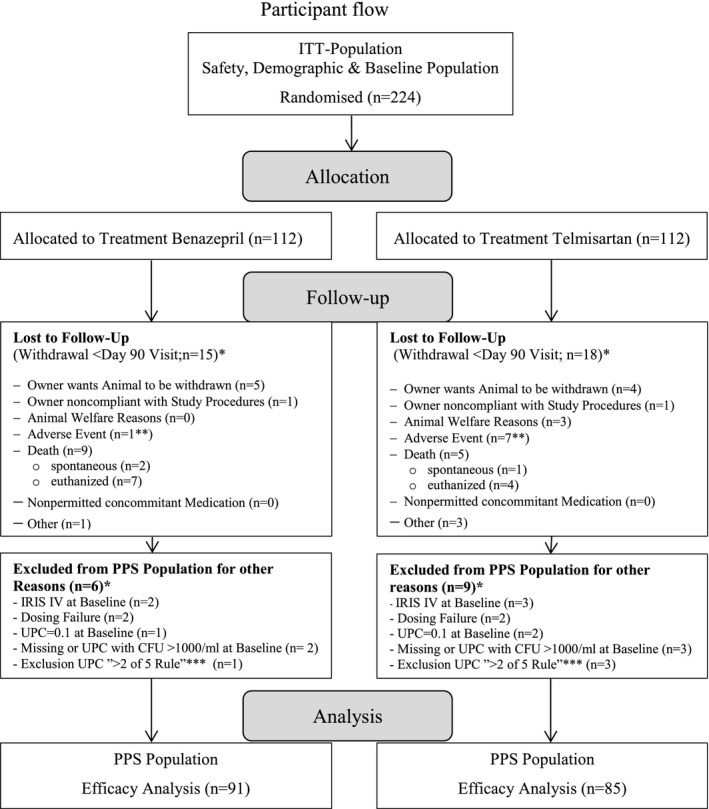
Participant flow. *More than 1 reason for exclusion possible. **Benazepril group: anemia; telmisartan group: increase in renal parameters (2 cats), uremic crisis, anorexia, cerebellar mass on MRI, intra‐abdominal neoplasia, hind limb ataxia (1 cat for each). ***Exclusion UPC “>2 of 5” Rule: For the first 5‐study days at most 2 missing or excluded because of bacteria contamination (CFU >1,000/mL) UPC values are considered to be acceptable to yield reliable AUC values.
The same approach was followed as used in the reference study,28 in which benazepril was shown to be superior to placebo in a randomized controlled trial assessing the effect of treatment on time‐averaged proteinuria (area under the curve [AUC] UP/C). Because internal pilot and published data4 showed a skewed distribution of the calculated AUC UP/C values, sample size determination and statistical analysis of the primary efficacy variable were based on the log transformed AUC UP/C values. For the primary efficacy analysis, a summary value based on a log transformed weighted average of UP/C change from baseline (AUC UP/C 0→t/t) in percentage of each cat over time was calculated (Day 0 = 100%). The AUC principle was used to account for progressive loss of cats to follow‐up as used in the reference study.28 Noninferiority to benazepril was based on a confidence interval (CI) approach using the normal approximation.29 The null hypothesis “inferiority of treatment to positive control” was rejected if the 95% 2‐sided CI of the log (AUC UP/C 0→t/t) difference lay entirely above the noninferiority margin Δ(−0.108 to 0.116). A noninferiority margin of Δ = 0.108 on the log scale, corresponding to a ratio AUC(IVP)/AUC(CP) of approximately 1.11, was assumed and determined from an internal pilot study with 37 animals over 8–12 weeks in which an average AUC 0→t/t value of 106.83% with a SD of 28.28% was observed. Based on these data, an absolute margin of 119% corresponding to a relative margin of approximately 1.11 was defined a priori and was accepted as clinically meaningful by the licensing authorities.
In addition, absolute change in UP/C from baseline was analyzed within each group using a 1‐sample signed‐rank test. P‐values were adjusted for multiplicity by the Bonferroni–Holm procedure.26
Other secondary efficacy variables, including behavioral changes and number of treatment failures, were summarized statistically for the PPS population. Secondary efficacy variables were not compared between groups statistically.
In accordance with the recommendations of CONSORT,30 a valid assessment of this noninferiority study is provided. Statistical analyses were performed using the SAS System Version 9.2.4 P < .05 was considered to indicate significance.
Results
Cats were recruited between March 2008 and August 2010, with case follow‐up completed in January 2011. More than 2000 cats were screened and 224 cats (ITT population) were recruited with 112 cats randomized to each group (Fig 1). Clinical signs suggestive of CKD were present for >3 months in most cats before inclusion. The baseline demographics, and clinical and laboratory characteristics were distributed homogeneously between groups (Table 1). Cats >11 years predominated in both groups, which had similar mean and median ages (Table 1). Most cats (approximately 80%) were classified as IRIS CKD stage 2 (84/112 and 91/112 for telmisartan and benazepril, respectively). Four cats in IRIS CKD stage 4 were included in the study as a protocol deviation (Table 2), the remainder being IRIS CKD stage 3. Most cats enrolled were borderline proteinuric (UP/C 0.2–0.4), with similar numbers in each treatment group; 3 cats with UP/C < 0.2 were included as a protocol deviation because all other criteria for CKD were fulfilled (Table 2). Fifty‐one percent of the cats had SBP results in the mild to moderate hypertension class (IRIS classification; 150–179 mmHg; Table 2). A small number of cats with SBP >170 but <180 mmHg that were stabilized on amlodipine treatment before Day 0 was included in both groups (Table 1). Approximately, 30% of cats in both groups were being fed a renal diet at inclusion (Table 1). Deviations from planned time schedule were unavoidable under field conditions. They did not exceed 12 days and were considered to be of no influence on the general study outcome.
Table 1.
Baseline characteristics at inclusion (intention‐to‐treat population)
| Variable | Benazepril (n = 112) | Telmisartan (n = 112) | ||
|---|---|---|---|---|
| Mean (min–max) | No (%) | Mean (min–max) | No (%) | |
| Age (years) | 12.8 (2–22) | 13.6 (3–21) | ||
| Weight (kg) | 4.3 (2–11) | 4.4 (2–9) | ||
| Gender | ||||
| Female | 58 (51,8) | 55 (49.1) | ||
| Male | 54 (48,2) | 57 (50.9) | ||
| Neutered | 104 (92,9) | 106 (94.6) | ||
| Breed | ||||
| DLH | 6 (5.4) | 9 (8.0) | ||
| DSH | 72 (64.3) | 75 (67.0) | ||
| Mixed | 9 (8.0) | 4 (3.6) | ||
| Other | 20 (17.9) | 16 (14.3) | ||
| Persian | 5 (4.5) | 8 (7.1) | ||
| Serum Biochemistry | % >ULN | % >ULN | ||
| TT4 (μg/dL) | 1.9 (0.3–5.4) | 0.9 | 1.8 (0.3–4.3) | 0.0 |
| Creatinine (mg/dL)a | 2.4 (1.6–6.6) | 58 | 2.5 (1.1–6.6) | 64.3 |
| Urea (BUN) (mg/dL) | 48.7 (21.3–139.8) | 84.8 | 52.1 (23.3–299.4) | 79.5 |
| Sodium (mEq/L) | 154.9 (147.0–167.0) | 0.9 | 155.3 (147.6–174.0) | 1.8 |
| Potassium (mEql/L) | 4.5 (3.0–6.4) | 7.1 | 4.5 (3.0–8.1b) | 3.6 |
| Calcium (mg/dL) | 10.4 (7.6–13.2) | 6.3 | 10.4 (8.0–13.2) | 2.7 |
| Phosphate (mg/dL) | 5.0 (2.2–10.2) | 7.1 | 5.0 (2.2–15.8) | 6.3 |
| Albumin (mg/dL) | 3.4 (2.2–4.6) | 0.0 | 3.4 (2.6–4.5) | 0.0 |
| ALT (U/L) | 65.2 (22.0–210.8) | 1.8 | 65.1 (21.4–279.4) | 1.8 |
| ALP (U/L) | 32.8 (10.0–111.8) | 0.9 | 30.4 (12.0–129.0) | 0.9 |
| CBC | % <LLN, >ULN | %<LLN, >ULN | ||
| RBCc 10 × 12/L | 7.5 (3.4–10.9) | <3.6, >6.3 | 8.0 (3.9–14.6) | <2.7, >10.7 |
| HGB g/L | 108.2 (55–170) | <17.3, >1.2 | 113.4 (67–170) | <11.5, >2.6 |
| HCT % | 34.9 (19.0–54.0) | <10.8, >1.8 | 37.3 (21.0–69.8) | <5.4, >3.6 |
| WBCc g/L | 10.7 (1.9–28.9) | <17.1, >18.9 | 9.6 (2.8–49.2) | <17.0, >8.0 |
| Urine Variables | ||||
| Specific gravityc | 1.022 | 1.022 | ||
| UPC | 0.41 (0.1–2.2) | 0.41 (0.1–3.0) | ||
| Systolic BP (mmHg) | 147.7 (95–180) | 146.2 (98–176) | ||
| Amlodipine treatmentc | 6 (5.4) | 10 (8.9) | ||
| Kidney diet | 36 (32.1) | 39 (34.8) | ||
| Disease History | Mean Duration (years) | Mean Duration (years) | ||
| PU/PD | 0.66 | 63 (56.3) | 0.56 | 71 (63.4) |
| Decreased appetite | 0.41 | 28 (25.0) | 0.36 | 33 (29.5) |
| Weight loss | 0.53 | 48c (42.9) | 0.52 | 56 (50.0) |
| Palpably small kidneys | 21 (18.8) | 18 (16.1) | ||
Upper reference limit (ULN) laboratory 2.0 mg/dL.
Laboratory error suspected at D0, at recheck examinations potassium levels of this patient were well within reference ranges.
One missing value, LLN/ULN = lower/upper reference limit.
Table 2.
Telmisartan and Benazepril Group (intention‐to‐treat population, n = 112 each), IRIS stage at baseline including substaging
| Telmisartan (n = 112)a | IRIS Stage 2a (n = 36) | IRIS Stage 2b (n = 48) | IRIS Stage 3 (n = 21) | IRIS Stage 4 (n = 3)b |
|---|---|---|---|---|
| Substaging by SBP | ||||
| <150 mmHg | 17 | 30 | 6 | 1 |
| 150–159 mmHg | 11 | 5 | 5 | 1 |
| 160–179 mmHg | 8 | 13 | 10 | 0 |
| ≥180 mmHg | 0 | 0 | 0 | 0 |
| Substaging by UP/Ca | ||||
| <0.2 | 1 | 1 | 0 | 0 |
| 0.2–0.4 | 26 | 40 | 13 | 0 |
| >0.4 | 9 | 8 | 2 | |
| Benazepril (n = 112) | IRIS Stage 2a (n = 47) | IRIS Stage 2b (n = 44) | IRIS Stage 3 (n = 19) | IRIS Stage 4 (n = 2) |
|---|---|---|---|---|
| Substaging by SBP | ||||
| <150 mmHg | 24 | 18 | 8 | 1 |
| 150–159 mmHg | 7 | 9 | 5 | 1 |
| 160–179 mmHg | 16 | 16 | 6 | 0 |
| ≥180 mmHg | 0 | 1 | 0 | 0 |
| Substaging by UP/C | ||||
| <0.2 | 0 | 1 | 0 | 0 |
| 0.2–0.4 | 42 | 31 | 15 | 1 |
| >0.4 | 5 | 12 | 4 | 1 |
IRIS stage 1 (n = 4) not shown.
One missing value for IRIS 4.
The primary variable log (AUC UP/C 0→t/t) based on the CI approach showed noninferiority of telmisartan compared to benazepril in the PPS population (n = 176) and in the ITT population (n = 224). Although the study was designed as a noninferiority study, telmisartan was close to being superior to benazepril (Fig 2).
Figure 2.
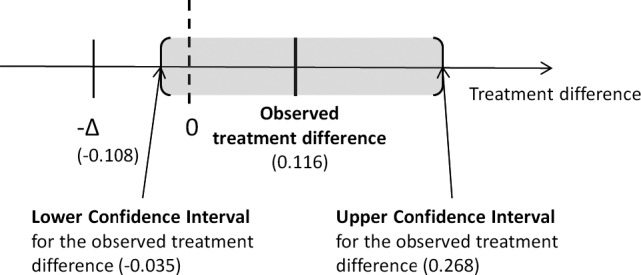
Results of the noninferiority and superiority analysis for the primary variable log AUC 0→t/t in the PPS‐population. The delta (−∆) indicates the noninferiority margin. The observed 95% confidence interval limits (CL) for the treatment difference are shown as the shaded area.
The changes from baseline over time are presented in Table 3. On Day 180, overall mean UP/C values were numerically lower in both groups compared to baseline. In the telmisartan group, the mean decrease (−0.05 ± 0.31) was significantly different from baseline (P = .016). In the benazepril group, the mean decrease (−0.02 ± 0.48) was not significantly different from baseline (P = .136). Furthermore, in the telmisartan group statistically significant decreases in UP/C were found at all assessment points, whereas in the benazepril group no statistical change in UP/C compared to baseline was found at any assessment point. These findings also are reflected in the number of cats that changed from being classified as proteinuric at baseline to borderline proteinuric or nonproteinuric at Day 180, which was 30.8% (4/13) in the benazepril and 57.2% (8/14) in the telmisartan group. In cats that were classified as borderline proteinuric at baseline the number of cats that either shifted to nonproteinuric (approximately 33%) or remained borderline proteinuric (approximately 50%) at Day 180 was comparable in both groups with 85.7% (54/63) in benazepril and 87.9% (51/58) in telmisartan‐treated cats.
Table 3.
UP/C changes from baseline by treatment and study day for the PPS population
| Treatment | Day | N | Mean | SD | Median | Range | IQ Range | P‐value |
|---|---|---|---|---|---|---|---|---|
| Benazepril | 7 | 87 | −0.06 | 0.25 | 0.0 | −1.4 to 1.0 | 0.10 | .040 |
| 30 | 88 | −0.06 | 0.28 | 0.0 | −1.5 to 0.5 | 0.15 | .023 | |
| 60 | 87 | −0.07 | 0.38 | 0.0 | −1.6 to 1.1 | 0.10 | .046 | |
| 90 | 86 | +0.06 | 0.61 | 0.0 | −1.5 to 3.5 | 0.20 | .072 | |
| 120 | 85 | +0.06 | 0.77 | −0.1 | −1.6 to 5.2 | 0.20 | .143 | |
| 180 | 76 | −0.02 | 0.48 | 0.0 | −1.5 to 2.3 | 0.20 | .136 | |
| Telmisartan | 7 | 80 | −0.07 | 0.28 | −0.1 | −1.7 to 1.0 | 0.10 | <.0001a |
| 30 | 83 | −0.08 | 0.26 | −0.1 | −1.6 to 0.5 | 0.10 | <.0001a | |
| 60 | 82 | −0.09 | 0.33 | −0.1 | −1.7 to 0.8 | 0.10 | .0002a | |
| 90 | 83 | −0.08 | 0.31 | −0.1 | −1.4 to 1.3 | 0.20 | <.0001a | |
| 120 | 75 | −0.02 | 0.51 | −0.1 | −1.6 to 2.4 | 0.20 | .002a | |
| 180 | 72 | −0.05 | 0.31 | 0.0 | −1.1 to 1.1 | 0.10 | .016a |
The P‐values are adjusted for multiplicity by the Bonferroni–Holm procedure (P‐values sorted from lowest to highest and compared to the adjusted P‐values (ie, .0083; .01; .0125; .016; .025; .05).
Significantly different from baseline.
The majority of cats completed the study, and the proportion of cats removed from the study was comparable in both treatment groups (Fig 1). The percentage of treatment failures in the PPS population was 15.3% (13/85) for telmisartan and 19.8% (18/91) for benazepril‐treated cats. The number of deaths in telmisartan‐treated cats (ITT population) was 13/112 and 22/112 for benazepril‐treated cats. The changes in general behavior indicative of quality of life in treated cats were assessed relative to baseline to describe clinical benefit. Cat owners reported the most visible change in behavior during the first 60 days with a small numerical increase in active cats being reported in both groups. Most cats (approximately 80%) had normal appetite and little change in appetite was seen throughout the study.
Telmisartan and benazepril were well tolerated and most of the AEs reported were typical of the signs of CKD, and the majority was unlikely to be related to treatment. Adverse events that were reported in both groups (benazepril: 65 cats and telmisartan: 61 cats with ≥1 AE) included signs of deterioration of CKD, gastrointestinal disorders, and very few cats with urinary tract infections, cardiac, oral cavity, skin, or respiratory disorders, neurological signs, or neoplasia. Figure 1 gives details of the AEs that resulted in withdrawal of cases from the study before Day 90.
At the end of the study, the mean values for red blood cell count (RBC), hemoglobin (HGB) and hematocrit (HCT) were comparable between treatment groups. Clinical evidence of anemia was reported in 2 cats (1 in each group) with CKD; both also were receiving amlodipine. No clinically relevant changes in serum calcium, potassium, sodium, or phosphate concentrations were recorded in either treatment group.
Discussion
The aim of our study was to compare the efficacy of telmisartan against benazepril with respect to the primary endpoint, change in UP/C from baseline. Although the study was designed as a noninferiority study, telmisartan was close to meeting the a priori agreed upon criterion for superiority to benazepril.
The UP/C was significantly decreased compared to baseline at all assessment points in telmisartan‐treated cats, whereas statistical significance was not reached for benazepril‐treated cats.
Telmisartan1, a selective AT1 receptor antagonist, does not bind to AT2 receptors or other key physiologically important receptors or enzymes at therapeutic concentrations.31 Selective blockade of AT1 receptors offers an alternative approach to the modulation of the RAAS. Telmisartan is licensed for 2 indications in human adults: the treatment of essential hypertension, as well as the reduction in cardiovascular morbidity (cardiovascular prevention) in patients with atherothrombotic cardiovascular disease or type 2 diabetes mellitus with target organ damage. Renoprotective properties of telmisartan in humans are thought to be related to selective blockade of AT1 and partial agonism of PPAR‐γ receptors.23, 32 The apparently more pronounced decrease in proteinuria in cats in this study might be explained by this dual mode of action of telmisartan or circumvention of potential ACE‐escape mechanisms.
A large number of cats had to be screened to identify suitable cats with CKD for inclusion in this study. The study population (predominantly IRIS CKD stage 2) was considered to be appropriate for the intended target population because early treatment is logical if the therapeutic rationale is that of slowing progression. Cats in this study were older and had a slightly higher body weight compared to the reference study,28 findings that probably are indicative of recent demographic changes in the cat population.
According to the requirements of CONSORT,30 efficacy of the reference treatment had been established in a randomized placebo‐controlled study.28 For a valid assessment of noninferiority, it is necessary that participants, study conduct, and outcome measures are similar to the reference study.28 This study closely matched the reference study with respect to study design, target population, and outcome measures. The primary outcome, decrease in proteinuria, calculated by UP/C change from baseline (as assessed by the AUC UP/C 0→t/t), was chosen because it was used in the reference study. Furthermore, proteinuria has been previously demonstrated to be an independent predictor of progression and survival of cats with CKD.4, 11, 12, 13
Limitations reported for the reference study28 were addressed to allow an unbiased assessment of outcome in this study. Thus, before inclusion, urine culture and screening for hyperthyroidism were undertaken. Hyperthyroidism is a known comorbidity in cats with CKD and may influence proteinuria and liver enzyme activity.33 In order not to bias efficacy and safety conclusions, cats with a T4 concentration >3.1 μg/dL, which is within the upper third of the reference range, were excluded, with the aim of excluding cats with concomitant hyperthyroidism and CKD in which suppressed T4 concentrations may complicate diagnosis of hyperthyroidism.34
More than 90% of urine samples collected in this study was obtained by cystocentesis, which compares favorably with 60% collected by this method in the reference study.28 Abnormal urine sediment triggered urine culture in this study. In 14 cats (5 in the benazepril and 9 in the telmisartan group), bacterial urinary tract infection was confirmed leading to exclusion from the efficacy analysis.
Benazepril previously has been reported to decrease proteinuria in cats with CKD in comparison to placebo when assessed over a variable follow‐up period, but not to significantly improve survival time,28 or slow CKD progression.35 The reference study showed that time‐averaged UP/C increased above baseline in the placebo group, and benazepril seemed to prevent this increase. Similar to the reference study,28 in this study, benazepril prevented an increase in proteinuria while a decrease in proteinuria relative to baseline after 180 days was present only in telmisartan‐treated cats. This study was conducted for 180 days, thus the effect of telmisartan on survival of cats with CKD could not be evaluated. Additional studies are warranted to assess telmisartan's effect on survival time in cats with CKD.
It might be questioned whether the decrease in UP/C relative to baseline seen in this study is clinically relevant. A previous study demonstrated that high UP/C (median value of 0.26 versus 0.15 in progressive versus nonprogressive cases, respectively) and high plasma phosphate concentration at diagnosis are significant independent risk factors for progression of azotemia in cats with CKD and that small differences in UP/C at diagnosis are clinically relevant.12 In a postmortem study, proteinuria was associated with interstitial fibrosis and glomerular hypertrophy.36 These associations between proteinuria and negative CKD outcomes do not prove causality but provide the rationale for investigating telmisartan's effect on progression of CKD or survival time in cats because this study demonstrated telmisartan's efficacy in decreasing proteinuria. These associations also support the contention that, if a decrease in proteinuria is renoprotective and prolongs survival in the cats with CKD (which remains to be proven), relatively small decreases in UP/C (eg, from proteinuric to borderline proteinuric or from borderline proteinuric to nonproteinuric) or prevention of an increases in UP/C over time could be clinically relevant.
Unlike the reference study,28 in our study SBP was monitored at inclusion and at study conclusion, because hypertension is known to be associated with CKD and proteinuria4 in cats. The SBP of the PPS population at the end of the study was comparable to baseline in both groups. Our study was not designed to assess antihypertensive efficacy, and additional work is necessary to assess effects on blood pressure. In the ITT population, 16 cats (Table 1), and in the PPS population 11 cats were pretreated with amlodipine (3 and 8, in the benazepril and telmisartan group, respectively). To exclude a potential effect of amlodipine on the primary efficacy variable in the both groups, the same analysis was performed without the amlodipine‐treated cats, with almost the same results: observed treatment difference for the primary variable log AUC→t/t: 0.112, and significant change in overall mean UP/C value (P = .013) for the telmisartan but not (P = .16) for the benazepril group. Furthermore, the percentages of cats classified as proteinuric, borderline proteinuric or nonproteinuric at Day 180 in comparison to baseline were very similar when amlodipine‐treated cats were excluded.
Hematology variables were monitored to assess changes in RBC counts, which might be caused either by progression of CKD or by drug interaction with the RAAS. At baseline, approximately 3% of cats had RBC counts below the reference range (5.0 × 1012/L). In the reference study,28 approximately 10% of the cats had RBC counts below the reference range at baseline. The mean RBC counts were comparable between groups and within the reference range at the end of the study. The proportion of cats with RBC counts below the reference range slightly increased in both groups by study end. Clinical evidence of anemia was reported in 2 cats (1 per group). Both cats received concomitant amlodipine treatment, and thus the role of treatment could not be assessed. Medications that inhibit the RAAS may decrease red cell mass by blocking the effects of AT‐II on erythropoiesis.37, 38 The results of this study suggest this potential decrease in RBC count is not clinically relevant. Hypokalemia occurs in 20–25% of cats with CKD.39, 40 In this study, neither hypo‐ nor hyperkalemia was found in any cat over the study period.
Because a primary requirement for the administration of any medication is safety, our study also collected data on AEs. The number of deaths and withdrawals from the study was small in both groups. Adverse events reported in both groups were those expected for cats with CKD and in most cases were judged as unlikely to be treatment related.
In conclusion, telmisartan effectively decreased proteinuria and was safe for treatment of cats with CKD. For the primary variable, telmisartan was at least as effective as benazepril. Indeed, telmisartan's antiproteinuric effect appeared to be more pronounced because significant decreases in UP/C compared to baseline were identified for telmisartan but not benazepril. The results of our study indicated that telmisartan was a safe and effective treatment to decrease proteinuria associated with CKD in cats.
Acknowledgments
The authors thank the following veterinarians for their contributions and support: Luc Beco, Peggy Binaut, Frédéric Blouin, Evert‐Jan de Boer, Laurent Bourdenx, Laura Broschek, Martha Cannon, Michael Deinert, Nicolas Delamarche, Christel Delprat, Oriol Domenech, Roswitha Dorsch, Mark Evans, Alwyn Evans, Bernard Flasse, Alain Le Garreres, Peter Hettling, David Hodges, Barbara Kohn, Caroline Léger, Josh Lida, Francois Mens, Nicolas Mourlan, Ruth Negatsch, Isabelle Papadopulo, Nick Park, Wolfgang Paulenz, Félix Pradies, Sally Rackham, Boris Radicke, Stefan Reindl, Kathrin Reuter, Brice Reynolds, Bernhard Sörensen, Annetta Steger, Tiekie von Tonder, and Emil Visnjaric. The authors are particularly grateful to the owners of the enrolled cats, who made this study possible.
Conflict of Interest Declaration
This project was funded by Boehringer Ingelheim Vetmedica GmbH, a company representative read and approved the final draft. Ulrike Sent, Rüdiger Gössl and Tanja Zimmering are employees of Boehringer Ingelheim Pharma GmbH&Co.KG and Vetmedica GmbH, respectively. Prof. Elliott has acted as a paid independent consultant to Boehringer Ingelheim Vetmedica GmbH providing advice on study design and critically evaluating the results of the study for registration purposes.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
Footnotes
Semintra oral solution for cats (telmisartan 4 mg/mL), Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany
Fortekor 2.5, flavored benazepril, Novartis Santé Animale S.A.S Huningue Cedex, France
VICH GL9 (GCP), London, UK, July 1, 2001
SAS Institute Inc, Cary, NC
References
- 1. Polzin DJ, Osborne CA. Update—Conservative medical management of chronic renal failure In: Kirk RW, ed. Current Veterinary Therapy IX. Philadelphia, PA: Saunders; 1986:1167–1173. [Google Scholar]
- 2. Polzin DJ. Chronic kidney disease In: Ettinger SJ, Feldman EC, eds, Textbook of Veterinary Internal Medicine. Missouri: Saunders; 2010:1990–2021. [Google Scholar]
- 3. DiBartola SP, Rutgers HC, Zack PM, Tarr MJ. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973‐1984). J Am Vet Med Assoc 1987;190:1196–1202. [PubMed] [Google Scholar]
- 4. Syme HM, Markwell PJ, Pfeiffer D, Elliott J. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria. J Vet Intern Med 2006;20:528–535. [DOI] [PubMed] [Google Scholar]
- 5. Boyd LM, Langston C, Thompson K, et al. Survival in cats with naturally occurring chronic kidney disease (2000–2002). J Vet Intern Med 2008;22:1111–1117. [DOI] [PubMed] [Google Scholar]
- 6. Polzin DJ, Osborne CA, Ross S. Evidence‐based management of chronic kidney disease In: Bonagura JD, Twedt DC, eds. Kirk's Current Veterinary Therapy XIV. Philadelphia, PA: Saunders; 2009:872–879. [Google Scholar]
- 7. Navar LG. Intrarenal renin‐angiotensin system in regulation of glomerular function. Curr Opin Nephrol Hypertens 2014;23:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siragy HM, Carey RM. Role of the intrarenal renin‐angiotensin‐aldosterone system in chronic kidney disease. Am J Nephrol 2010;31:541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruester C, Wolf G. Renin‐angiotensin‐aldosterone system and progression of renal disease. J Am Soc Nephrol 2006;17:2985–2991. [DOI] [PubMed] [Google Scholar]
- 10. Mitani S, Yabuki A, Taniguchi K, Yamato O. Association between the intrarenal renin‐angiotensin system and renal injury in chronic kidney disease of dogs and cats. J Vet Med Sci 2013;75:127–133. [DOI] [PubMed] [Google Scholar]
- 11. Jepson RE, Brodbelt D, Vallance C, et al. Evaluation of predictors of the development of azotemia in cats. J Vet Intern Med 2009;23:806–813. [DOI] [PubMed] [Google Scholar]
- 12. Chakrabarti S, Syme HM, Elliott J. Clinicopathological Variables Predicting Progression of Azotemia in Cats with Chronic Kidney Disease. J Vet Intern Med 2012;26:275–281. [DOI] [PubMed] [Google Scholar]
- 13. King JN, Tasker S, Gunn‐Moore DA, Strehlau G. Prognostic factors in cats with chronic kidney disease. J Vet Intern Med 2007;21:906–916. [PubMed] [Google Scholar]
- 14. Siragy HM. The angiotensin II type 2 receptor and the kidney. J Renin Angiotensin Aldosterone Syst 2010;11:33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Danyel LA, Schmerler P, Paulis L, et al. Impact of AT2‐receptor stimulation on vascular biology, kidney function, and blood pressure. Integr Blood Press Control 2013;6:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Padia SH, Carey RM. AT2 receptors: Beneficial counter‐regulatory role in cardiovascular and renal function. Pflugers Arch 2013;465:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akasu M, Urata H, Kinoshita A, et al. Differences in tissue angiotensin II‐forming pathways by species and organs in vitro. Hypertension 1998;32:514–520. [DOI] [PubMed] [Google Scholar]
- 18. Ennezat PV, Berlowitz M, Sonnenblick EH, Le Jemtel TH. Therapeutic implications of escape from angiotensin‐converting enzyme inhibition in patients with chronic heart failure. Curr Cardiol Rep 2000;2:258–262. [DOI] [PubMed] [Google Scholar]
- 19. Aramaki Y, Uechi M, Takase K. Angiotensin converting enzyme and chymase activity in the feline heart and serum. J Vet Med Sci 2003;65:1115–1118. [DOI] [PubMed] [Google Scholar]
- 20. Aoki A, Ogawa T, Sumino H, et al. Long‐term effects of telmisartan on blood pressure, the renin‐angiotensinaldosterone system, and lipids in hypertensive patients. Heart Vessels 2010;25:195–202. [DOI] [PubMed] [Google Scholar]
- 21. Matsuo T, Miyata Y, Sagara Y, et al. Renoprotective effects of telmisartan after unilateral renal ablation in rats. Int J Nephrol Renovasc Dis 2013;6:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Noone D, Licht C. Chronic kidney disease: A new look at pathogenetic mechanisms and treatment options. Pediatr Nephrol 2014;29:779–792. [DOI] [PubMed] [Google Scholar]
- 23. Balakumar P, Bishnoi HK, Mahadevan N. Telmisartan in the management of diabetic nephropathy: A contemporary view. Curr Diabetes Rev 2012;8:183–190. [DOI] [PubMed] [Google Scholar]
- 24. Fukami K, Yamagishi SI, Kaifu K, et al. Telmisartan inhibits AGE‐induced podocyte damage and detachment. Microvasc Res 2013;88:79–83. [DOI] [PubMed] [Google Scholar]
- 25. Michel MC, Foster C, Brunner HR, Liu L. A systematic comparison of the properties of clinically used angiotensin II type 1 receptor antagonists. Pharmacol Rev 2013;65:809–848. [DOI] [PubMed] [Google Scholar]
- 26. Westfall PTRaWR . Multiple Comparisons and Multiple Tests Using SAS. Cary, NC: SAS® Press; 2011. [Google Scholar]
- 27. IRIS guidelines . Available at: http://www.iris-kidney.com/guidelines/staging.shtml. Accessed 21 January 2015.
- 28. King JN, Gunn‐Moore DA, Tasker S, et al. Tolerability and efficacy of benazepril in cats with chronic kidney disease. J Vet Intern Med 2006;20:1054–1064. [DOI] [PubMed] [Google Scholar]
- 29. Wellek S. Testing Statistical Hypotheses of Equivalence and Noninferiority, 2nd ed Boca Raton: Chapman & Hall/CRC; 2010. [Google Scholar]
- 30. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int J Surg 2012;10:28–55. [DOI] [PubMed] [Google Scholar]
- 31. Wienen W, Entzeroth M, Van Meel JCA, et al. A review on telmisartan: A novel, long‐acting angiotensin II‐receptor antagonist. Cardiovasc Drug Rev 2000;18:127–156. [Google Scholar]
- 32. Ladino M, Schulman IH. Renovascular and renoprotective properties of telmisartan: Clinical utility. Int J Nephrol Renovasc Dis 2010;3:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Williams TL, Peak KJ, Brodbelt D, et al. Survival and the development of azotemia after treatment of hyperthyroid cats. J Vet Intern Med 2010;24:863–869. [DOI] [PubMed] [Google Scholar]
- 34. Wakeling J, Moore K, Elliott J, Syme H. Diagnosis of hyperthyroidism in cats with mild chronic kidney disease. J Small Anim Pract 2008;49:287–294. [DOI] [PubMed] [Google Scholar]
- 35. Mizutani H, Koyama H, Watanabe T, et al. Evaluation of the clinical efficacy of benazepril in the treatment of chronic renal insufficiency in cats. J Vet Intern Med 2006;20:1074–1079. [DOI] [PubMed] [Google Scholar]
- 36. Chakrabarti S, Syme HM, Brown CA, Elliott J. Histomorphometry of feline chronic kidney disease and correlation with markers of renal dysfunction. Vet Pathol 2013;50:147–155. [DOI] [PubMed] [Google Scholar]
- 37. Robles NR, Angulo E, Grois J, Barquero A. Comparative effects of fosinopril and irbesartan on hematopoiesis in essential hypertensives. Ren Fail 2004;26:399–404. [DOI] [PubMed] [Google Scholar]
- 38. Pratt MC, Lewis‐Barned NJ, Walker RJ, et al. Effect of angiotensin converting enzyme inhibitors on erythropoietin concentrations in healthy volunteers. Br J Clin Pharmacol 1992;34:363–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Elliott J, Barber PJ. Feline chronic renal failure: Clinical findings in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract 1998;39:78–85. [DOI] [PubMed] [Google Scholar]
- 40. Dow SW, Fettman MJ. Chronic renal disease and potassium depletion in cats. Semin Vet Med Surg (Small Anim) 1992;7:198–201. [PubMed] [Google Scholar]


