Abstract
Tinnitus is a phantom auditory sensation that reduces quality of life for millions worldwide and for which there is no medical cure. Most cases are associated with hearing loss caused by the aging process or noise exposure. Because exposure to loud recreational sound is common among youthful populations, young persons are at increasing risk. Head or neck injuries can also trigger the development of tinnitus, as altered somatosensory input can affect auditory pathways and lead to tinnitus or modulate its intensity. Emotional and attentional state may play a role in tinnitus development and maintenance via top-down mechanisms. Thus, military in combat are particularly at risk due to combined hearing loss, somatosensory system disturbances and emotional stress. Neuroscience research has identified neural changes related to tinnitus that commence at the cochlear nucleus and extend to the auditory cortex and brain regions beyond. Maladaptive neural plasticity appears to underlie these neural changes, as it results in increased spontaneous firing rates and synchrony among neurons in central auditory structures that may generate the phantom percept. This review highlights the links between animal and human studies, including several therapeutic approaches that have been developed, which aim to target the neuroplastic changes underlying tinnitus.
Introduction
Tinnitus, the perception of sound in the absence of a corresponding external auditory stimulus, is a phantom sensation (ringing of the ears) that reduces quality of life for millions worldwide and for which at present there is no medical cure. While most common after the age of 60 where 8–20% of individuals are affected, chronic tinnitus can occur at any age [1] and is a major service-related disability for soldiers returning from Iraq and Afghanistan [2]. More than one billion dollars was disbursed in disability payments by the United States Government in 2011 to members of the military suffering from tinnitus. Of the general population approximately 1–2% of individuals suffer from unremitting tinnitus to the extent that they seek assistance from the health professions including family physicians, otolaryngologists, audiologists, psychiatrists, and neurologists [1, 3].
In this review we focus on what is currently known about tinnitus-triggering factors, its psychoacoustic properties, and the neural mechanisms underlying its generation and associated symptomatology. We also discuss treatment approaches which, while not fully effective in eliminating the tinnitus, have promise for reducing its impact on quality of life for many tinnitus sufferers.
Triggering Factors and Associated Conditions
The circumstances and conditions associated with tinnitus are numerous. The most common associated condition is the presence of hearing loss as assessed by the clinical audiogram. Hearing loss is present in up to 90% of cases [4] [5, 6] and may result from recreational or occupational noise exposure or the aging process. Other factors associated with the onset of tinnitus include head and neck injuries, ototoxic drug use, infections, and a range of medical conditions that can affect hearing. While most tinnitus sufferers describe their tinnitus as a steady tonal or hissing percept depending on its bandwidth, more complex sounds such as insect sounds, chimes, running water, or multiple sounds are also reported, although some of this variability may relate to the descriptors that tinnitus patients choose to describe their percept rather than to variability in the percept itself [7]. Tinnitus varies in the circumstances associated with its onset (for example, noise exposure, whiplash or head injuries), its time course (continuous or intermittent), its spatial attributes (whether experienced in one or both ears or perceived in the head), its degree of intrusiveness, and with respect to whether hyperacusis (increased sensitivity to ordinary environmental sounds) is also present. Anxiety, sleeplessness, and depression are common comorbidities especially in the early stages of tinnitus. The extent of this variability has sparked investigation into whether subtypes can be identified that may be associated with a specific etiology and pathophysiology [8] notwithstanding that, because tinnitus is an auditory percept, some communalities must exist in its underlying neural mechanisms. Identification of subtypes may be worthwhile insofar as clinical management can be optimized for typical cases or etiologies identified that enable effective treatment in rare cases [9–11].
Neuroscience research conducted in the last decade has shown that most cases of chronic tinnitus do not arise from increased activity in the cochlear nerve driven by the damaged cochlea, but rather develop as a consequence of changes that occur in central auditory pathways and other brain regions when the brain loses its input from the ear. Clinical observations support this conclusion. Tinnitus is a predictable outcome when the auditory nerve is sectioned during surgery for the removal of acoustic neuromas and is typically not eliminated in preexisting cases [12]. While exceptions to these principles have been reported, which may involve pathology in the olivocochlear efferent system or other factors [13, 14], section of the auditory nerve is not a recommended procedure for the treatment of tinnitus. On the contrary, when hearing function is augmented by cochlear implants in individuals suffering sensorineural hearing loss, the tinnitus associated with the hearing loss is frequently reduced and sometimes even eliminated [15].
While deafferentation of auditory pathways thus appears to be a critical triggering factor in tinnitus, nonetheless about 10–15% of tinnitus sufferers present with normal clinical audiograms up to 8 kHz [16, 17], while many more individuals experience high-frequency hearing loss with aging but do not have tinnitus [18]. What these cases tell us about tinnitus is currently debated. Recent advances in cochlear neuroscience conducted in animals exposed to noise trauma suggest that cases of tinnitus without auditory threshold shift may involve neuropathic changes in the cochlea consequent to noise exposure or the aging process that are not expressed in hearing thresholds, but rather exhibit themselves when suprathreshold hearing is tested [19]. While damage to the cochlear transduction mechanism (inner and outer hair cells on the basilar membrane of the inner ear and their associated stereocilia) often recovers after noise exposure, synapses connecting auditory nerve fibers (ANFs) to the inner hair cells appear to be more vulnerable to damage by noise exposure [20] as well as to the effects of aging [21]. Especially vulnerable are synapses on ANFs that have high thresholds for depolarization and are tuned to frequencies commencing typically one to two octaves above the noise exposure frequencies [22, 23]. This pattern of synaptic pathology is relevant to tinnitus without threshold shift, because its presence would not affect the detection of low level sounds (thus exempting the audiogram) but would affect ANFs with higher frequency tuning which is where tinnitus percepts lie in individuals with tinnitus and clinically normal hearing [17] or with audiometric hearing loss (see below). The presence of hidden hearing loss in tinnitus is supported by evidence that Wave I (reflecting auditory nerve responses) of the Auditory Brainstem Response (ABR) to suprathreshold sounds, is reduced in human tinnitus sufferers with normal audiograms compared to control subjects with normal hearing [17, 24]. In contrast, Wave V reflecting processing in the auditory midbrain, is either normal [17] or enhanced [24] in tinnitus subjects, revealing increased central gain. To the extent that deafferentation, whether hidden or expressed in the audiogram, is a critical triggering factor in tinnitus, understanding the role of hearing loss in tinnitus is important for gaining a wider perspective on the condition.
Properties of Tinnitus Related to Audiometric Hearing Loss
When subjects with sloping, high frequency hearing loss expressed in the audiogram are asked to rate several sound frequencies for similarity to their tinnitus, similarity judgments typically commence near the edge of normal hearing and increase in proportion with the depth of threshold shift, yielding a “tinnitus spectrum” that spans the hearing impaired region [25–28]. Similarly, tinnitus can be transiently suppressed for 30–60 seconds after presentation of a band-limited masking noise, a phenomenon known as “residual inhibition” (RI) in the tinnitus literature [29]. This forward masking effect is optimal when the center frequency of the band-limited masking noise is also in the tinnitus frequency region aligning with the tinnitus spectrum [18]. However, if hearing loss in the tinnitus region is deep maskers centered at lower frequencies may be somewhat more effective [14]. These results apply to cases of notched hearing loss expressed in the audiogram [25, 29] and likely to “hidden” hearing loss as well, where tinnitus spectra shift inversely with respect to audiometric thresholds even when thresholds remain <20 dBHL up to 8 kHz [17]. Psychoacoustic properties of tinnitus are relevant to understanding neural mechanisms of tinnitus [17] because they suggest that aberrant changes taking place among neurons tuned to the hearing-impaired frequencies in central auditory structures underlie tinnitus, and disrupting these neural changes suppresses it. What are these neural changes, and where are they occurring in the auditory projection pathway?
These questions have been addressed by animal studies that have examined the neural effects of noise trauma (or other procedures such as salicylate injections, which are beyond the scope of this review) that are known to impair the cochlear transduction mechanism. Noise exposure can yield either a permanent threshold shift (PTS) or a temporary threshold shift (TTS), depending on the noise exposure intensity and duration. The presence or absence of tinnitus in animals exposed to these putative tinnitus-inducing procedures is assessed either by measuring under conditions of silence changes in conditioned behavioral responses that were established by prior training to sound stimuli, or by measuring the extent to which silence modulates reflexive behavioral responses evoked by an unexpected suprathreshold sound (see [30] for review). An example of the latter method used by many but not all of the studies reviewed below is the “Gap Prepulse Inhibition of Acoustic Startle (GPIAS)” procedure developed by Turner and colleagues for rats and mice [31, 32] and modified for use in guinea pigs [33, 34]. In this method startle suppression by an acoustic prepulse verifies that functional hearing is present after noise exposure, while the failure of a silent gap to do the same is taken to indicate that the silent interval has been filled by a tinnitus sound (Insert Figure 1 here). Tinnitus and no-tinnitus animals are segregated on the basis to which startle suppression by the silent gap has fallen beneath a specified criterion. Many animal studies using GPIAS and other models of tinnitus have used noise-exposure levels that induce TTS not PTS, so that functional hearing is preserved. While the validity of animal models is not without challenge [35–37], an increasing number of studies are demonstrating the usefulness of these procedures by providing consistent neurophysiological patterns in animals that express behavioral evidence of tinnitus as compared to those that do not, when all animals have been treated similarly. The findings give greater assurance that neural changes are being measured that are inextricably related to tinnitus, and that the changes can be differentiated from those that may relate only to hearing loss or hyperacusis. Using behavioral models three types of neural changes have been identified that appear to accompany tinnitus, namely, increases in the spontaneous activity of auditory neurons in subcortical and cortical structures, increased burst firing in these structures, and increased synchronous activity among neurons affected by threshold shifts or suprathreshold hearing impairments.
Figure 1.
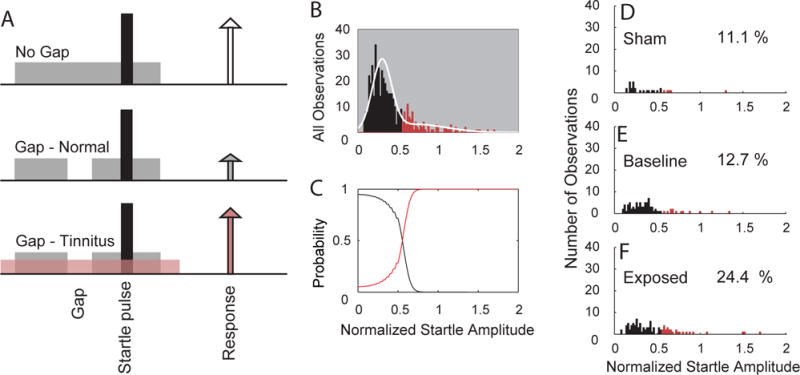
A. Schematic describing the GPIAS assay for tinnitus. A startle stimulus (black) is inserted into a background noise without a gap (no gap; top row) and with a gap (middle and lower rows; 50 msec. gap, 50 ms before the startle sound) are presented to the animal. Each trial consists of a continuous 60 dB background sound (grey bar) with a 10 msec., 115 dB startle pulse embedded (black bar). The guinea pig startles in response to the startle stimulus, with the amplitude of the response shown by the height of each arrow. In animals without tinnitus, the gap suppresses the startle response (middle row). In animals with tinnitus, the gap is filled by the tinnitus (pink) and the startle response shows less reduction relative to the no gap startle response (white arrow). B–F. Gaussian-mixture model analysis partitions the normalized startle distribution into normal and tinnitus distributions. B. Histogram of the normalized startle distribution (white line) partitioned into two distributions: no evidence for tinnitus (black bars) and evidence for tinnitus (red bars). C. The probability distributions of normalized startle values belonging to the tinnitus or non-tinnitus distributions. D. Histogram of the partitioned distribution of post-exposure normalized startle observations for sham animals. E. Histogram of the partitioned distribution of normalized startle observations for baseline (pre-exposure) observations from sham and exposed animals. F. Histogram of the partitioned distribution of post-exposure normalized startle observations from exposed animals. The percentage of observations placed into the tinnitus group is shown on each panel in D–F.
Adapted from ([41]).
Neurophysiological Alterations in Animal Models of Tinnitus
Frequency-specific increases in Spontaneous Firing Rates
Altered neuronal activity is detected as a physiological correlate of tinnitus in the first structure of the central auditory pathway, the cochlear nucleus (CN). (Insert Figure 2 here). The identification of frequency-specific increases in spontaneous firing rates (SFR) in neurons with best frequencies (BFs) close to the noise exposure spectrum, which correlate with behavioral evidence of tinnitus was first shown in the dorsal CN (DCN) using operant conditioning [38]. Subsequent studies using different operant techniques or GPIAS confirmed that after pure tone or band limited noise-exposure, the principal output neurons of the DCN, the fusiform cells, show increased SFRs at frequencies close to the noise-exposure frequency and the behaviorally- determined tinnitus frequencies [39–41]. Some of these studies used noise-exposure levels that only produced TTS so that sound thresholds had recovered by the time of tinnitus testing [40, 41], which would be consistent with the studies showing tinnitus in humans with clinically normal audiograms [17, 42]. The presence in animal studies of frequency-specific increases in SFRs in the CN close to the noise-exposure frequencies [40, 41] also suggests that there is a loss of ANF input to the CN from high threshold ANFs even after recovered audiometric thresholds [20, 23]. Other evidence indicates that increased SFRs can occur in the ventral CN (VCN) after various types of hearing impairment [43–45], which suggests that the VCN could also be a site of hyperactivity-initiation in the brain. Human studies examining the auditory brainstem responses of tinnitus patients corroborate the possible involvement of the VCN in tinnitus development [24]. However, studies using a behavioral tinnitus model to examine tinnitus-related activity in the VCN after noise-exposure have yet to be performed. Thus, at present, the DCN can be considered to be the site where diminished auditory nerve input initiates hyperactivity, the first physiological hallmark of tinnitus, which is then conveyed to higher brainstem and cortical regions [46, 47].
Figure 2.
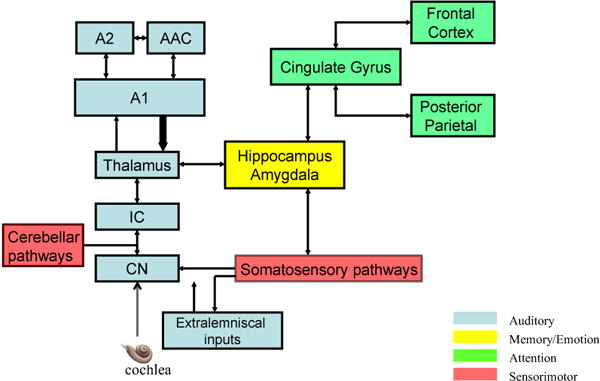
Simplified representation of auditory and non-auditory pathways discussed in this article and revealed by functional imaging studies to be involved in aspects of tinnitus. Blue identifies principal structures in the auditory pathway commencing with the cochlear nucleus (CN) and projecting through the inferior colliculus (IC) to the thalamus and auditory cortex (A1, primary auditory cortex A2; secondary auditory cortex; AAC, anterior auditory field). Return projections to the thalamus (these projections more numerous than forward projections) and subcortical structures in the auditory pathway are represented by a thickened arrow. Output from auditory pathways distributes to several major nonauditory regions of the brain, here simplified by their putative functional roles in normal information processing as identified by neuroscience research (see color code). Prominent structures involved in memory and emotion include the parahippocampal gyrus, amygdala, and limbic region including the insula (yellow). Participating in attention and consciousness (green) are the anterior and posterior cingulate regions, orbitofrontal cortex, prefrontal cortex (dorsomedial and ventromedial divisions), the subcallosal region (nucleus accumbens), and posterior partietal cortex including the precuneus. Sensori-motor pathways (red) include somatosensory ganglia, brainstem somatosensory pathways, primary and secondary somatosensory cortex, and the cerebellum. In this summary diagram connections among these regions are portrayed by arrows, but the connections among them are densely parallel and reciprocal mediated by cortico-cortico projections directly or via the thalamus as well as by multisensory interactions that occur in subcortical auditory structures discussed in the article. A2, secondary auditory cortex; AAC, anterior auditory field; A1, primary auditory cortex; IC, inferior colliculus; CN, cochlear nucleus.
Several studies at the next auditory center of the brain stem, the inferior colliculus (IC), have demonstrated increased SFRs just below, within and just above the noise-damaged region of the cochlea, which correlated with presence of tinnitus using GPIAS [48, 49], but in some studies [50, 51] the increased spontaneous activity was not dependent on the presence of tinnitus. Nonetheless, increases in IC spontaneous activity appear to be dependent on that transmitted from the DCN since DCN ablation prior to noise damage prevents IC hyperactivity as well as tinnitus development [52]. Furthermore, DCN ablation after noise damage immediately abolishes IC hyperactivity [53]. However, it must be noted that this finding contradicts another study [54] that demonstrated that tinnitus persists after DCN ablation, suggesting the possibility that higher structures are recruited in tinnitus maintenance. Nevertheless, it is unlikely that IC maintains hyperactivity independently as it can be abolished by cochlear ablation for up to 6 weeks post exposure but not if ablation occurs later [55]. This contrasts with DCN, in which established hyperactivity is not affected by subsequent elimination of either afferent or efferent inputs 4 to 6 weeks post-exposure [56, 57]. In addition, the time course and tonotopicity of hyperactivity in IC mimics that of DCN [58]. We can conclude from these studies that elevated spontaneous activity in DCN is probably transmitted to IC, and then to the thalamus from IC or perhaps independently through direct projections from DCN [59–61].
Tinnitus-related hyperactivity continues to be observed at the level of the auditory thalamus. Here, neurons in the medial geniculate body show frequency-specific increased SFRs after noise damage that correlate with the animals’ degree of tinnitus measured using GPIAS [62]. Neurons in the primary auditory cortex, earlier shown to have increased spontaneous activity after noise damage [4, 63], were confirmed to have tinnitus-related increases in SFR in later studies that used GPIAS to confirm the presence of tinnitus [64, 65].
Neural Synchrony and burst-firing
Two other markers that have been suggested as physiological correlates of tinnitus are increased synchrony between neurons or increased bursting in a specific auditory structure. Increased synchrony between neurons could create perceptual grouping of auditory objects [66] and thus it is feasible that increased synchrony in the absence of a physical auditory stimulus, could lead to the perception of a phantom sound [67]. A recent study has indeed reported increased SFRs, bursting and neural synchrony in the fusiform cells of the DCN, establishing the presence of all three correlates in the earliest central auditory region that correlate with tinnitus [68]. In the IC, increased bursting and synchrony across multi-unit clusters was shown in animals with behaviorally-confirmed tinnitus using an operant conditioning model [69]. The bursting and synchronous firing were not confined to the central nucleus of the IC, but were also evident in regions surrounding the central nucleus, particularly the dorsal cortex. In other research using GPIAS, tinnitus-related maladaptive plastic changes of MGB single unit responses were demonstrated in sound-exposed adult rats with behavioral evidence of tinnitus. In addition to increased SFRs, the MGB units in animals with tinnitus exhibited altered burst-firing properties, which correlated with the degree of tinnitus measured [62].
Changes in the MGB by tinnitus-inducing procedures would be expected to influence the response properties of neurons in primary auditory cortex. In primary auditory cortex, after noise over-exposure that elevated ABR thresholds above the exposure frequency, neurons in the hearing loss region shifted their preferred tuning to frequencies near the audiometric edge, such that these frequencies became over-represented in the cortical tonotopic map [70, 71]. Neurons in primary auditory cortex also showed increases in SFR, increased synchronization, and increased burst firing [71]. Increased SFR and synchrony were observed primarily at frequencies within the hearing loss region, with increased synchrony confined to this region. Burst firing increased immediately after noise trauma but subsided to normal over the measurement period of a few hours while the changes in SFR and synchrony persisted. While tinnitus was not measured in these experiments, subsequent studies confirmed that there was increased cortical synchrony and SFR in animals with verified tinnitus, using the GPIAS, giving credence to the validity of hyperactivity and synchrony as neural correlates of tinnitus [72].
Mechanisms of Increased SFR and Synchrony
Neural plasticity beginning at brain stem
A plethora of studies demonstrate that homeostatic and long-term plasticity change in the CN after cochlear damage. Even a partial reduction of auditory nerve inputs to the dorsal and ventral divisions of the cochlear nucleus results in decreases in inhibitory neurotransmitters including glycine and GABA as well as in changes of their receptors [73, 74]. Additionally, there is an increase in excitatory neurotransmission after severe and partial cochlear damage [75, 76] and an upregulation of excitatory non-auditory projections [77]. Decreased inhibition combined with increased excitation could result in hyperactivity of CN neurons that could be transmitted through the IC, or bypass the IC [60, 78], to the MGB. At the level of the MGB, however, there is little evidence of tinnitus-related decreases in GABAergic neurotransmission [79]. Rather, at this level, tinnitus measured with GPIAS was associated with increases in tonic extra synaptic GABAAR currents in action potentials/evoked bursts, and in GABAAR δ-subunit gene expression. These findings are consistent with thalamocortical dysrhythmia, which results from abnormal theta-range resonant interactions between thalamus and cortex, due to neuronal hyperpolarization and the initiation of low-threshold calcium spike bursts [80].
Spike timing dependent plasticity (STDP) in vitro, [81] and its macroscopic in vivo correlate, stimulus timing dependent plasticity (StDP) [82], occur in the DCN. In the normal system, this form of long-term plasticity presents as Hebbian plasticity in the principal output neurons of the DCN in vitro. Hebbian plasticity is the process by which synapses are strengthened when presynaptic activation precedes post synaptic activation, while anti-Hebbian plasticity results in a weakening of synapses under these conditions [83]. StDP is governed not only by the temporal order of pre-and post-synaptic activity but also by NMDA and acetylcholine functional modulation [84]. In vivo, fusiform cells in the DCN, as well as neurons in primary auditory cortex demonstrate primarily Hebbian plasticity in normal animals [82] (Insert Figure 3 here). However, animals with tinnitus, assessed by GPIAS, show primarily anti-Hebbian plasticity in both regions. In contrast, animals that did not develop tinnitus showed increased long-term depression [41, 65, 85]. Altered acetylcholine-mediated neuromodulation, NMDA receptor changes along with increased glutamatergic transmission and decreased glycinergic and/or GABAergic transmission contribute to these changes [86, 87] [77] [73] [88] (see Figure 3). Computational studies indicate that STDP can alter synchronization, suggesting that the changes in StDP demonstrated with tinnitus may lead to the alterations in synchrony observed in animals with tinnitus [68, 89, 90].
Figure 3. Mechanisms of tinnitus initiation in the dorsal cochlear nucleus.
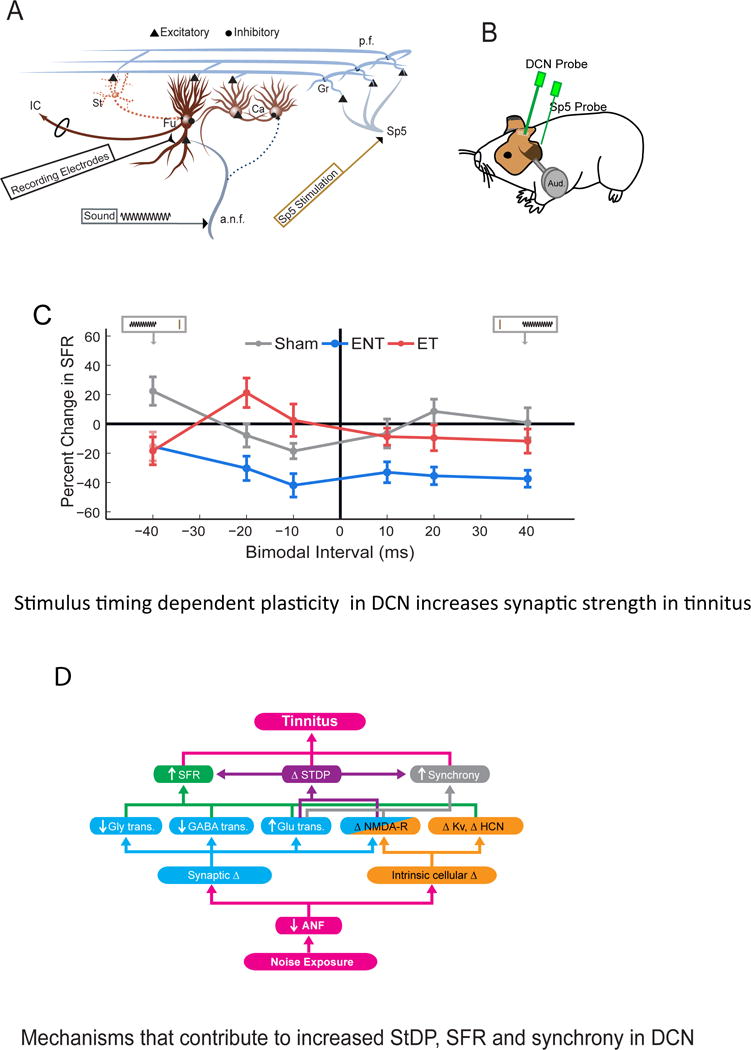
A. Schematic of DCN circuitry showing the principal output neurons, fusiform cells and inhibitory interneurons, the cartwheel cells. Electrical stimulation and recording locations are shown in spinal trigeminal nucleus (Sp5) and DCN for measuring StDP. The thirty two-channel recording electrodes (black) spanned the tonotopic axis of the DCN. Short current pulses delivered via a bipolar stimulating electrode (brown) placed into Sp5 activated parallel-fiber inputs to DCN that activate fusiform cells subsequent to granule-cell activation. Tones were delivered through calibrated, hollow ear bars. Ca - cartwheel cell; Fu - fusiform cell; Gr - granule cell; St – Stellate cell; IC - inferior colliculus; Sp5 - spinal trigeminal nucleus; a.n.f - auditory nerve fiber; p.f - parallel fiber. ET- Exposed with tinnitus; ENT – exposed without tinnitus.
B. Cartoon of the locations of the recording and stimulating electrodes relative to the animal’s head.
C. Bimodal plasticity of spontaneous firing rates (SFR) shifts from predominantly Hebbian in shams (where somatosensory preceding auditory produces facilitation at 20 ms) to anti-Hebbian rules in guinea pigs with tinnitus (facilitation now seen when auditory precedes somatosensory stimulation at −20 ms) and suppressive at all pairing intervals in guinea pigs without tinnitus. Mean timing rules are shown for SFRs for units from Sham (gray), ENT (pink), and ET (red) guinea pigs. Cartoon inset at top represents the relative order of Sp5 and sound stimuli. The brown vertical line indicates the Sp5 stimulation, and the sinusoid represents the tone stimulus. Mean timing rules were computed for all measurements from DCN fusiform cell units. Error bars indicate SEM. Adapted from ([41]).
D. Putative molecular mechanisms underlying changes in StDP, SFR and synchrony of DCN principal output neurons (fusiform cells) in initiating tinnitus. Noise over-exposure triggers auditory nerve fiber (ANF) deafferentation or neuropathy leading to alterations in the DCN circuitry via the following processes: Synaptic changes include decreased glycinergic (Gly), GABAergic, and increased glutamatergic (Glu) neurotransmission; intrinsic cellular changes include NMDA receptor (NMDA-R), voltage-gated potassium channel (Kv), and hyperpolarization-activated cyclic nucleotide-gated (HCN) channel properties. Each of these processes has been shown to affect one or more tinnitus phenotypes in DCN: increased SFR, increased neural synchrony, and inverted STDP.
What we can learn from animals and humans that do not develop tinnitus
While most studies on tinnitus mechanisms focus on animals or humans that develop tinnitus, equally important is the information provided by those that do not develop, or are resistant to tinnitus development after the same noise-exposure conditions. Studies with PTS that compared animals with and without tinnitus have shown important differences between these groups that would not have been discerned if animals had been divided into only noise-exposed and normal groups. An exemplary study demonstrated that after a mild PTS, animals showing tinnitus (using GPIAS) demonstrated an increased ABR wave V amplitude at suprathreshold levels in contrast to a reduced wave V amplitude in the non-tinnitus animals [91]. The tinnitus animals showed no significant change in cortical activity measured with local field potentials, but showed a significant increase in wave V ABR amplitude representing synchronous activity of neurons in the inferior colliculus. This is consistent with another study demonstrating a reduction in levels of the activity-regulated cytoskeletal protein, Arc, in auditory cortex in animals that developed tinnitus, but not in those that did not develop tinnitus, measured in this case with an operant conditioning method [92]. Other studies demonstrated that animals developing tinnitus differed in KCNQ 2/3 and HCN channel activity from those without tinnitus after PTS, suggesting that the effect of noise trauma on intrinsic membrane properties also plays a role in the development of tinnitus (see Figure 3) [93, 94].
Studies using a TTS or ‘hidden hearing loss’ model are especially useful in this regard, as central effects can be more purely attributed to central homeostatic or timing-dependent plasticity mechanisms in the absence of differences in audiometric hearing level. Two studies in which there was only a temporary threshold shift after noise exposure for all animals in the study, report that those that did not develop tinnitus (using GPIAS) showed more long-term depression than long-term potentiation in the DCN. In contrast, the animals that developed tinnitus exhibited more long-term potentiation and less long-term depression [40, 41]. Other studies using TTS models have correlated the GPIAS tinnitus index with other physiological or molecular changes and have shown that the greater the tinnitus index, the greater the likelihood of observing increased SFR or bursting in the CN and MGB [62] [86]. In addition, MGB neurons from animals with tinnitus fired more spikes per burst relative to non-tinnitus MGB neurons, suggesting a tinnitus-related increase in intrinsic membrane excitability [79].
Role of Non-Auditory Structures
Somatosensory Pathways
Integration of auditory and somatosensory afferent projections occurs in peripheral brain structures as early as the CN, where projections from the AN and trigeminal and dorsal column ganglia and brain stem nuclei converge [95, 96]. These projection neurons terminate primarily on the CN granule cells, whose parallel-fiber axons terminate on the apical dendrites of DCN fusiform cells [97–99]. Auditory nerve fibers, bringing input from the cochlea, terminate on the basal dendrites of the fusiform cells. Fusiform cells are therefore ideally placed for multisensory integration and do so using mechanisms of long-term plasticity, which is stimulus timing dependent [82].
After cochlear damage- reducing auditory nerve input to the CN, somatosensory inputs are upregulated over a time interval of days [75, 77, 100] resulting in heightened fusiform cell responses to somatosensory stimulation [101]. This effect is a consequence of an increase in glutamatergic neurotransmission from somatosensory fibers following loss of input from auditory pathways [102]. Interestingly, the upregulation of glutamatergic inputs from the somatosensory system occurs after a ‘threshold’ level of cochlear damage, beyond which, no further changes occur [103]. Tinnitus-related changes in auditory-somatosensory integration by the fusiform cells include increased long-term potentiation [41], likely mediated by the increased non-auditory glutamatergic innervation after cochlear damage [75, 76]. Importantly, animals that did not develop tinnitus instead displayed increased long-term depression at fusiform synapses. The differences between animals with tinnitus, showing more long-term potentiation and those without tinnitus, showing more long-term depression, involve a complex interplay between multiple mechanisms involved in homeostatic and timing dependent plasticity. Given the significant alterations in processing involved in somatosensory integration in the CN, which are transmitted to the auditory cortex [104], it is not surprising that a majority of tinnitus sufferers can manipulate the intensity and frequency of their tinnitus by stimulating or moving their face and neck [105, 106], regions providing trigeminal and dorsal column inputs to the CN [99, 107, 108]. This so-called “somatosensory tinnitus” or “somatic tinnitus” occurs in up to two thirds of humans with tinnitus [105, 106].
Non-Auditory Brain Networks
Animal studies and human neuroimaging studies [92, 109] [110–112] have confirmed tinnitus–related changes in several non-auditory brain areas. The results suggest that tinnitus is accompanied by structural and functional alterations in prefrontal cortex, parietal cortex, cingulate cortex, amygdala, hippocampus, nucleus accumbens, insula, thalamus and the cerebellum [110, 113], (Figure 2). However, while many of the changes seen in these regions may relate to tinnitus, a challenge is to disambiguate effects attributable to the presence of tinnitus from those related to comorbidities of hearing loss, hyperacusis, and distress behavior that are often experienced by tinnitus sufferers. Melcher et al. [114] attempted to resolve conflicting reports of differences in grey matter volume in the subcallosal region between tinnitus patients and controls that in the previous studies had been matched hearing level to the clinical standard of 8 kHz[115, 116]. In their study no definitive group differences in gray matter volume or concentration were found; however, grey matter concentration was negatively correlated with threshold increase at frequencies >8kHz, which was not measured by previous studies.[114] Another study found that abnormal sound level tolerance correlated with increased fMRI responses evoked by suprathreshold sounds in the auditory midbrain when the presence of tinnitus was held constant between groups, whereas tinnitus by itself (with abnormal sound level tolerance held constant between groups) was associated with increased activity only in primary auditory cortex[117]. Moreover it remains to be elucidated to which extent the changes in non-auditory brain areas reflect a predisposition to or consequences of tinnitus development. In spite of these challenges we can conclude from the neuroimaging literature that tinnitus-related changes in brain structure and function extend well beyond classical auditory pathways when tinnitus and control groups are compared, even if the precise functional role of the different non-auditory structures in tinnitus is not yet unambiguously elucidated.
Changes in functional connectivity among brain regions have also been extensively investigated in tinnitus. Increased connectivity between the auditory cortices and a frontoparietal attention network was found by several EEG, MEG, and resting-state fMRI studies [113, 118–120]. The results are consistent with the hypothesis that the conscious perception of sound including the phantom sound of tinnitus requires long-range connectivity between auditory and attention-related areas [121]. Distress involved in tinnitus (measured by tinnitus severity questionnaires) has been associated with enhanced activity and connectivity between auditory and stress-related brain areas [122–124]. A notable brain area consistently highlighted in functional imaging studies of tinnitus is the parahippocampal region [118, 120, 125–128] (a structure known to be involved in memory) which has increased connectivity with the auditory cortex in tinnitus subjects in resting-state-EEG [125, 127] and fMRI data [118, 129]. This finding is consistent with the hypothesis that auditory perception is based on predictions about the external world that require information about the organism’s history with sound [4, 120, 130]. A prediction based on auditory memory encoded before the hearing loss would not correspond with input from the damaged cochlea, which may then activate frontoparietal attention mechanisms in an attempt to resolve these disparities. Other brain areas showing increased activation in individuals with tinnitus are the anterior cingulate cortex (ACC) and insula [122, 126]. As these two areas are key regions of the ‘salience network’ [131] it is assumed that increased activity in ACC and insula reflects the attribution of salience to the tinnitus sound. Based on these findings it has been proposed that tinnitus involves abnormal activity in multiple overlapping networks in the brain [120]. Some of the heterogeneity seen in tinnitus patients particularly with respect to comorbid distress behavior may reflect variation in the involvement of individual specific networks.
Treatment of Tinnitus
For the treatment of tinnitus many different pharmacological and non-pharmacological approaches have been tried, but none has shown convincing meta-analytic evidence for reducing the tinnitus percept. The best-established treatment thus far is cognitive behavioral therapy (CBT) for which a Cochrane meta-analysis found an improvement in quality of life and reduction of depression scores, even if CBT did not reduce tinnitus loudness or eliminate the percept [132]. In clinical practice CBT is frequently combined with sound therapy (see below). A recent large randomized clinical trial has shown that a multidisciplinary stepped care approach involving counseling, sound therapy and elements of CBT results in a significant benefit in tinnitus severity, tinnitus impairment and health related quality of life as compared to treatment as usual [133].
Several types of sound therapy have been devised with the aim of masking tinnitus or suppressing it through putative neuroplastic mechanisms. One approach provides music modified to compensate for the individual’s pattern of hearing loss, while in another, music is “notched” to exclude energy close to the tinnitus frequency [134, 135]. The resulting edges in the frequency spectrum are thought to distribute lateral inhibition into the notched region, suppressing tinnitus-related neural activity [136]. Coordinated Reset Neuromodulation consists of auditory stimuli presented as short tones in a random varying sequence above and below the tinnitus frequency, with the goal of reducing tinnitus-related neuronal hypersynchrony. A pilot study with this method found significant reductions of tinnitus loudness and annoyance as well as reduced abnormal oscillatory activity measured by EEG in tinnitus patients [137]. In contrast to these methods in which sound is presented passively, several forms of active auditory training have been explored in an attempt to induce neuroplastic changes. These studies have employed sounds of varying spectral complexity with frequencies within or just below the hearing loss or tinnitus frequency (see [138] for overviews). Based on results obtained from an animal model of tinnitus using GPIAS [64], vagus nerve stimulation paired with sound in the region of functional hearing aimed at normalizing tonotopic map organization [139] and has shown positive initial results in human studies [139]. Another novel approach applies paired auditory and somatosensory stimulation at timing orders and intervals chosen to suppress SFR and synchrony in auditory pathways, exploiting the StDP demonstrated in DCN in animal studies [41]. Even though these innovative forms of sound therapy have shown some positive pilot data, they are considered as experimental until results are confirmed in large randomized controlled trials.
As hearing loss represents the most important trigger for tinnitus, restoration of auditory input should reduce tinnitus. Accordingly, in patients with both uni- and bilateral, profound sensorineural hearing loss and tinnitus, cochlear implantation can suppress tinnitus perception in a majority of cases [15]. The efficacy of hearing aids for tinnitus reduction is less clear [140], probably because hearing aids cannot restore auditory nerve signals in case of inner hair cell or ribbon synapse loss. Moreover, amplification of sound by hearing aids is limited in the higher frequency range, where most tinnitus patients have their hearing loss (and their tinnitus percept). Accordingly, recent studies have only shown a benefit in those patients with a tinnitus pitch below 6 kHz [141, 142].
Many pharmacological agents have been investigated for tinnitus treatment but none has provided convincing reduction of tinnitus impact in excess of placebo effects [143]. Treatment with antidepressants improved comorbid depressive or anxiety disorders [144], but did not reduce tinnitus [145]. A meta-analysis of anticonvulsant treatment with carbamazepine, gabapentin and lamotrigine did not reveal additional benefits compared to placebo in controlled studies [146]. For benzodiazepines beneficial effects on tinnitus distress were reported [147], but long-term data are missing and in light of the dependency risk of regular benzodiazepine intake their routine use cannot be recommended [148, 149]. New approaches currently under study include potassium channel modulators [62, 93] and the intra-tympanic application of the NMDA receptor antagonist s-ketamine [150]. Because multiple signalling pathways may be involved in supporting brain network activity in tinnitus, it has been suggested that therapeutic shotguns could prove to be more effective at suppressing tinnitus than agents that target specific receptors [143].
With increasing knowledge about neuronal mechanisms of tinnitus new techniques are increasingly being investigated as potential therapeutic approaches. Repetitive transcranial magnetic stimulation (rTMS) uses the rhythmic application of brief magnetic pulses delivered by a coil placed on the scalp to modulate auditory cortical activity. A recent meta-analysis demonstrates beneficial effects for this approach, but the effect sizes are small, and the duration of treatment effects remains often limited (Soleimani et al. 2015). Better results were obtained with additional stimulation of prefrontal brain areas (Lehner et al. 2012). At this time it is unknown whether further developments of these and other novel treatment approaches will deliver results superior to the modest improvements in tinnitus behaviour and perception reported by previous studies. However, their guidance from neuroscience research is an encouraging development.
Looking Ahead: Complementary Animal and Human Studies
Behavioral and functional imaging studies of human tinnitus sufferers have corroborated several of the changes reported above from animal studies, including increased gain or hyperactivity in central pathways [17, 151], reduced inhibition in the auditory cortex [152], and macroscopic tonotopic map reorganization in primary auditory cortex when audiometric hearing loss is present [153] although not among tinnitus sufferers without audiometric hearing loss [154]. The latter finding suggests that macroscopic cortical map reorganization may be more closely related to hearing loss than to tinnitus, although to the extent that cortical neurons lose their input from the ear, some degree of tuning shift could be expected to accompany the presence of tinnitus. Changes in sound-evoked responses generated by neural sources in the tinnitus frequency region of primary auditory cortex have been found to track tinnitus suppression during RI in tinnitus sufferers [155]. This result is consistent with studies in humans [117] and animals [65] that found tinnitus to be associated with neural changes in this brain region. Interestingly, sound-driven responses from secondary (nonprimary) auditory cortex did not track RI depth [155], although these responses were larger in tinnitus than control subjects. It was suggested that neural changes occurring in nonprimary auditory cortex reflect disinhibition of this region by attention, prediction failure, or deafferentation. Persistent disinhibition of the auditory cortex in tinnitus [117] may explain why individuals with tinnitus do not perform as well as controls on tasks requiring top-down modulation of attention [156].
As reviewed here, a major contribution from human research has been to identify brain areas outside of auditory pathways that are involved in tinnitus. Investigation of non-auditory structures was motivated initially by surprising results from functional imaging studies [111, 112] and by prescient models of tinnitus that proposed an involvement of limbic [157, 158] and somatosensory structures [106] in tinnitus. More recent magnetoencephalography (MEG) studies of brain network activity in tinnitus have been guided by the concept of thalamocortical dysrhythmia [80], which proposed that tinnitus is generated by changes in oscillatory brain activity that occur in the thalamus and cortex when thalamic neurons are hyperpolarized by deafferentation of auditory pathways. As reported earlier, evidence for hyperpolarization and a shift to burst firing of MGB neurons has been reported in animals expressing behavioral evidence of tinnitus. Early MEG research in humans following this approach reported increased delta and reduced alpha oscillations in the auditory cortex of tinnitus subjects, as well as increased gamma oscillations [153, 159–161], although more recent research has questioned these associations with tinnitus [162]. Gamma oscillations likely reflects local communication within auditory structures while slow wave delta oscillations likely reflect communication between the auditory cortex and non-auditory regions involved in tinnitus network activity. In this regard, one notable study found that delta oscillations associated with increased tinnitus perception extended beyond circumscribed regions of auditory cortex to encompass large parts of temporal, parietal, and sensorimotor cortex and as well as limbic regions [163]. Investigation of non-auditory regions in animal studies is a recent direction that may afford insight into the role of non-auditory structures in tinnitus. STDP is likely to be engaged by brain network activity in non-auditory brain regions, although to date its role in tinnitus has been investigated only in auditory and somatosensory pathways. Coupling among brain regions expressed in electrical oscillatory activity has not yet been investigated by animal models. A continuing challenge for research in human and animal models will be to disambiguate neural changes essential for tinnitus perception from those related to hearing loss, hyperacusis, or distress behaviour that are often seen in tinnitus patients.
Key Points.
Tinnitus is prevalent in up to 15% of the world population
Tinnitus is linked to hearing loss
Loss of input from the cochlea triggers neural plastic changes resulting in hyperactivity and synchrony in affected regions of the auditory system.
Neurons in non-auditory regions are also affected by tinnitus
An understanding of neural mechanisms of tinnitus is essential for developing efficacious treatments.
Acknowledgments
This work was supported by National Institutes of Health Grants NIHR01-DC004825 (SES) NIH P30-DC05188 (SES), the tinnitus research initiative (SES, LER, BL); the Natural Sciences and Engineering Research Council of Canada and the Canadian Institutes of Health Research and the American Tinnitus Association (LER) and from the European Commission (TINNET COST Action BM 1306) (BL). We thank Calvin Wu and Amarins Heeringa for excellent assistance with graphics.
Biographies
Susan E Shore received her bachelor’s and Master’s degrees from the University of Witwatersrand in Johannesburg, S. Africa, and her Ph.D. in physiology at the Kresge Hearing research laboratory of the South in New Orleans, LA. Her research interests include the structure and function of the cochlear nucleus, neural plasticity, multisensory integration and neural tinnitus mechanisms.
Larry E Roberts received his bachelor’s and PhD degrees from the University of Minnesota (Minneapolis). He has studied neural plasticity in the human auditory system using laboratory training methods and musicians as models, and has investigated the role of neural plasticity in tinnitus, publishing extensively in both domains.
Berthold Langguth received his MD from the Ludwig-Maximilian-University of Munich (Germany). He has completed training in neurology, psychiatry, psychotherapy and pain medicine at the University of Regensburg (Germany). His research interests include brain imaging and stimulation for assessing and inducing neuroplastic changes in tinnitus and other neuropsychiatric disorders as well as clinical assessment and clinical trial management.
Footnotes
Conflict of Interest Statements
Berthold Langguth received honoraria for speaking and consultancy from ANM, Astra Zeneca, Autifony, Gerson Lehrman Group, Lundbeck, McKinsey, Merz, Magventure, Novartis, Neuromod Devices, Pfizer and Servier, grants and support for research from the Tinnitus Research Initiative, the German Forschungsgemeinschaft, the German Bundesministerium für Bildung und Forschung, the American Tinnitus Association, Astra Zeneca, Cerbomed, Deymed, Magventure, Sivantos and Otonomy, and travel and accommodation payments from the European Union (COST), Lilly, Servier and Pfizer.
Berthold Langguth holds patents for the use of neuronavigation for transcranial magnetic stimulation for tinnitus treatment and for the use of cyclobenzaprine for tinnitus treatment
Contributor Information
Susan E Shore, Email: sushore@umich.edu.
Larry E. Roberts, Email: roberts@mcmaster.ca.
Berthold Langguth, Email: Berthold.Langguth@medbo.de.
References
- 1.Axelsson A, Ringdahl A. Tinnitus–a study of its prevalence and characteristics. Br J Audiol. 1989;23(1):53–62. doi: 10.3109/03005368909077819. [DOI] [PubMed] [Google Scholar]
- 2.Park B, et al. Analysis of the prevalence of and risk factors for tinnitus in a young population. Otol Neurotol. 2014;35(7):1218–22. doi: 10.1097/MAO.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 3.Gallus S, et al. Prevalence and Determinants of Tinnitus in the Italian Adult Population. Neuroepidemiology. 2015;45(1):12–9. doi: 10.1159/000431376. [DOI] [PubMed] [Google Scholar]
- 4.Roberts LE, et al. Ringing ears: the neuroscience of tinnitus. J Neurosci. 2010;30(45):14972–9. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reed GF. An audiometric study of two hundred cases of subjective tinnitus. AMA Arch Otolaryngol. 1960;71:84–94. doi: 10.1001/archotol.1960.03770010088009. [DOI] [PubMed] [Google Scholar]
- 6.Vernon J. Attemps to relieve tinnitus. J Am Audiol Soc. 1977;2(4):124–31. [PubMed] [Google Scholar]
- 7.Hallberg LR, Erlandsson SI. Tinnitus characteristics in tinnitus complainers and noncomplainers. Br J Audiol. 1993;27(1):19–27. doi: 10.3109/03005369309077885. [DOI] [PubMed] [Google Scholar]
- 8.Landgrebe M, et al. The Tinnitus Research Initiative (TRI) database: a new approach for delineation of tinnitus subtypes and generation of predictors for treatment outcome. BMC Med Inform Decis Mak. 2010;10:42. doi: 10.1186/1472-6947-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Ridder D, et al. Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci U S A. 2011;108(20):8075–80. doi: 10.1073/pnas.1018466108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lockwood AH, Salvi RJ, Burkard RF. Tinnitus. N Engl J Med. 2002;347(12):904–10. doi: 10.1056/NEJMra013395. [DOI] [PubMed] [Google Scholar]
- 11.Mardini MK. Ear-clicking “tinnitus” responding to carbamazepine. N Engl J Med. 1987;317(24):1542. doi: 10.1056/nejm198712103172418. [DOI] [PubMed] [Google Scholar]
- 12.House JW, Brackmann DE. Tinnitus: surgical treatment. Ciba Found Symp. 1981;85:204–216. doi: 10.1002/9780470720677.ch12. [DOI] [PubMed] [Google Scholar]
- 13.Baguley DM, et al. The effect of vestibular nerve section upon tinnitus. Clin Otolaryngol. 2002;27(4):219–26. doi: 10.1046/j.1365-2273.2002.00566.x. [DOI] [PubMed] [Google Scholar]
- 14.Soleymani T, et al. Surgical approaches to tinnitus treatment: A review and novel approaches. Surg Neurol Int. 2011;2:154. doi: 10.4103/2152-7806.86834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blasco MA, Redleaf MI. Cochlear implantation in unilateral sudden deafness improves tinnitus and speech comprehension: meta-analysis and systematic review. Otol Neurotol. 2014;35(8):1426–32. doi: 10.1097/MAO.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 16.Barnea G, et al. Tinnitus with normal hearing sensitivity: extended high-frequency audiometry and auditory-nerve brain-stem-evoked responses. Audiology. 1990;29(1):36–45. doi: 10.3109/00206099009081644. [DOI] [PubMed] [Google Scholar]
- 17.Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci. 2011;31(38):13452–7. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts LE, et al. Residual Inhibition Functions Overlap Tinnitus Spectra and the Region of Auditory Threshold Shift. J Assoc Res Otolaryngol. 2008 doi: 10.1007/s10162-008-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29(45):14077–85. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furman AC, Kujawa SG, Liberman MC. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol. 2013;110(3):577–86. doi: 10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sergeyenko Y, et al. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci. 2013;33(34):13686–94. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear Res. 2015 doi: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin HW, et al. Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol. 2011;12(5):605–16. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu JW, et al. Brainstem Auditory Evoked Potentials Suggest a Role for the Ventral Cochlear Nucleus in Tinnitus. J Assoc Res Otolaryngol. 2012 doi: 10.1007/s10162-012-0344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norena A, et al. Psychoacoustic characterization of the tinnitus spectrum: implications for the underlying mechanisms of tinnitus. Audiol Neurootol. 2002;7(6):358–69. doi: 10.1159/000066156. [DOI] [PubMed] [Google Scholar]
- 26.Sereda M, et al. Re-examining the relationship between audiometric profile and tinnitus pitch. Int J Audiol. 2011;50(5):303–12. doi: 10.3109/14992027.2010.551221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X, et al. Impaired cochlear function correlates with the presence of tinnitus and its estimated spectral profile. Hear Res. 2011;277(1–2):107–16. doi: 10.1016/j.heares.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Heijneman KM, et al. Can the tinnitus spectrum identify tinnitus subgroups? Noise Health. 2013;15(63):101–6. doi: 10.4103/1463-1741.110290. [DOI] [PubMed] [Google Scholar]
- 29.Roberts LE. Residual inhibition. Prog Brain Res. 2007;166:487–95. doi: 10.1016/S0079-6123(07)66047-6. [DOI] [PubMed] [Google Scholar]
- 30.Heffner HE, Heffner RS. Behavioral Tests for Tinnisus in Animals. In: Eggermont JJ, et al., editors. Tinnitus. Springer; New York: 2012. [Google Scholar]
- 31.Turner J, et al. Time course of tinnitus development following noise exposure in mice. J Neurosci Res. 2012;90(7):1480–8. doi: 10.1002/jnr.22827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner JG, et al. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci. 2006;120(1):188–95. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- 33.Dehmel S, Eisinger D, Shore SE. Gap prepulse inhibition and auditory brainstem-evoked potentials as objective measures for tinnitus in guinea pigs. Front Syst Neurosci. 2012;6:42. doi: 10.3389/fnsys.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger JI, et al. A novel behavioural approach to detecting tinnitus in the guinea pig. J Neurosci Methods. 2013;213(2):188–95. doi: 10.1016/j.jneumeth.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayes SH, et al. Behavioral models of tinnitus and hyperacusis in animals. Front Neurol. 2014;5:179. doi: 10.3389/fneur.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von der Behrens W. Animal models of subjective tinnitus. Neural Plast. 2014;2014:741452. doi: 10.1155/2014/741452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galazyuk A, Hebert S. Gap-Prepulse Inhibition of the Acoustic Startle Reflex (GPIAS) for Tinnitus Assessment: Current Status and Future Directions. Front Neurol. 2015;6:88. doi: 10.3389/fneur.2015.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaltenbach JA, et al. Activity in the dorsal cochlear nucleus of hamsters previously tested for tinnitus following intense tone exposure. Neurosci Lett. 2004;355(1–2):121–5. doi: 10.1016/j.neulet.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 39.Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22(6):2383–90. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dehmel S, et al. Noise overexposure alters long-term somatosensory-auditory processing in the dorsal cochlear nucleus–possible basis for tinnitus-related hyperactivity? J Neurosci. 2012;32(5):1660–71. doi: 10.1523/JNEUROSCI.4608-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koehler SD, Shore SE. Stimulus timing-dependent plasticity in dorsal cochlear nucleus is altered in tinnitus. J Neurosci. 2013;33(50):19647–56. doi: 10.1523/JNEUROSCI.2788-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong H, et al. Hidden hearing loss in tinnitus patients with normal audiograms: implications for the origin of tinnitus. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;27(7):362–5. [PubMed] [Google Scholar]
- 43.Vogler DP, Robertson D, Mulders WH. Hyperactivity following unilateral hearing loss in characterized cells in the inferior colliculus. Neuroscience. 2014;265C:28–36. doi: 10.1016/j.neuroscience.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 44.Sumner CJ, Tucci DL, Shore SE. Responses of ventral cochlear nucleus neurons to contralateral sound after conductive hearing loss. J Neurophysiol. 2005;94(6):4234–43. doi: 10.1152/jn.00401.2005. [DOI] [PubMed] [Google Scholar]
- 45.Bledsoe SC, Jr, et al. Ventral cochlear nucleus responses to contralateral sound are mediated by commissural and olivocochlear pathways. J Neurophysiol. 2009;102(2):886–900. doi: 10.1152/jn.91003.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaltenbach JA. Summary of evidence pointing to a role of the dorsal cochlear nucleus in the etiology of tinnitus. Acta Otolaryngol Suppl. 2006;556:20–6. doi: 10.1080/03655230600895309. [DOI] [PubMed] [Google Scholar]
- 47.Wu C, et al. Tinnitus: Maladaptive auditory-somatosensory plasticity. Hear Res. 2015 doi: 10.1016/j.heares.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robertson D, et al. Spontaneous hyperactivity in the auditory midbrain: relationship to afferent input. Hear Res. 2013;295:124–9. doi: 10.1016/j.heares.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Mulders WH, Barry KM, Robertson D. Effects of furosemide on cochlear neural activity, central hyperactivity and behavioural tinnitus after cochlear trauma in guinea pig. PLoS One. 2014;9(5):e97948. doi: 10.1371/journal.pone.0097948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ropp TJ, et al. Effects of unilateral acoustic trauma on tinnitus-related spontaneous activity in the inferior colliculus. J Assoc Res Otolaryngol. 2014;15(6):1007–22. doi: 10.1007/s10162-014-0488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coomber B, et al. Neural changes accompanying tinnitus following unilateral acoustic trauma in the guinea pig. Eur J Neurosci. 2014 doi: 10.1111/ejn.12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brozoski TJ, et al. Bilateral Dorsal Cochlear Nucleus Lesions Prevent Acoustic-Trauma Induced Tinnitus in an Animal Model. J Assoc Res Otolaryngol. 2011 doi: 10.1007/s10162-011-0290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manzoor NF, et al. Noise-induced hyperactivity in the inferior colliculus: its relationship with hyperactivity in the dorsal cochlear nucleus. J Neurophysiol. 2012;108(4):976–88. doi: 10.1152/jn.00833.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brozoski TJ, Bauer CA. The effect of dorsal cochlear nucleus ablation on tinnitus in rats. Hear Res. 2005;206(1–2):227–36. doi: 10.1016/j.heares.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 55.Mulders WH, et al. Relationship between auditory thresholds, central spontaneous activity, and hair cell loss after acoustic trauma. J Comp Neurol. 2011;519(13):2637–47. doi: 10.1002/cne.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zacharek MA, et al. Effects of cochlear ablation on noise induced hyperactivity in the hamster dorsal cochlear nucleus: implications for the origin of noise induced tinnitus. Hear Res. 2002;172(1–2):137–43. doi: 10.1016/s0378-5955(02)00575-0. [DOI] [PubMed] [Google Scholar]
- 57.Zhang JS, et al. Origin of hyperactivity in the hamster dorsal cochlear nucleus following intense sound exposure. J Neurosci Res. 2006;84(4):819–31. doi: 10.1002/jnr.20985. [DOI] [PubMed] [Google Scholar]
- 58.Manzoor NF, et al. Comparison and contrast of noise-induced hyperactivity in the dorsal cochlear nucleus and inferior colliculus. Hear Res. 2013;295:114–23. doi: 10.1016/j.heares.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anderson LA, et al. Evidence for a direct, short latency projection from the dorsal cochlear nucleus to the auditory thalamus in the guinea pig. Eur J Neurosci. 2006;24(2):491–8. doi: 10.1111/j.1460-9568.2006.04930.x. [DOI] [PubMed] [Google Scholar]
- 60.Malmierca MS, et al. Direct projections from cochlear nuclear complex to auditory thalamus in the rat. J Neurosci. 2002;22(24):10891–7. doi: 10.1523/JNEUROSCI.22-24-10891.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schofield BR, Mellott JG, Motts SD. Subcollicular projections to the auditory thalamus and collateral projections to the inferior colliculus. Front Neuroanat. 2014;8:70. doi: 10.3389/fnana.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalappa BI, et al. Single unit hyperactivity and bursting in the auditory thalamus of awake rats directly correlates with behavioural evidence of tinnitus. J Physiol. 2014;592(Pt 22):5065–78. doi: 10.1113/jphysiol.2014.278572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27(11):676–82. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 64.Engineer ND, et al. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470(7332):101–4. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Basura G, Koehler S, S SE. Stimulus-timing dependence of auditory-somatosensory plasticity in auditory cortex neurons after noise induced temporary threshold shifts and tinnitus. Journal of Neurophysiology. 2015 In Press. [Google Scholar]
- 66.Ilin V, et al. Fast Computations in Cortical Ensembles Require Rapid Initiation of Action Potentials. Journal of Neuroscience. 2013;33(6):2281–2292. doi: 10.1523/JNEUROSCI.0771-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaltenbach JA. Tinnitus: Models and mechanisms. Hear Res. 2011;276(1–2):52–60. doi: 10.1016/j.heares.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu c, Martel D, Shore S. Indreased synchrony and bursting of dorsal cochlear nucleus fusiform cells correlates with tinnitus. Journal of Neuroscience. 2015 doi: 10.1523/JNEUROSCI.3960-15.2016. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bauer CA, et al. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008;86(11):2564–78. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robertson D, Irvine DR. Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. J Comp Neurol. 1989;282(3):456–71. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- 71.Norena AJ, Tomita M, Eggermont JJ. Neural changes in cat auditory cortex after a transient pure-tone trauma. J Neurophysiol. 2003;90(4):2387–401. doi: 10.1152/jn.00139.2003. [DOI] [PubMed] [Google Scholar]
- 72.Basura GJ, Koehler SD, Shore SE. Bimodal Stimulus Timing Dependent Plasticity in Primary Auditory Cortex is Altered After Noise Exposure With and Without Tinnitus. J Neurophysiol. 2015 doi: 10.1152/jn.00319.2015. p. jn 00319 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang H, et al. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009;164(2):747–59. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Middleton JW, et al. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proc Natl Acad Sci U S A. 2011;108(18):7601–6. doi: 10.1073/pnas.1100223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeng C, et al. Cochlear damage changes the distribution of vesicular glutamate transporters associated with auditory and nonauditory inputs to the cochlear nucleus. J Neurosci. 2009;29(13):4210–7. doi: 10.1523/JNEUROSCI.0208-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barker M, et al. Acoustic overexposure increases the expression of VGLUT-2 mediated projections from the lateral vestibular nucleus to the dorsal cochlear nucleus. PLoS One. 2012;7(5):e35955. doi: 10.1371/journal.pone.0035955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zeng C, et al. Somatosensory projections to cochlear nucleus are upregulated after unilateral deafness. J Neurosci. 2012;32(45):15791–801. doi: 10.1523/JNEUROSCI.2598-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schofield BR, et al. Projections from the dorsal and ventral cochlear nuclei to the medial geniculate body. Front Neuroanat. 2014;8:10. doi: 10.3389/fnana.2014.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sametsky EA, et al. Enhanced GABAA-Mediated Tonic Inhibition in Auditory Thalamus of Rats with Behavioral Evidence of Tinnitus. J Neurosci. 2015;35(25):9369–80. doi: 10.1523/JNEUROSCI.5054-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Llinas RR, et al. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci USA. 1999;96:15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tzounopoulos T, et al. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci. 2004;7(7):719–25. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- 82.Koehler SD, Shore SE. Stimulus-timing dependent multisensory plasticity in the guinea pig dorsal cochlear nucleus. PLoS One. 2013;8(3):e59828. doi: 10.1371/journal.pone.0059828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roberts PD, Leen TK. Anti-hebbian spike-timing-dependent plasticity and adaptive sensory processing. Front Comput Neurosci. 2010;4:156. doi: 10.3389/fncom.2010.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stefanescu RA, Shore SE. NMDA receptors mediate stimulus timing dependent plasticity and neural synchrony in dorsal cochlear nucleus. Front Syst Neurosci. 2015 doi: 10.3389/fncir.2015.00075. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu C, Martel D, Shore S. Transcutaneous induction of stimulus timing dependent plasticity in dorsal cochlear nucleus. Front Syst Neurosci. 2015 doi: 10.3389/fnsys.2015.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaltenbach JA, Zhang J. Intense sound-induced plasticity in the dorsal cochlear nucleus of rats: evidence for cholinergic receptor upregulation. Hear Res. 2007;226(1–2):232–43. doi: 10.1016/j.heares.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 87.Jin YM, et al. Effects of intense tone exposure on choline acetyltransferase activity in the hamster cochlear nucleus. Hear Res. 2006;216–217:168–75. doi: 10.1016/j.heares.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 88.D’Amour JA, Froemke RC. Inhibitory and excitatory spike-timing-dependent plasticity in the auditory cortex. Neuron. 2015;86(2):514–28. doi: 10.1016/j.neuron.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tass PA, Popovych OV. Unlearning tinnitus-related cerebral synchrony with acoustic coordinated reset stimulation: theoretical concept and modelling. Biol Cybern. 2012;106(1):27–36. doi: 10.1007/s00422-012-0479-5. [DOI] [PubMed] [Google Scholar]
- 90.Talathi SS, Hwang DU, Ditto WL. Spike timing dependent plasticity promotes synchrony of inhibitory networks in the presence of heterogeneity. J Comput Neurosci. 2008;25(2):262–81. doi: 10.1007/s10827-008-0077-7. [DOI] [PubMed] [Google Scholar]
- 91.Tziridis K, et al. Noise Trauma Induced Neural Plasticity Throughout the Auditory System of Mongolian Gerbils: Differences between Tinnitus Developing and Non-Developing Animals. Front Neurol. 2015;6:22. doi: 10.3389/fneur.2015.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singer W, et al. Noise-induced inner hair cell ribbon loss disturbs central arc mobilization: a novel molecular paradigm for understanding tinnitus. Mol Neurobiol. 2013;47(1):261–79. doi: 10.1007/s12035-012-8372-8. [DOI] [PubMed] [Google Scholar]
- 93.Li S, Choi V, Tzounopoulos T. Pathogenic plasticity of Kv7.2/3 channel activity is essential for the induction of tinnitus. Proc Natl Acad Sci U S A. 2013;110(24):9980–5. doi: 10.1073/pnas.1302770110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li S, Kalappa BI, Tzounopoulos T. Noise-induced plasticity of KCNQ2/3 and HCN channels underlies vulnerability and resilience to tinnitus. Elife. 2015;4 doi: 10.7554/eLife.07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dehmel S, Cui YL, Shore SE. Cross-modal interactions of auditory and somatic inputs in the brainstem and midbrain and their imbalance in tinnitus and deafness. Am J Audiol. 2008;17(2):S193–209. doi: 10.1044/1059-0889(2008/07-0045). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu C, et al. Listening to another sense: somatosensory integration in the auditory system. Cell Tissue Res. 2014 doi: 10.1007/s00441-014-2074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haenggeli CA, et al. Projections from the spinal trigeminal nucleus to the cochlear nucleus in the rat. J Comp Neurol. 2005;484(2):191–205. doi: 10.1002/cne.20466. [DOI] [PubMed] [Google Scholar]
- 98.Wright DD, Ryugo DK. Mossy fiber projections from the cuneate nucleus to the cochlear nucleus in the rat. J Comp Neurol. 1996;365(1):159–72. doi: 10.1002/(SICI)1096-9861(19960129)365:1<159::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 99.Zhan X, Pongstaporn T, Ryugo DK. Projections of the second cervical dorsal root ganglion to the cochlear nucleus in rats. J Comp Neurol. 2006;496(3):335–48. doi: 10.1002/cne.20917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zeng C, Shroff H, Shore SE. Cuneate and spinal trigeminal nucleus projections to the cochlear nucleus are differentially associated with vesicular glutamate transporter-2. Neuroscience. 2011;176:142–51. doi: 10.1016/j.neuroscience.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shore SE, et al. Dorsal cochlear nucleus responses to somatosensory stimulation are enhanced after noise-induced hearing loss. Eur J Neurosci. 2008;27(1):155–68. doi: 10.1111/j.1460-9568.2007.05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou J, Nannapaneni N, Shore S. Vessicular glutamate transporters 1 and 2 are differentially associated with auditory nerve and spinal trigeminal inputs to the cochlear nucleus. J Comp Neurol. 2007;500(4):777–87. doi: 10.1002/cne.21208. [DOI] [PubMed] [Google Scholar]
- 103.Heeringa A, et al. Altered vesicular glutamate transporter distributions in the mouse cochlear nucleus following cochlear insult. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.12.009. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Basura GJ, Koehler SD, Shore SE. Multi-sensory integration in brainstem and auditory cortex. Brain Res. 2012;1485:95–107. doi: 10.1016/j.brainres.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sanchez TG, Rocha CB. Diagnosis and management of somatosensory tinnitus: review article. Clinics (Sao Paulo) 2011;66(6):1089–94. doi: 10.1590/S1807-59322011000600028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Levine RA, Abel M, Cheng H. CNS somatosensory-auditory interactions elicit or modulate tinnitus. Exp Brain Res. 2003;153(4):643–648. doi: 10.1007/s00221-003-1747-3. [DOI] [PubMed] [Google Scholar]
- 107.Zhou J, Shore S. Projections from the trigeminal nuclear complex to the cochlear nuclei: a retrograde and anterograde tracing study in the guinea pig. J Neurosci Res. 2004;78(6):901–7. doi: 10.1002/jnr.20343. [DOI] [PubMed] [Google Scholar]
- 108.Zhou J, Shore S. Convergence of spinal trigeminal and cochlear nucleus projections in the inferior colliculus of the guinea pig. J Comp Neurol. 2006;495(1):100–12. doi: 10.1002/cne.20863. [DOI] [PubMed] [Google Scholar]
- 109.Wallhausser-Franke E, et al. Expression of c-fos in auditory and non-auditory brain regions of the gerbil after manipulations that induce tinnitus. Exp Brain Res. 2003;153:649–654. doi: 10.1007/s00221-003-1614-2. [DOI] [PubMed] [Google Scholar]
- 110.Adjamian P, et al. Neuroanatomical abnormalities in chronic tinnitus in the human brain. Neurosci Biobehav Rev. 2014;45C:119–133. doi: 10.1016/j.neubiorev.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lockwood AH, et al. The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology. 1998;50(1):114–20. doi: 10.1212/wnl.50.1.114. [DOI] [PubMed] [Google Scholar]
- 112.Pinchoff RJ, et al. Modulation of tinnitus by voluntary jaw movements. Am J Otol. 1998;19(6):785–9. [PubMed] [Google Scholar]
- 113.Vanneste S, De Ridder D. The auditory and non-auditory brain areas involved in tinnitus. An emergent property of multiple parallel overlapping subnetworks. Front Syst Neurosci. 2012;6:31. doi: 10.3389/fnsys.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Melcher JR, Knudson IM, Levine RA. Subcallosal brain structure: correlation with hearing threshold at supra-clinical frequencies (>8 kHz), but not with tinnitus. Hear Res. 2013;295:79–86. doi: 10.1016/j.heares.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 115.Landgrebe M, et al. Structural brain changes in tinnitus: grey matter decrease in auditory and non-auditory brain areas. Neuroimage. 2009;46(1):213–218. doi: 10.1016/j.neuroimage.2009.01.069. [DOI] [PubMed] [Google Scholar]
- 116.Muhlau M, et al. Structural brain changes in tinnitus. Cereb Cortex. 2006;16(9):1283–1288. doi: 10.1093/cercor/bhj070. [DOI] [PubMed] [Google Scholar]
- 117.Gu JW, et al. Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J Neurophysiol. 2010;104(6):3361–3370. doi: 10.1152/jn.00226.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maudoux A, et al. Auditory resting-state network connectivity in tinnitus: a functional MRI study. PLoS One. 2012;7(5):e36222. doi: 10.1371/journal.pone.0036222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schlee W, et al. Mapping cortical hubs in tinnitus. BMC Biol. 2009;7:80. doi: 10.1186/1741-7007-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.De Ridder D, et al. An integrative model of auditory phantom perception: Tinnitus as a unified percept of interacting separable subnetworks. Neurosci Biobehav Rev. 2014;44C:16–32. doi: 10.1016/j.neubiorev.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 121.de Lafuente V, Romo R. Neuronal correlates of subjective sensory experience. Nat Neurosci. 2005;8(12):1698–703. doi: 10.1038/nn1587. [DOI] [PubMed] [Google Scholar]
- 122.Golm D, et al. Neural correlates of tinnitus related distress: an fMRI-study. Hear Res. 2013;295:87–99. doi: 10.1016/j.heares.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 123.Vanneste S, et al. The neural correlates of tinnitus-related distress. Neuroimage. 2010;52(2):470–80. doi: 10.1016/j.neuroimage.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 124.Schecklmann M, et al. Auditory cortex is implicated in tinnitus distress: a voxel-based morphometry study. Brain Struct Funct. 2013;218(4):1061–70. doi: 10.1007/s00429-013-0520-z. [DOI] [PubMed] [Google Scholar]
- 125.Vanneste S, et al. The difference between uni- and bilateral auditory phantom percept. Clin Neurophysiol. 2011;122(3):578–87. doi: 10.1016/j.clinph.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 126.Carpenter-Thompson JR, et al. Alterations of the emotional processing system may underlie preserved rapid reaction time in tinnitus. Brain Res. 2014;1567:28–41. doi: 10.1016/j.brainres.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 127.Vanneste S, Heyning PV, Ridder DD. Contralateral parahippocampal gamma-band activity determines noise-like tinnitus laterality: a region of interest analysis. Neuroscience. 2011;199:481–490. doi: 10.1016/j.neuroscience.2011.07.067. [DOI] [PubMed] [Google Scholar]
- 128.Schecklmann M, et al. Neural correlates of tinnitus duration and distress: a positron emission tomography study. Hum Brain Mapp. 2013;34(1):233–40. doi: 10.1002/hbm.21426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Maudoux A, et al. Connectivity graph analysis of the auditory resting state network in tinnitus. Brain Res. 2012;1485:10–21. doi: 10.1016/j.brainres.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 130.Roberts LE, Husain FT, Eggermont JJ. Role of attention in the generation and modulation of tinnitus. Neurosci Biobehav Rev. 2013;37(8):1754–73. doi: 10.1016/j.neubiorev.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 131.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Martinez-Devesa P, et al. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010;(9):CD005233. doi: 10.1002/14651858.CD005233.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cima RF, et al. Specialised treatment based on cognitive behaviour therapy versus usual care for tinnitus: a randomised controlled trial. Lancet. 2012;379(9830):1951–1959. doi: 10.1016/S0140-6736(12)60469-3. [DOI] [PubMed] [Google Scholar]
- 134.Davis PB, Paki B, Hanley PJ. Neuromonics Tinnitus Treatment: third clinical trial. Ear Hear. 2007;28(2):242–59. doi: 10.1097/AUD.0b013e3180312619. [DOI] [PubMed] [Google Scholar]
- 135.Vanneste S, et al. Does enriched acoustic environment in humans abolish chronic tinnitus clinically and electrophysiologically? A double blind placebo controlled study. Hear Res. 2013;296:141–8. doi: 10.1016/j.heares.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 136.Okamoto H, et al. Listening to tailor-made notched music reduces tinnitus loudness and tinnitus-related auditory cortex activity. Proc Natl Acad Sci USA. 2010;107(3):1207–1210. doi: 10.1073/pnas.0911268107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tass PA, et al. Counteracting tinnitus by acoustic coordinated reset neuromodulation. Restor Neurol Neurosci. 2012;30(2):137–59. doi: 10.3233/RNN-2012-110218. [DOI] [PubMed] [Google Scholar]
- 138.Hoare DJ, et al. Sound therapy for tinnitus management: practicable options. J Am Acad Audiol. 2014;25(1):62–75. doi: 10.3766/jaaa.25.1.5. [DOI] [PubMed] [Google Scholar]
- 139.De Ridder D, et al. Safety and efficacy of vagus nerve stimulation paired with tones for the treatment of tinnitus: a case series. Neuromodulation. 2014;17(2):170–9. doi: 10.1111/ner.12127. [DOI] [PubMed] [Google Scholar]
- 140.Hoare DJ, et al. Amplification with hearing aids for patients with tinnitus and co-existing hearing loss. Cochrane Database Syst Rev. 2014;1:CD010151. doi: 10.1002/14651858.CD010151.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schaette R, et al. Acoustic stimulation treatments against tinnitus could be most effective when tinnitus pitch is within the stimulated frequency range. Hear Res. 2010;269(1–2):95–101. doi: 10.1016/j.heares.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 142.McNeill C, et al. Tinnitus pitch, masking, and the effectiveness of hearing aids for tinnitus therapy. Int J Audiol. 2012;51(12):914–9. doi: 10.3109/14992027.2012.721934. [DOI] [PubMed] [Google Scholar]
- 143.Langguth B, Elgoyhen AB. Current pharmacological treatments for tinnitus. Expert Opin Pharmacother. 2012;13(17):2495–509. doi: 10.1517/14656566.2012.739608. [DOI] [PubMed] [Google Scholar]
- 144.Zoger S, Svedlund J, Holgers KM. The effects of sertraline on severe tinnitus suffering–a randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2006;26(1):32–39. doi: 10.1097/01.jcp.0000195111.86650.19. [DOI] [PubMed] [Google Scholar]
- 145.Baldo P, et al. Antidepressants for patients with tinnitus. Cochrane Database Syst Rev. 2012;9:CD003853. doi: 10.1002/14651858.CD003853.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hoekstra CE, et al. Anticonvulsants for tinnitus. Cochrane Database Syst Rev. 2011;(7):CD007960. doi: 10.1002/14651858.CD007960.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Han SS, et al. Clonazepam Quiets tinnitus: a randomised crossover study with Ginkgo Biloba. J Neurol Neurosurg Psychiatry. 2012;83(8):821–827. doi: 10.1136/jnnp-2012-302273. [DOI] [PubMed] [Google Scholar]
- 148.Tunkel DE, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(2 Suppl):S1–S40. doi: 10.1177/0194599814545325. [DOI] [PubMed] [Google Scholar]
- 149.Zenner HP, et al. On the interdisciplinary S3 guidelines for the treatment of chronic idiopathic tinnitus. HNO. 2015;63(6):419–27. doi: 10.1007/s00106-015-0011-z. [DOI] [PubMed] [Google Scholar]
- 150.van de Heyning P, et al. Efficacy and safety of AM-101 in the treatment of acute inner ear tinnitus–a double-blind, randomized, placebo-controlled phase II study. Otol Neurotol. 2014;35(4):589–97. doi: 10.1097/MAO.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hebert S, Fournier P, Norena A. The Auditory Sensitivity is Increased in Tinnitus Ears. J Neurosci. 2013;33(6):2356–64. doi: 10.1523/JNEUROSCI.3461-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Diesch E, et al. Interaction among the components of multiple auditory steady-state responses: enhancement in tinnitus patients, inhibition in controls. Neuroscience. 2010;167(2):540–53. doi: 10.1016/j.neuroscience.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 153.Wienbruch C, et al. Frequency organization of the 40-Hz auditory steady-state response in normal hearing and in tinnitus. Neuroimage. 2006;33(1):180–94. doi: 10.1016/j.neuroimage.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 154.Langers DR, de Kleine E, van Dijk P. Tinnitus does not require macroscopic tonotopic map reorganization. Front Syst Neurosci. 2012;6:2. doi: 10.3389/fnsys.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Roberts LE, et al. Evidence for differential modulation of primary and nonprimary auditory cortex by forward masking in tinnitus. Hear Res. 2015;327:9–27. doi: 10.1016/j.heares.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 156.Paul BT, et al. Modulation of electrocortical brain activity by attention in individuals with and without tinnitus. Neural Plast. 2014;2014:127824. doi: 10.1155/2014/127824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jastreboff PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res. 1990;8:221–254. doi: 10.1016/0168-0102(90)90031-9. [DOI] [PubMed] [Google Scholar]
- 158.Leaver AM, et al. Cortico-limbic morphology separates tinnitus from tinnitus distress. Front Syst Neurosci. 2012;6:21. doi: 10.3389/fnsys.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Weisz N, et al. The neural code of auditory phantom perception. J Neurosci. 2007;27(6):1479–84. doi: 10.1523/JNEUROSCI.3711-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Weisz N, et al. Tinnitus perception and distress is related to abnormal spontaneous brain activity as measured by magnetoencephalography. PLoS Med. 2005;2(6):e153. doi: 10.1371/journal.pmed.0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Adjamian P, et al. Neuromagnetic indicators of tinnitus and tinnitus masking in patients with and without hearing loss. J Assoc Res Otolaryngol. 2012;13(5):715–31. doi: 10.1007/s10162-012-0340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pierzycki RH, et al. Whole scalp resting state EEG of oscillatory brain activity shows no parametric relationship with psychoacoustic and psychosocial assessment of tinnitus: A repeated measures study. Hear Res. 2015 doi: 10.1016/j.heares.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 163.Sedley W, et al. Intracranial Mapping of a Cortical Tinnitus System using Residual Inhibition. Curr Biol. 2015;25(9):1208–14. doi: 10.1016/j.cub.2015.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]


