Abstract
Introduction:
Albizzia lebbeck (L.) Benth. (Family - Leguminosae) extract is a proven mast cell stabilizing agent. Mast cells are involved in the inflammatory processes leading to the diabetes mellitus.
Aim:
To evaluate the effect of A. lebbeck against experimentally induced type 2 diabetes mellitus in rats.
Materials and Method:
Female Sprague-Dawley rats were randomly allocated to six groups (n = 6). Diabetes was induced by single intraperitoneal injection of streptozotocin (50 mg/kg) given after 15 min of nicotinamide administration (110 mg/kg). Treatment with methanolic extract of A. lebbeck bark (MEAL) and metformin drug as standard was given for 21 days. Serum glucose (GLU) levels were measured on the 0 day and on 1st, 7th, 14th and 21st day after diabetes induction. After completion of study period, various biochemical parameters in serum such as - GLU, lipid profile, urea and creatinine were estimated. One-way analysis of variance followed with post-hoc Dunnett's test was used to analyse the data. Statistical significance for the values was set at P< 0.05.
Results:
MEAL significantly decreased the level of serum GLU, creatinine, urea, cholesterol, triglycerides, low-density lipoprotein-cholesterol, very low-density lipoprotein-cholesterol and increased high-density lipoprotein levels.
Conclusion:
A. lebbeck bark extract showed antihyperglycaemic activity along with antihyperlipidemic effect.
Keywords: Albizzia lebbeck, mast cell stabilizer, metformin, type II diabetes mellitus
Introduction
Three hundred eighty-two million people world over have diabetes and by 2035 this number is expected to be 592 million. India is second only to China in having high number of diabetic patients. According to latest report of International Diabetic Federation, 63 million people in India are diabetic. With over 1 million diabetes-related deaths reported in 2013, dreadful diabetes poses a huge health and financial burden.[1]
Type II diabetes mellitus results from less insulin secretion from pancreatic beta cells and impaired insulin sensitivity or development of resistance by cells against insulin action. Weight gain and hypoglycemia like side-effects have hastened the need of new therapies for hyperglycemia.[2]
Mast cells are inflammatory cells and diabetes being inflammatory disease, it was discovered recently that mast cells play a direct role in obesity and diabetes. Study with two groups of mice one lacking mast cell and other with mast cell stabilization showed decreased weight gain, glucose (GLU) and insulin tolerance.[3,4] A satisfactory diabetic treatment still eludes modern allopathic system. Traditional medicines which offer cheaper treatment option have been approved by WHO for the healthcare program.[5]
Albizzia lebbeck (L.) Benth. plant of the leguminous family is commonly known as Shirisha in Sanskrit. It is tall deciduous tree distributed throughout India. In Ayurveda, expressed juice of flower is used in poisoning, and for Hikka (hiccup) and Shwasa (asthma) in the form of Leha and in eye disease in form of Anjana. It has been proved to be useful in various conditions. It is reported to have anticancer, antiprotozoal, antioxidant, hepatoprotective, and antiarthritic activities.[6,7,8,9]
In addition, it also possesses astringent property and protective effect in bronchospasm. Methanolic bark extract of A. lebbeck has shown maximum mast cell stabilizing activity in in-vitro study, which was comparable to disodium cromoglycate.[10]
Thus, this study was undertaken with the purpose to evaluate the effect of mast cell stabilizer action of A. lebbeck in experimentally induced diabetes mellitus in rats.
Materials and Methods
Identification and collection of plant material
Bark of A. lebbeck was collected from Dilip Ayurvedic Nursery, Nadiad and plant was identified and authenticated by Botanist, B.R. Doshi School of Biosciences, Sardar Patel University, Bakrol, Gujarat, India (Herbarium specimen no: APCH33).
Extract preparation
The collected bark material was reduced to the coarse powder in a blender. The powder (20–25 g) was then extracted with 90% methanol in a soxhlet apparatus. Methanol was used for extraction as it gives high yields.[9] Using rotary vacuum evaporator under reduced pressure all the solvent was evaporated to obtain the solid extract. The obtained solid extract (15.2% yield) was labeled as Methanolic extract of A. lebbeck (MEAL) and stored in air tight container. Phytochemical tests revealed the presence of saponin, flavonoids, carbohydrates and tannins.
Drugs and chemicals
Metformin was obtained as a gift sample from Sun Pharma, Vadodara, Gujarat, India. Nicotinamide (Batch No.: B11Z/1310/0409/62) and all other chemicals used in this project were of analytical grade and were obtained from Astron Chemicals, Ahmedabad and SD Fine Chemicals, Mumbai, India. Streptozotocin (STZ) was obtained as pure (>98%) chemical from Sigma Aldrich. All the biochemical tests were performed using the standard reagent kits (GLU 011, TGL 014, CHO 014, HDL 020, CKB 012, and URE 022) purchased from Coral Clinical Systems, Tulip Group, Goa, India.
Animals
Healthy Female Sprague Dawley rats weighing 280–350 g obtained from Animal house of Anand Pharmacy College, Anand, Gujarat, India, were used as experimental animals for the study. The animals were housed in a group of 3 rats per cage under well-controlled conditions of temperature (22°C ± 2°C), humidity (55 ± 5%) and 12:12 h light-dark cycle. Animals had free access to conventional laboratory diet (Pranav Agro Industries, Delhi, India) and R.O. water ad libitum. The protocol of the experiment was approved by Institutional Animal Ethics Committee (IAEC) as per the guidance of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India (APC/2012-IAEC/1208).
Experimental procedure
Streptozotocin (STZ) - nicotinamide (NAD) induced diabetic model
Diabetes was induced by single intraperitoneal (i.p) injection of STZ (50 mg/kg body weight) which was freshly prepared in normal 0.9% saline and was given after 15 min of single i.p injection of NAD (110 mg/kg body weight), which was also prepared freshly in normal 0.9% saline. Diabetes was confirmed after 3rd day of STZ + NAD administration by measuring fasting serum glucose (GLU) level. More than 200 mg/dl was set as cut off sugar level.[11]
Experimental group
Animals were randomly allocated based on serum GLU levels into six groups, with n = 6 animals in each group, as follows:
Diabetes was induced in all groups except group-I which served as normal control (NC) and received saline p.o. Group II served as diabetic model control (MC) while Group III which served as standard control received metformin dissolved in water at a dose of 45 mg/kg/day; p.o. Group IV-VI treatment control received drug extraction 2% tween 80 solution-MEAL: 200, 350 and 620 mg/kg/day, p.o. respectively.
After diabetes induction, treatment was given from next day and it lasted for 21 days. Normal fasting blood GLU level was measured on the 0 day and then after induction of diabetes fasting blood GLU level was measured on 1st, 7th, 14th and 21st day.
Collection of biological samples
At the end of treatment, blood was collected retro-orbitally under anesthetic conditions and animals were sacrificed by 100 mg/kg i.p phenobarbital anesthesia. Serum was separated by centrifugation (PlastocraftsRota 4R-V/FA) at 3000 rpm for 15 min and stored at −20°C until the analysis was carried out. Serum samples were analysed for different biochemical parameters like GLU, triglyceride (TG), total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-c), low-density lipoprotein-cholesterol (LDL-c), very low-density lipoprotein (VLDL-c), creatinine and urea.
Statistical analysis
Represented data are mean ± standard error of the mean, results are analysed by one-way analysis of variance followed by post-hoc Dunnett's test with statistical significance set at a value of P < 0.05. Statistical analysis was performed using GraphPad Prism software, version 5.03 (GraphPad Software, Inc.).
Results
Effect on serum glucose level
As shown in Figure 1, the serum GLU levels in MC animal showed significant (P < 0.05) difference compared to NC animals at day 1, 7, 14 and 21 of diabetes induction. Standard control-metformin (SCM) group showed significant (P < 0.05) decrease in serum GLU levels compared to MC. Treatment with MEAL plummeted the serum GLU levels significantly (P < 0.05) as compared to MC, with maximum reduction on day 21 by MEAL 620.
Figure 1.
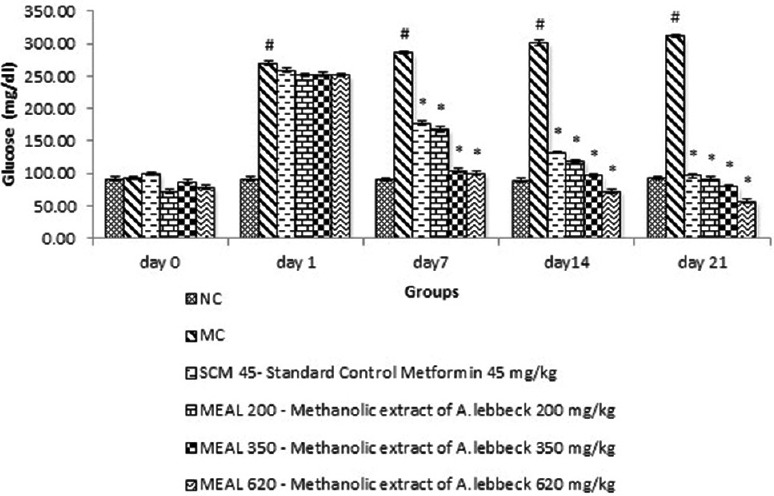
Effect on serum glucose level: Data are represented as mean ± SEM, n = 6; #P < 0.05 as compared to normal control (group I); *P < 0.05 as compared to model control (group II), NC: Normal control, MC: Model control, SCM 45: Standard control metformin 45 mg/kg, MEAL 200, 350 and 620 – Methanol extract of Albizzia lebbeck 200, 350 and 620 mg/kg respectively
Effect on total cholesterol level
Model control showed significant (P < 0.05) increase in TC level compared to NC. SCM treatment reduced the TC significantly (P < 0.05) compared to MC. MEAL treatment at all dose levels abated elevation in TC levels significantly (P < 0.05) compared to MC, as depicted in Figure 2.
Figure 2.
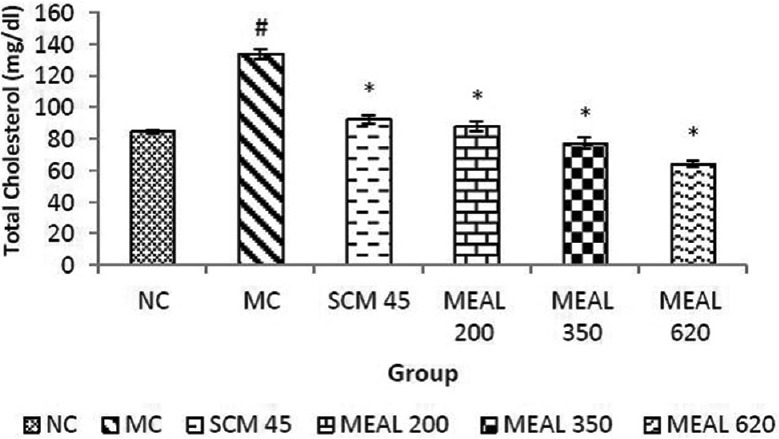
Effect on total cholesterol level: Data are represented as mean ± SEM, n = 6; #P < 0.05 as compared to normal control (group I); *P < 0.05 as compared to model control (group II), NC: Normal control, MC: Model control, SCM 45: Standard control metformin 45 mg/kg, MEAL 200, 350 and 620 – Methanol extract of Albizzia lebbeck 200, 350 and 620 mg/kg respectively
Effect on serum high-density lipoprotein-cholesterol level
Significantly (P < 0.05) lower HDL-c levels were observed in MC compared to NC. Treatment with SCM and MEAL increased HDL-c levels significantly (P < 0.05) compared to MC, as represented graphically in Figure 3.
Figure 3.
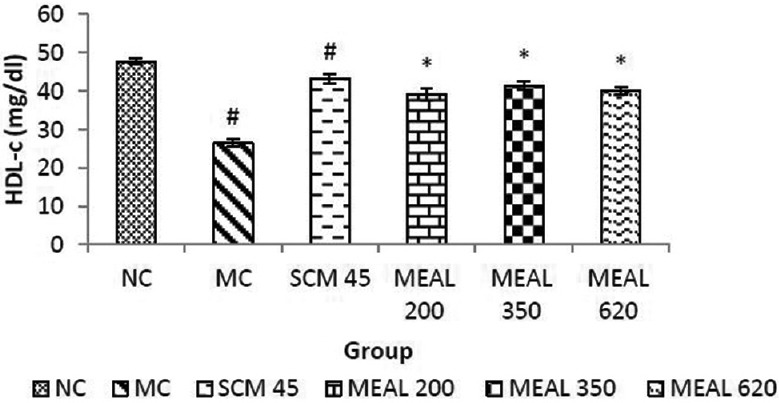
Effect on HDL-c level: Data are represented as mean ± SEM, n = 6; #P < 0.05 as compared to normal control (group I); *P < 0.05 as compared to model control (group II), NC: Normal control, MC: Model control, SCM 45: Standard control metformin 45 mg/kg, MEAL 200, 350 and 620 – Methanol extract of Albizzia lebbeck 200, 350 and 620 mg/kg respectively
Effect on serum triglyceride level
As shown in Figure 4, significant (P < 0.05) increase in TG levels were observed in MC as compared to NC. Significant (P < 0.05) reduction was obtained in MEAL treated group compared to MC. Similar reduction were obtained in SCM, which was also significant (P < 0.05) as compared to MC.
Figure 4.
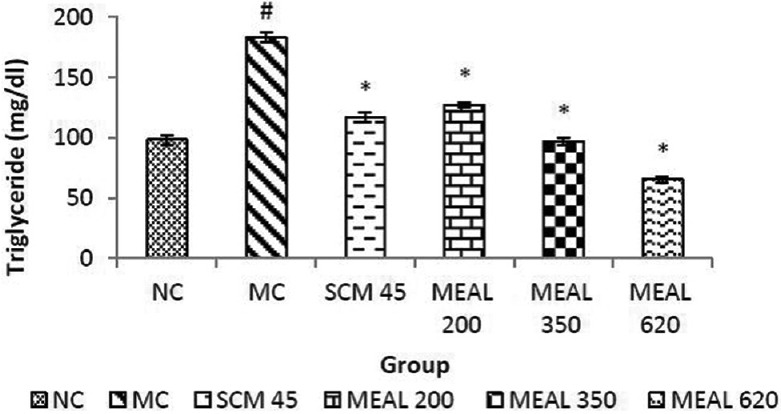
Effect on triglyceride level: Data are represented as mean ± SEM, n = 6; #P < 0.05 as compared to normal control (group I); *P < 0.05 as compared to model control (group II), NC: Normal control, MC: Model control, SCM 45: Standard control metformin 45 mg/kg, MEAL 200, 350 and 620 – Methanol extract of Albizzia lebbeck 200, 350 and 620 mg/kg respectively
Effect on serum low-density lipoprotein-cholesterol level
Low-density lipoprotein-cholesterol levels were significantly (P < 0.05) higher in MC as compared to NC. SCM group treated with metformin showed a significant reduction (P < 0.05) compared to MC. MEAL treated groups showed a greater significant reduction (P < 0.05) as compared to MC, as shown in Figure 5.
Figure 5.
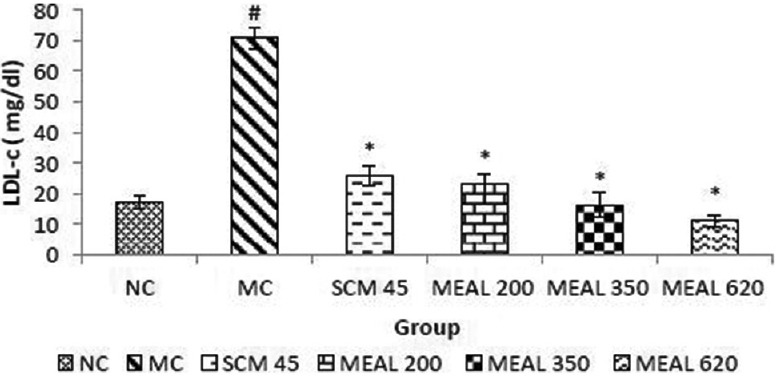
Effect on LDL-c level: Data are represented as mean ± SEM, n = 6; #P < 0.05 as compared to normal control (group I); *P < 0.05 as compared to model control (group II), NC: Normal control, MC: Model control, SCM 45: Standard control metformin 45 mg/kg, MEAL 200, 350 and 620 – Methanol extract of Albizzia lebbeck 200, 350 and 620 mg/kg respectively
Effect on serum very low-density lipoprotein-cholesterol level
Increase in VLDL-c levels as demonstrated by MC was significantly (P < 0.05) different from NC. SCM group showed significantly (P < 0.05) decreased VLDL-c levels as compared to MC. MEAL treatment greatly reduced VLDL-c levels, which were significantly (P < 0.05) different as compared to MC, depicted in Figure 6.
Figure 6.
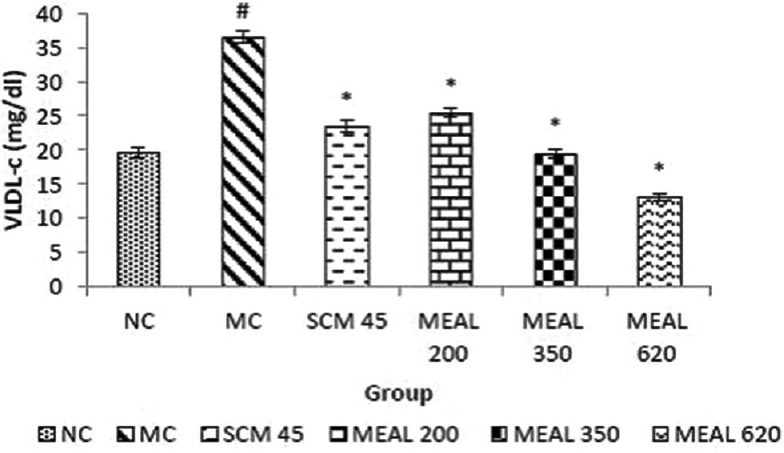
Effect on VLDL-c level: Data are represented as mean ± SEM, n = 6; #P < 0.05 as compared to normal control (group I); *P < 0.05 as compared to model control (group II), NC: Normal control, MC: Model control, SCM 45: Standard control metformin 45 mg/kg, MEAL 200, 350 and 620 – Methanol extract of Albizzia lebbeck 200, 350 and 620 mg/kg respectively
Effect on serum creatinine level
Creatinine levels were found to be higher significantly (P < 0.05) in MC as compared to NC. SCM control showed significant (P < 0.05) reduction in creatinine levels. Treatment with MEAL produced reduction in elevated creatinine, which was significantly (P < 0.05) different from MC, as observed in Figure 7.
Figure 7.
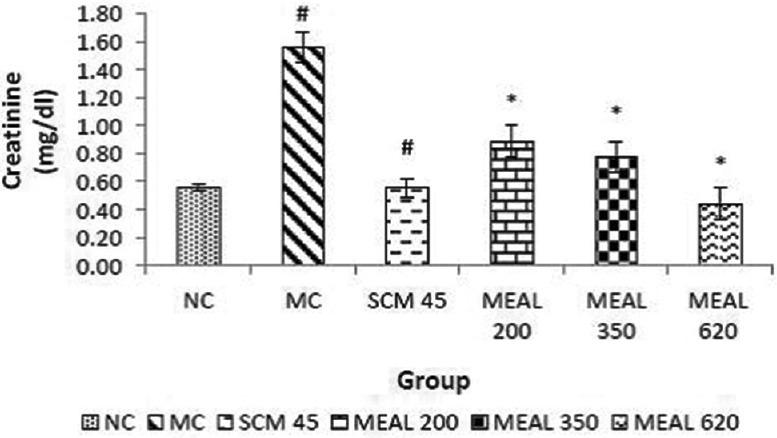
Effect on serum creatinine level: Data are represented as mean ± SEM, n = 6; #P < 0.05 as compared to normal control (group I); *P < 0.05 as compared to model control (group II), NC: Normal control, MC: Model control, SCM 45: Standard control metformin 45 mg/kg, MEAL 200, 350 and 620 – Methanol extract of Albizzia lebbeck 200, 350 and 620 mg/kg respectively
Effect on serum urea level
As illustrated in Figure 8, elevation was observed in serum urea levels of MC, significantly (P < 0.05) different from NC. SCM reduced the level significantly (P < 0.05) as compared to MC. Treatment with MEAL abated the elevated levels of serum urea significantly (P < 0.05) as compared to MC.
Figure 8.
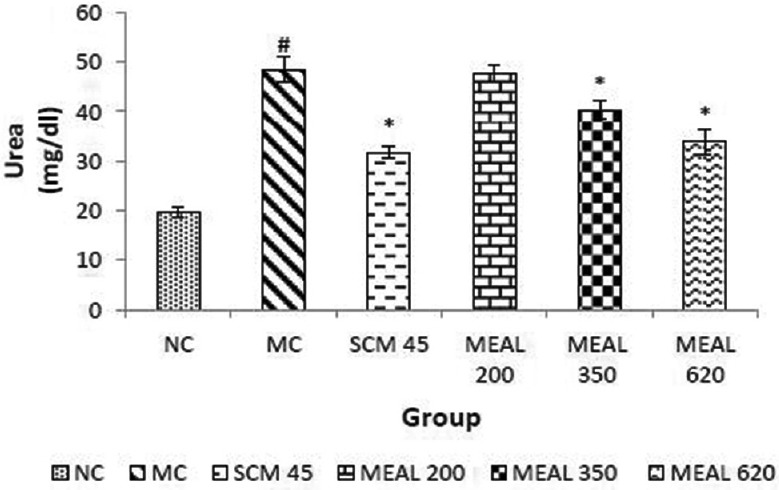
Effect on blood urea level: Data are represented as mean ± SEM, n = 6; #P < 0.05 as compared to normal control (group I); *P < 0.05 as compared to model control (group II), NC: Normal control, MC: Model control, SCM 45: Standard control metformin 45 mg/kg; MEAL 200, 350 and 620 – Methanol extract of Albizzia lebbeck 200, 350 and 620 mg/kg respectively
Discussion
Streptozotocin is the most widely used experimental chemical to induce diabetes in animals. STZ is a GLU analog, which was obtained for the 1st time from the cultures of Streptomyces achromogenes. The glucose transporter-2, which leads to GLU uptake into pancreatic β-cells also mediates the cellular uptake of STZ. Once inside the pancreatic β-cells, STZ fragments DNA through the formation of free alkylating radicals leading to a reduction in cellular levels of nucleotides and related compounds, particularly NAD+, eventually causing a rapid necrosis of β-cells. A water-soluble Vitamin B3-Nicotinamide is a biochemical precursor of nicotinamide adenine dinucleotide (NAD). It has been shown to inhibit apoptosis, improve energy status in ischemic tissues, exhibit antioxidant properties and metabolic improvements. Nicotinamide has well proven free radical scavenging activity and consequently could reduce DNA damage, and thereby it protects pancreatic β-cells against STZ by inhibiting nitric oxide (NO) mediated damage. Hence STZ-NAD model does not completely damage β-cells, but it is a valuable model for induction of type II diabetes mellitus.[12]
Hyperglycemia so induced by STZ-NAD administration increases the inflammatory markers: Tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1) and IL-6. This proinflammatory cytokine production in excess exaggerates the inflammatory stress, which contributes to the pathogenesis of diabetes. IL-2 plays an important role in the pathogenesis of insulin resistance.[13,14,15,16] Mechanistic studies suggest that mast cells participate in metabolic disorders by affecting energy expenditure, protease expression, angiogenesis, apoptosis, and preadipocyte differentiation. Mast cells being the major source of proinflammatory cytokines production, their stabilization or prevention of degranulation serve as a good target for antidiabetic drug.[3,4]
In the present study, hyperglycemia was observed after 72 h of STZ-NAD administration. After induction of diabetes with STZ-NAD rats showed higher blood GLU levels when compared with the normal rats. Treatment with MEAL 200, MEAL 350 and MEAL 620 mg/kg showed significant decrease in fasting serum GLU level. The improvement in serum GLU level indicates antihyperglycemic activity of MEAL. Mechanism involved may be potentiation of plasma insulin effect by increasing insulin secretion from the pancreatic β cells or may be it increases the peripheral uptake of GLU. Another possible mechanism of MEAL is stimulation of insulin mediated GLU metabolism and inhibit gluconeogenesis in liver due to mast cell stabilizing activity because mast cell stabilizer decreases the level of IL-6 and TNF-α which is responsible for insulin resistance.
Hypercholesterolemia is primary factor involved in the development of atherosclerosis and coronary heart disease which are the secondary complications of diabetes. Increased fatty acid concentrations also increase the β-oxidation of fatty acids, producing more acetyl-CoA and cholesterol in diabetics. STZ-NAD induced diabetic rats showed significant increase in cholesterol (57.73%) and TG (86.21%) levels. When diabetic rats were treated with MEAL 200, MEAL 350, MEAL 620, it produced significant decrease in serum TC (43.62%, 55.94% and 67.02%), LDL-c (67.10%, 78.76% and 90.05%) VLDL-c (31.11%, 46.36% and 65.44%) and TG (31.11%, 46.36% and 65.44%) levels. Administration of MEAL 200, MEAL 350and MEAL 620 to the diabetic rat significantly elevated the decreased HDL-c level by 15.48%, 17.06%, and 21.01% respectively. The observed antihyperlipidemic effect of MEAL may be because of decreased lipolysis and fatty acid synthesis.
Mast cell degranulation leads to the release of various mediators, such as TGF-β, chymase, tryptase, cathepsin G, rennin, and many others that may contribute to renal diseases.[17] Diabetic nephropathy is an important cause of morbidity and mortality. Hyperglycaemia causes degranulation of mast cells that releases inflammatory mediators such as histamine, cytokines IL-1, IL-2, IL-6 and TNF-α and TGF-β, that augment the reactive oxygen species production and inflammation. A. lebbeck bark extract has been proven to be a mast stabilizing agent.[10] The diabetic rats, in this study, showed increased levels of serum urea and serum creatinine, which are considered as significant markers of renal insufficiency. Urea is the major nitrogen containing metabolic product of protein metabolism; creatinine is endogenously produced and released into body fluids and its clearance measured as an indicator of renal function. STZ-NAD induced rats showed significant increase in serum creatinine level (92.59%) and serum urea level (110.86%). Treatment with MEAL 200, 350, 620 significantly decreased elevated serum creatinine (20%, 30% and 60%) and MEAL 350 and MEAL 620 significantly decreased urea level (28.31%and 40.52%) respectively which indicate its beneficial effect on kidney. While MEAL 200 insignificantly decreased serum urea (19.15%) as compared to MC. Decrease in serum urea and creatinine level with MEAL could be due to its antioxidant activity and may be due to decreased disturbances in protein and nucleic acid metabolism owing to better glycaemic control.
Conclusion
From the results obtained in this study, it can be concluded that A. lebbeck has antidiabetic activity owing to its ability to reduce blood GLU level in the animal model. The mechanism behind the anti-hyperglycemic effect of A. lebbeck may be attributed to its proven mast cell stabilizing activity. It also normalizes the dyslipidemic profile. Thus, the preliminary study done using animal model for diabetes shows promising results but further extensive advanced investigations need to be done to ascertain its beneficial role before it can be extrapolated for human therapeutics.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.International Diabetes Federation. [Accessed on 2014 Aug 17]. Available from: http://www.idf.org/diabetesatlas .
- 2.Tahrani AA, Piya MK, Kennedy A, Barnett AH. Glycaemic control in type 2 diabetes: Targets and new therapies. Pharmacol Ther. 2010;125:328–61. doi: 10.1016/j.pharmthera.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–5. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Shi GP. Mast cell stabilization: Novel medication for obesity and diabetes. Diabetes Metab Res Rev. 2011;27:919–24. doi: 10.1002/dmrr.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson M, Zhang X. The World Medicines Situtiation 2011-Traditional Medicines: Global Situation, Issues and Challenges. Geneva: World Health Organization; 2011. pp. 1–4. [Google Scholar]
- 6.Gupta RS, Kachhawa JB, Chaudhary R. Antifertility effects of methanolic pod extract of Albizzia lebbeck (L.) Benth in male rats. Asian J Androl. 2004;6:155–9. [PubMed] [Google Scholar]
- 7.Chulet R, Pankaj P, Sharma S, Jhajharia M. Phytochemical screening and antimicrobial activity of Albizzia lebbeck. J Chem Pharm Res. 2010;2:476–84. [Google Scholar]
- 8.Patel T, Shirode D, Roy SP, Kumar S, Setty SR. Evaluation of antioxidant and hepatoprotective effects of 70% ethanolic bark extract of Albizzia lebbeck in rats. Int J Res Pharm Sci. 2010;1:270–6. [Google Scholar]
- 9.Pathak NL, Patel N, Kasture S, Jivani N, Bhalodia Y, Malavia S. Free radical scavenging activity of Albizzia lebbeck methanolic extract in arthritic rats. Int J Pharm Res Dev. 2010;1:1–8. [Google Scholar]
- 10.Shashidhara S, Bhandarkar AV, Deepak M. Comparative evaluation of successive extracts of leaf and stem bark of Albizzia lebbeck for mast cell stabilization activity. Fitoterapia. 2008;79:301–2. doi: 10.1016/j.fitote.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Punitha IS, Shirwaikar A, Shirwaikar A. Antidiabetic activity of benzyl tetra isoquinoline alkaloid berberine in streptozotocin-nicotinamide induced type 2 diabetic rats. Diabetol Croat. 2005;34:117–28. [Google Scholar]
- 12.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–46. [PubMed] [Google Scholar]
- 13.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet. 2005;365:1333–46. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 14.Murugan P, Pari L. Antioxidant effect of tetrahydrocurcumin in streptozotocin-nicotinamide induced diabetic rats. Life Sci. 2006;79:1720–8. doi: 10.1016/j.lfs.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 16.Shi YC, Liao JW, Pan TM. Antihypertriglyceridemia and anti-inflammatory activities of monascus-fermented dioscorea in streptozotocin-induced diabetic rats. Exp Diabetes Res 2011. 2011 doi: 10.1155/2011/710635. 710635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Shi GP. Mast cells and metabolic syndrome. Biochim Biophys Acta. 2012;1822:14–20. doi: 10.1016/j.bbadis.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


