Abstract
Phytophthora pathogens secrete an array of specific effector proteins to manipulate host innate immunity to promote pathogen colonization. However, little is known about the host targets of effectors and the specific mechanisms by which effectors increase susceptibility. Here we report that the soybean pathogen Phytophthora sojae uses an essential effector PsAvh262 to stabilize endoplasmic reticulum (ER)-luminal binding immunoglobulin proteins (BiPs), which act as negative regulators of plant resistance to Phytophthora. By stabilizing BiPs, PsAvh262 suppresses ER stress-triggered cell death and facilitates Phytophthora infection. The direct targeting of ER stress regulators may represent a common mechanism of host manipulation by microbes.
 Phytophthora pathogens manipulate host plant immune responses by secreting effector proteins into plant cells. Here, Jing et al. show that the Phytophthora sojae Avh262 effector negatively regulates plant resistance by binding to host BiP proteins and suppressing ER-stress induced cell death
Phytophthora pathogens manipulate host plant immune responses by secreting effector proteins into plant cells. Here, Jing et al. show that the Phytophthora sojae Avh262 effector negatively regulates plant resistance by binding to host BiP proteins and suppressing ER-stress induced cell death
Plants have evolved a two-layer surveillance system to protect themselves against pathogens. In one layer, plant basal defence responses are activated upon the perception of pathogen-associated molecular patterns (PAMP) by pattern recognition receptors at the cell surface, resulting in PAMP-triggered immunity (PTI) that prevents infection by most microbes1. However, successful pathogens deliver effectors into plant cells to suppress PTI and establish parasitism1. In response, plants evolved a second layer of immunity, called effector-triggered immunity (ETI), that is initiated upon recognition of effectors by specific intracellular receptors1. Pathogen effectors can also attenuate ETI2,3,4,5,6. Thus, research on effector functions has not only elucidated the pathogenic mechanisms of pathogens, but also has identified novel components of plant immune systems.
Fungi and oomycetes, are among the most damaging pathogens to agriculture. For example, Phytophthora sojae (P. sojae) is one of the most important pathogens of soybean. Oomycetes secrete RxLR effectors that are translocated into plant cells during infection3,7. The conserved N-terminal RxLR motif is required to mediate the delivery of these virulence proteins into host cells, probably via specific infection structures called haustoria and directly from hyphae2,3. Phytophthora genomes each encode about 300–700 RxLR effectors8,9,10,11. RxLR effectors of P. sojae suppress plant immunity via transcriptional programming and functional cooperation12. Many Phytophthora RxLR effectors can interfere with plant immunity by modifying host targets. For example, Phytophthora infestans (P. infestans) Avrblb2 focally accumulates near haustoria and blocks the secretion of the plant papain-like cysteine protease C14 into the apoplast13. P. sojae Avr3b functions as an ADP-ribose/NADH pyrophosphorylase and promotes virulence by its enzyme activity14. Two P. sojae RxLR effectors, PSR1 and PSR2, suppress RNA silencing in plants by inhibiting the biogenesis of small RNAs15. To date, the plant targets and molecular mechanisms of the vast majority of RxLR effectors are still unknown.
The endoplasmic reticulum (ER) is a membrane-bound compartment that mediates cellular processes such as calcium homoeostasis and protein processing16,17. In the ER, proteins that are improperly folded or assembled are recognized by the ER quality control (ERQC) system and transported into the cytoplasm for ER-associated degradation (ERAD)18,19. The ERQC system consists of three pathways, one of which relies on the binding immunoglobulin protein (BiP) complex20. As the most abundant ER chaperones and key components of the ERQC machinery, BiPs play an important role in the unfolded protein response (UPR) by regulating stress transducers, such as the activating transcription factor 6 (ATF6), protein kinase RNA-like ER kinase (PERK) and inositol-requiring enzyme 1 (IRE1)21,22.
Emerging evidence indicates that ER stress-related cell death is associated with microbe infection. For example, the endophytic fungus Piriformospora indica activates ER stress-mediated cell death by inhibiting the UPR-related pro-survival machinery23. Thus ER-stress pathways could be targeted by pathogens to facilitate infection24. BiPs can regulate plant responses to abiotic and biotic stresses. For example, overexpression of GmBiP4 in soybean (Glycine max) inhibited the UPR and increased tolerance to water loss25, and suppressed cell death associated with senescence and osmotic stress26, but at the same time intensified cell death and induction of SA-mediated responses triggered by avirulent Pseudomonas species26. AtBiP2 of Arabidopsis thaliana is involved in folding and secretion of pathogenesis-related (PR) proteins during systemic acquired resistance (SAR), as loss-of-function mutants of AtBiP2 are defective in salicylic acid-elicited PR1 protein secretion27. Overexpression of a Nicotiana benthamiana BiP gene eliminated the triple gene block protein 3 (TGBp3)-induced hypersensitive response (HR), which is consistent with the cyto-protective role of the NbBiP in virus-infected leaves of N. benthamiana28.
Here we report the identification of BiPs as novel targets of a P. sojae RxLR effector, PsAvh262 that is essential for P. sojae infection. Ectopic expression of BiPs in both soybean hairy roots and N. benthamiana leaves enhanced susceptibility, suggesting that BiPs negatively regulate plant defence against Phytophthora infection. Our results suggest that PsAvh262 may promote infection by binding and stabilizing BiPs, resulting in attenuated plant defence responses.
Results
PsAvh262 is required for full virulence of P. sojae
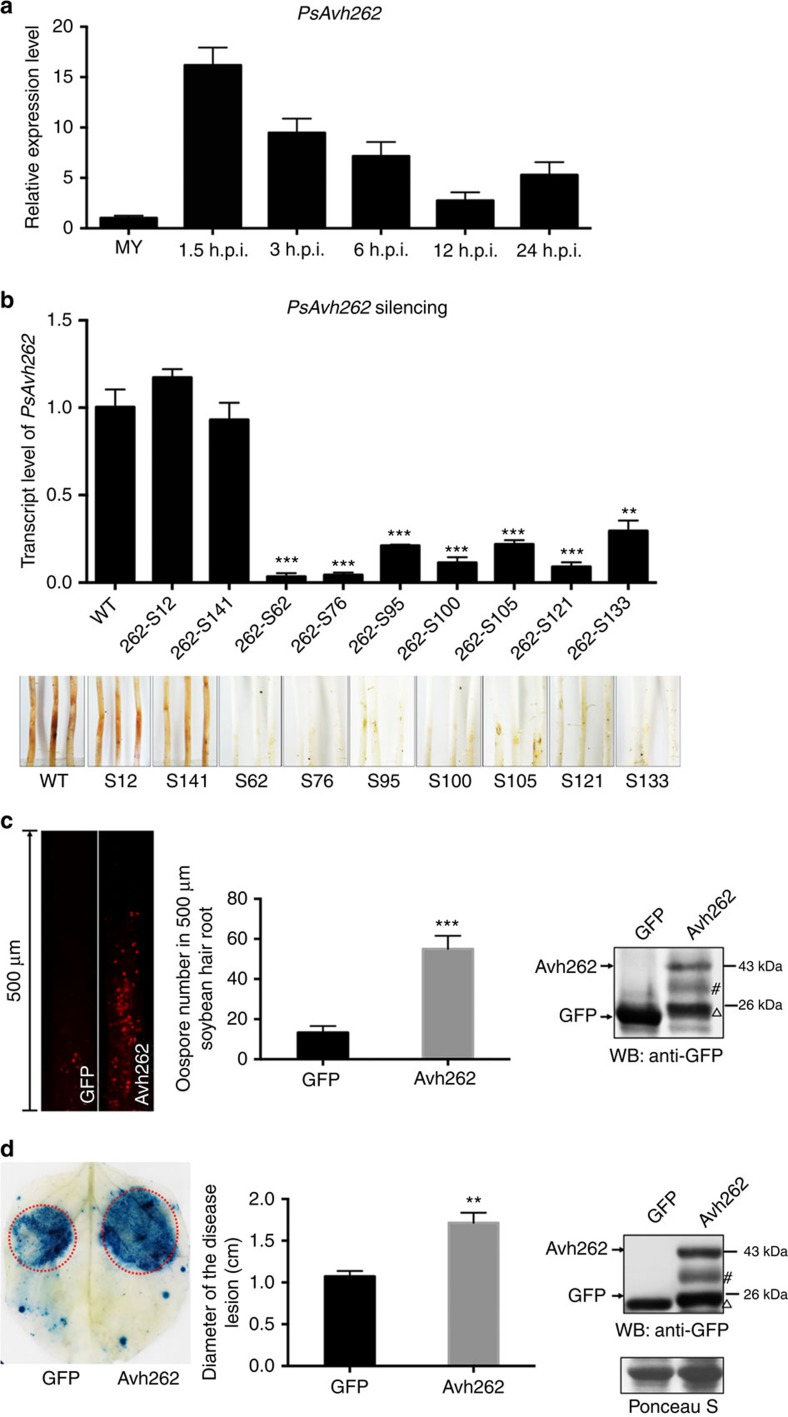
PsAvh262 encodes a 123-amino-acid protein that has a secretion signal peptide and an RxLR motif12. PsAvh262 is conserved in various P. sojae strains (Supplementary Fig. 1a). To determine the possible role of PsAvh262 during P. sojae infection, we first analysed the expression patterns of PsAvh262 at 1.5, 3, 6, 12 and 24 h post inoculation (h.p.i.) onto soybean hypocotyls. PsAvh262 was highly expressed at early stages of infection with the maximal expression level observed at 1.5 h.p.i. (Fig. 1a).
Figure 1. PsAvh262 is an essential virulence factor of Phytophthora sojae.
(a) Expression profile of PsAvh262 during P. sojae strain P6497 infection of soybean hypocotyls. The susceptible soybean cultivar Williams was used as the host. Total RNA was extracted from mycelia (MY) or infected soybean leaves at 1.5, 3, 6, 12 and 24 h post inoculation (h.p.i.). Transcript levels of PsAvh262 were determined by qRT–PCR. The P. sojae actin gene (VMD GeneID: 108986) was used as the pathogen internal control gene, (b) Silencing of PsAvh262 in P. sojae greatly impaired the virulence in soybean hypocotyls. Relative transcript levels of PsAvh262 (upper panel) in the P. sojae transformants were determined by qRT–PCR. Disease symptoms (lower panel) in etiolated hypocotyls were observed. Pictures were taken at 7 days post inoculation (d.p.i.). S12 and S141 were non-silenced transformants carrying the same silencing construct. (c) Expression of PsAvh262 in soybean hairy roots enhanced P. sojae infection. Hairy roots expressing GFP-PsAvh262 or GFP were inoculated with mycelia plugs of RFP-labelled wild-type P. sojae strain P6497 (P6497-RFP). Oospore production in the infected hair roots was observed under a confocal microscope (left panel), and lesion length was determined (middle panel) at 48 h.p.i. Expression of GFP or GFP-PsAvh262 was confirmed by western blotting using an anti-GFP antibody (right panel). (d) Expression of PsAvh262 in N. benthamiana enhanced infection of Phytophthora capsici. Leaf regions transiently expressing PsAvh262 or GFP, were inoculated with mycelia plugs of P. capsici. Infected leaves were stained with Trypan blue at 36 h.p.i. to visualize disease lesions (left panel) and the sizes of the lesions were determined (middle panel). Expression of GFP or PsAvh262-GFP was confirmed by western blotting using an anti-GFP antibody (right panel). Δ, non-specific band when using anti-GFP, which are common contaminants of western blots present in many published articles detecting GFP-fused proteins expressed in the plant cells13,64; #, PsAvh262 derived band; this may due to some unknown modification or degradation. Error bars represent the mean±s.d.(n=3) and asterisks (**or ***) denote significant differences (P<0.01 or P<0.001, assessed with one-way ANOVA). All experiments were repeated three times with similar results.
We then investigated the contribution of PsAvh262 to P. sojae virulence through RNA silencing. Seven PsAvh262-silenced transformants of P. sojae strain P6497 (wild type) were obtained using polyethylene glycol (PEG)-mediated transformation. Quantitative RT–PCR (qRT–PCR) confirmed that the PsAvh262 transcript levels in these transformants were significantly decreased, from 32 to 8% of the wild-type strain (Fig. 1b). All seven PsAvh262-silenced transformants showed reduced virulence on etiolated soybean seedlings, whereas two non-silenced transformants showed similar virulence as the wild-type strain (Fig. 1b). In addition, intense cell death symptoms were observed from soybean hypocotyls 9 h after inoculation with the PsAvh262-silenced transformants, whereas similar symptoms were not observed with the wild-type strain (Supplementary Fig. 1b). Silencing of PsAvh262 did not change any developmental phenotypes of P. sojae (Supplementary Fig. 2). Taken together, these results indicate that PsAvh262 is an essential virulence effector that is required for P. sojae infection, possibly through its ability to suppress plant cell death.
To further examine the virulence function of PsAvh262, we transiently expressed it in soybean hairy roots and in agroinfiltrated N. benthamiana leaves, then inoculated the plant tissues with Phytophthora. Soybean hairy roots expressing PsAvh262-GFP or GFP were inoculated with mycelia of P. sojae strain P6497RFP, which constitutively expresses red fluorescent protein. As shown in Fig. 1c, the number of oospores formed on 500-μm-long hairy roots expressing PsAvh262 was over three-fold more than on roots expressing GFP. Consistent with these results, transient expression of PsAvh262 in N. benthamiana leaves also increased their susceptibility to P. capsici; lesions on leaves transiently expressing PsAvh262 were significantly larger (P<0.01, one-way analysis of variance (ANOVA), n=5) than those on leaves expressing GFP (Fig. 1d).
In addition, PsAvh262 was able to suppress cell death in N. benthamiana triggered by a variety of elicitors, including the pro-apoptotic protein BAX, the P. infestans PAMP INF1 and the P. sojae RxLR effector Avh241 (Supplementary Fig. 1c). Collectively, these results establish that PsAvh262 can promote Phytophthora infection and can act as an inhibitor of programmed cell death.
PsAvh26260–82 is essential for its virulence function
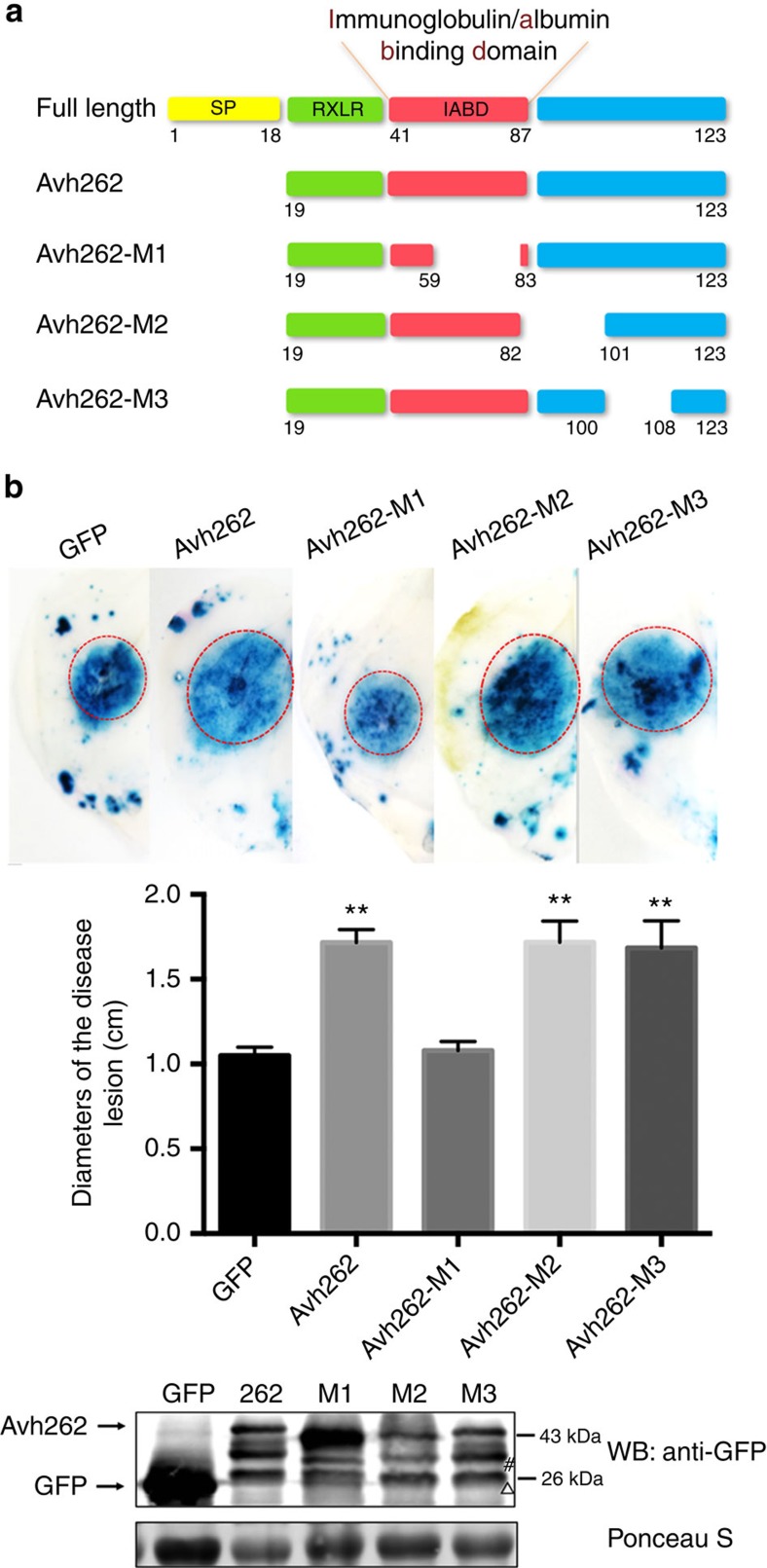
To identify functional domains in PsAvh262, we characterized potential motifs and its predicted secondary structure. A weak match to the immunoglobulin/albumin-binding domain (IPR009063; IABD) was identified within residues 41–87 using the Protein Homology/analog Y Recognition Engine V 2.0 (Phyre 2, http://www.sbg.bio.ic.ac.uk/phyre2/)29 (Supplementary Fig. 3a). This small three-helical domain has been found in various surface proteins in Gram-positive bacteria and shown to promote growth and virulence of Peptostreptococcus magnus (P. magnus)30. We examined three deletion mutants: PsAvh262-M1 (deletion of 60–82), which removed most of the possible IABD motif; PsAvh262-M2 (deletion of 83–100); and PsAvh262-M3 (deletion of 101–107) (Fig. 2a; Supplementary Fig. 3b). These mutants were transiently expressed in N. benthamiana, and then the plants were challenged with P. capsici. PsAvh262-M2 and PsAvh262-M3 functioned similarly to the wild-type PsAvh262 in promoting Phytophthora infection, whereas PsAvh262-M1 lost this virulence-promoting activity (Fig. 2b). These results suggest that the region 60–82 aa is essential for the virulence function of PsAvh262.
Figure 2. The potential immunoglobulin/albumin-binding domain is essential for the virulence function of PsAvh262.
(a) Schematic diagram showing the protein structures of PsAvh262 and its deletion mutants. The putative immunoglobulin/albumin-binding domain (IABD) motif is deleted in PsAvh262-M1. SP, N-terminal secretion signal peptide. Numbers underneath each construct indicate amino acid positions. (b) PsAvh262-M1 lost the ability to promote P. capsici infection in N. benthamiana. Leaves transiently expressing GFP, PsAvh262 or PsAvh262 mutants were inoculated with P. capsici. The pictures were taken at 36 h.p.i. after disease lesions were stained with Trypan blue (upper panel). Sizes of the lesions were measured and subjected to statistical analysis (middle panel). Error bars represent the mean±s.d.(n=3), and asterisks (**) denote significant differences (P<0.01) between samples. The statistical significances of the pairwise differences between PsAvh262/mutants and the GFP control were assessed with one-way ANOVA. Similar phenotypes were observed in at least three independent experiments. Expression of GFP, GFP-PsAvh262 and the deletion mutants was confirmed by western blotting using an anti-GFP antibody. WB, western blot; M1, M2 and M3 represent PsAvh262-M1, PsAvh262-M2 and PsAvh262-M3, respectively. Δ, non-specific band when using anti-GFP; #, PsAvh262 derived band.
PsAvh262 associates with plant ER-luminal BiPs
To identify the host targets of PsAvh262, N-terminal GFP-tagged PsAvh262 (without the N-terminal secretion signal peptide 1–18 aa) was transiently expressed in N. benthamiana leaves followed by immunoprecipitation (IP) using anti-GFP affinity beads. Immuno-purified proteins were then analysed by LC-MS/MS using the genome sequences of the closely related Nicotiana tabacum13. Among the N. benthamiana proteins that potentially associated with PsAvh262 (Supplementary Table 1), a protein similar to an ER-luminal BiP5 in N. tabacum (GenBank accession #729623) was selected for further analysis. Four BiPs, that is, NbBiP1, NbBiP2, NbBiP3 and NbBiP4, have been reported in N. benthamiana31. A search of the Sol Genomics Network (http://solgenomics.net/) using the NtBiP5 sequence as query identified an analogous protein (ID: NbS00040865g0006.1) in the N. benthamiana database with only one amino acid difference (a ‘V' versus an ‘I' at the position 13, 99.85% identity) (Supplementary Fig. 3). We therefore designated the PsAvh262-associated N. benthamiana protein as NbBiP5, which shares 76–96% identity in full-length amino-acid sequences with NbBiP1–NbBiP4, respectively (Supplementary Fig. 3). NbBiP5 also has homologues in soybean. The soybean BiP gene family consists of at least four members, GmBiP1 (Glyma08g02940), GmBiP2 (Glyma08g02960), GmBiP3 (Glyma05g36600) and GmBiP4 (Glyma05g36620), all of which are induced upon ER stress32. NbBiP5 shares 93.41, 91.77, 92.66 and 93.87% identity with GmBiP1–GmBiP4, respectively (Supplementary Fig. 4). Transcriptome analysis showed that GmBiP1 has the highest transcription level, followed by GmBiP4 (Supplementary Fig. 5). These two GmBiPs were utilized further in our study.
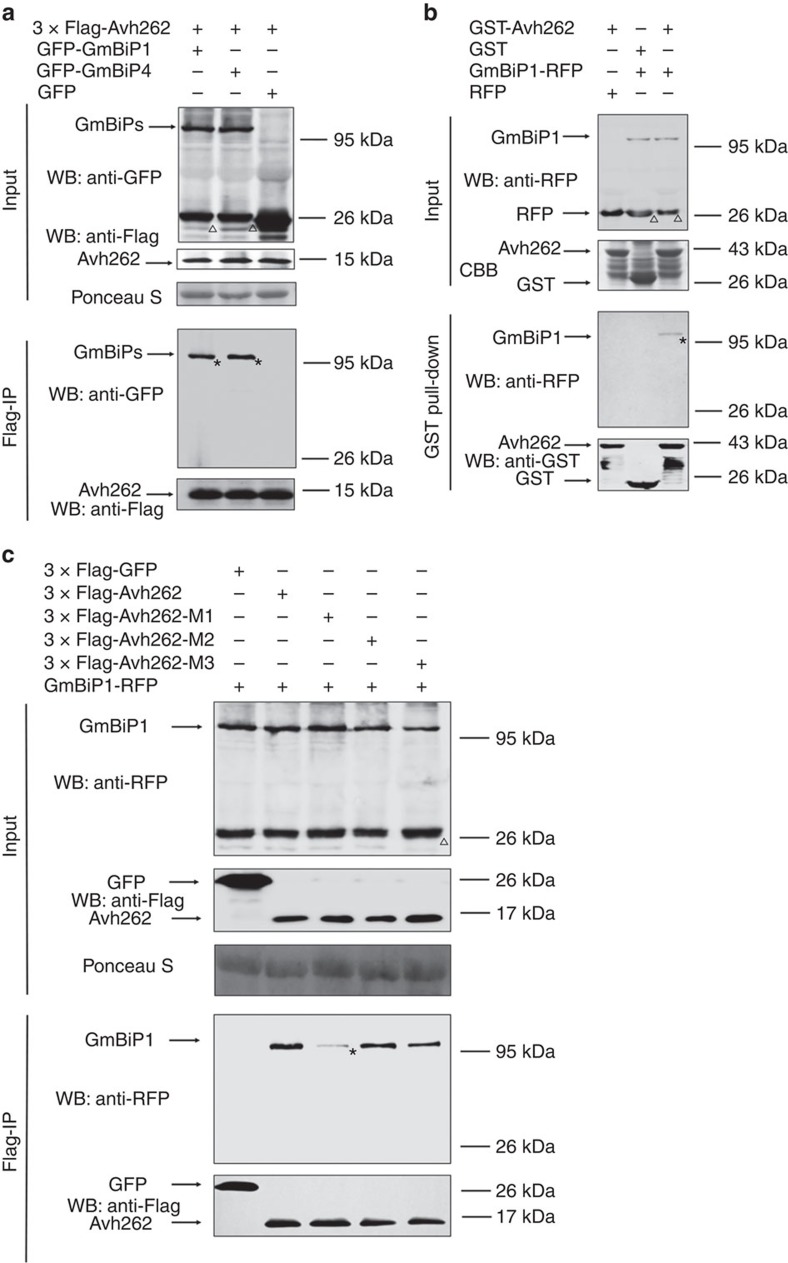
To validate the association of PsAvh262 with NbBiP5 and GmBiPs, we carried out Co-IP assays (Fig. 3; Supplementary Fig. 6). GFP-PsAvh262 was co-expressed with NbBiP5-RFP or RFP in N. benthamiana. Total proteins were extracted from the infiltrated leaves and incubated with GFP-Trap_A beads (Chromotek, Germany). NbBiP5-RFP, but not RFP, was significantly enriched in the GFP-PsAvh262 precipitates (Supplementary Fig. 6a). Consistent with this result, GFP-GmBiP1, GFP-GmBiP2, GFP-GmBiP3 and GFP-GmBiP4, but not GFP, were also enriched in the 3 × Flag-PsAvh262 precipitates (Fig. 3a; Supplementary Fig. 6b) following transient expression in N. benthamiana. We next confirmed this interaction by a semi-in vivo GST-pull-down assay because we were unable to express NbBiP5 or GmBiPs in Escherichia coli (E. coli). GmBiP1-RFP was expressed in N. benthamiana leaves and the total protein extracts were incubated with purified glutathione S-transferase (GST)-PsAvh262 expressed in E. coli. As showed in Fig. 3b, GmBiP1-RFP, but not RFP, was specifically enriched in GST-PsAvh262-bound glutathione beads. PsAvh262 also interacted with OsBiP3 from rice (Os02g02410) (Supplementary Fig. 6c). The interaction between PsAvh262-M1 and GmBiP1 was much weaker than the interaction with WT PsAvh262 (Fig. 3c), indicating that this region plays a key role in mediating the interaction between PsAvh262 and BiPs.
Figure 3. PsAvh262 interacts with plant endoplasmic reticulum luminal BiPs.
(a) PsAvh262 interacts with soybean BiPs in planta. Total proteins were extracted from N. benthamiana leaves expressing 3 × Flag-PsAvh262 together with GFP-GmBiP1 or GFP-GmBiP4 (GFP was attached to the N terminus of the signal peptides of the GmBiPs). Protein complexes were pulled down using anti-Flag agarose beads and the co-precipitation of GFP-GmBiP1 or GFP-GmBiP4 was detected by western blotting using an anti-GFP antibody. (b) Proteins extracted from N. benthamiana leaves expressing GmBiP1-RFP (RFP was fused to the C-terminal HDEL sequence of GmBiP1) or RFP were incubated with E. coli homogenate containing GST-PsAvh262 or GST. Enrichment of GmBiP1-RFP in GST-PsAvh262-bound glutathione resins was detected using an anti-RFP antibody. (c) M1 deletion greatly reduces the interaction of PsAvh262 with GmBiP1. 3 × Flag-PsAvh262 or its mutants were co-expressed in N. benthamiana with GmBiP1-RFP. Protein complexes were pulled down using anti-Flag agarose gel, and co-precipitations of PsAvh262 and its mutants with GmBiP1 were detected using immunoblots. Membranes were stained with Ponceau S to confirm equal loading. *, the objective bands of GmBiPs. Δ, non-specific bands of GmBiPs when using anti-GFP or anti-RFP, similar to GFP-PsAvh262 protein. Similar results were obtained in at least three independent experiments.
PsAvh262 and GmBiP1 co-localize in the endoplasmic reticulum
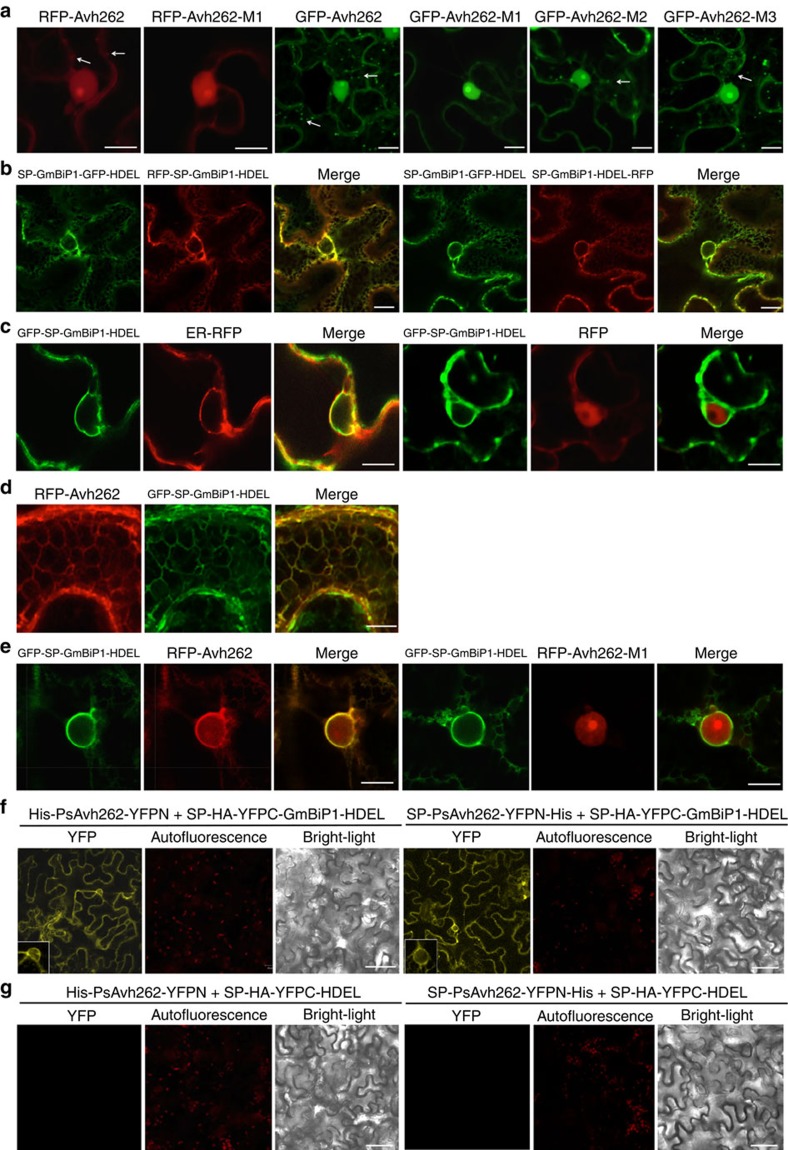
To gain insights into the functional significance of the PsAvh262-GmBiP1 interaction, we determined the localization patterns of the proteins in plant cells. Wild-type or mutant PsAvh262 proteins (lacking the signal peptide) were co-expressed with GmBiP1 as a variety of GFP or RFP fusion proteins by agroinfiltration in N. benthamiana. Confocal microscopy showed that RFP-PsAvh262, GFP-PsAvh262 and their mutants (the FPs were attached to the N terminus of PsAvh262) were primarily localized in the nucleus when expressed in the absence of co-expressed BiPs (Fig. 4a). RFP-PsAvh262 and GFP-PsAvh262 also were present in punctate structures in the cell cortex33 (Fig. 4a). PsAvh262-M2 and PsAvh262-M3, but not PsAvh262-M1, also were present in punctate structures (Fig. 4a), indicating that the region deleted in M1 is required for the localization of PsAvh262 into the punctate structures. Consistent with the previously observed localization of BiPs to the ER31,34,35,36, we found that SP-GmBiP1-GFP-HDEL (that is, GFP inserted between BiP and its HDEL motif) localized in an ER-like network of subcellular structures including the perinuclear ER (Fig. 4b,c). Surprisingly, the localization of BiP was unchanged if the FP tag was placed onto the N terminus of the signal peptide or onto the C terminus of the HDEL motif (Fig. 4b) indicating that the localization of GmBiP1 is primarily controlled by sequences other than these two signals. Co-expression of GFP-SP-GmBiP1-HDEL with an ER marker (SP-RFP-HDEL) confirmed that the localization was to the ER (Fig. 4c).
Figure 4. PsAvh262 and GmBiP1 co-localize to the endoplasmic reticulum.
In all panels, proteins were expressed in N. benthamiana through agroinfiltration. Fluorescence was detected in epidermal cells of the infiltrated leaves by confocal microscopy at 48 h after agroinfiltration. Scale bars, 10 μm. (a) Localization of RFP- and GFP-fusions of PsAvh262 and mutants in the absence of GmBiP1. FPs were attached to the N terminus of PsAvh262 without its signal peptide. PsAvh262 and mutants M2 and M3 were primarily located in the nucleus and nucleolus, but also in punctate structures in the cell cortex. RFP-PsAvh262-M1 was no longer present in cell cortex. (b,c) Localization of GmBiP1 FP fusions to the ER. GmBiP1 fusions were targeted to ER-like structures irrespective of the placement of the FP (b). GFP-SP-GmBiP1-HDEL co-localizes with SP-RFP-HDEL, but not with RFP (c). (d,e) RFP-PsAvh262 and GFP-SP-GmBiP1-HDEL co-localize to the ER. Co-localization to the ER network (d) and to the peri-nuclear ER (e). The M1 deletion eliminates co-localization to the peri-nuclear ER. (f,g) Co-localization of PsAvh262 and GmBiP1 detected by bimolecular fluorescence complementation (BiFC) using the constructs shown in Supplementary Fig. 7a. Complementation is only observed with GmBiP1 constructs (f) but not control constructs (g).
Notably, when co-expressed with GFP-SP-GmBiP1-HDEL in N. benthamiana, RFP-PsAvh262 was co-localized with GFP-SP-GmBiP1-HDEL to the ER network (Fig. 4d) including the perinuclear ER (Fig. 4e). This re-localization in the presence of GmBiP1 was not observed for RFP-PsAvh262-M1 (Fig. 4e) or RFP (Fig. 4c). To further confirm the location of the GmBiP1-PsAvh262 interaction in plant cells, we conducted a bimolecular fluorescence complementation (BiFC) experiment. PsAvh262 (PsAvh262-YFPN, with or without its signal peptide) and GmBiP1 (SP-YFPC-GmBiP1-HDEL) were co-expressed in N. benthamiana. YFP fluorescence was observed exclusively from the ER (Fig. 4f), suggesting that PsAvh262 could reach and bind to BiP located inside the ER whether PsAvh262 was targeted to the cytoplasm or whether it was targeted to the ER by a signal peptide. No fluorescence complementation was observed when BiP was removed from the YFPC constructs (Fig. 4g). Taken together, our results suggest that the GmBiP1-PsAvh262 interaction occurs in the ER, though we cannot rule out that the interaction (also) occurs in the cytoplasm and that the complex subsequent can relocate to the ER.
PsAvh262 and GmBiP1 co-localize around haustoria
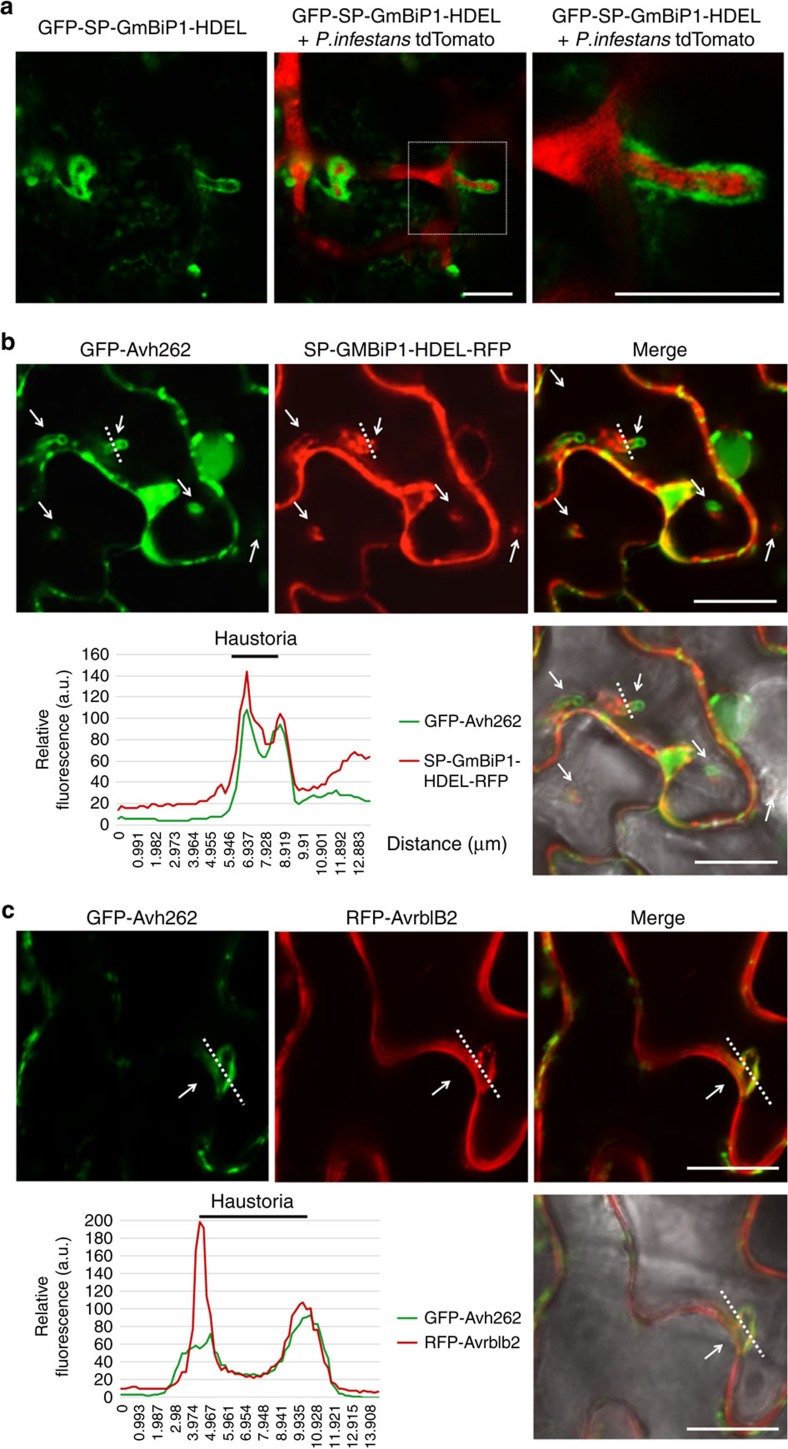
To investigate the localization patterns of GmBiP1 and PsAvh262 during Phytophthora infection, we inoculated N. benthamiana leaves expressing GFP-SP-GmBiP1-HDEL with P. infestans strain 88069td tagged with cytoplasmic tdTomato. Confocal microscopy showed preferential accumulation of GFP-SP-GmBiP1-HDEL around haustoria in the infected leaves (Fig. 5a), suggesting that relocation of BiPs to the peri-haustorial region is associated with plant responses to infection. To determine PsAvh262 and BiPs were co-localized during infection, GFP-PsAvh262 (lacking its signal peptide) and SP-GmBiP1-HDEL-RFP were co-expressed in N. benthamiana leaves infected by untagged P. infestans strain T30-4. The results showed that both PsAvh262 and GmBiP1 localized to the perihaustorial region, though the distribution of GmBiP1 appeared biased towards the necks of the haustoria, while PsAvh262 appeared biased towards the tips (Fig. 5b). To determine if PsAvh262 was localized to the extra-haustorial membrane (EHM), we co-expressed GFP-PsAvh262 with the P. infestans RxLR effector Avrblb2 (RFP-Avrblb2), since Avrblb2 was reported to associate with the extra-haustorial membrane13,37. As shown in Fig. 5c, GFP-PsAvh262 localized around the haustoria coincident with Avrblb2, demonstrating that PsAvh262 was associated with the EHM. Interestingly, in contrast to Fig. 4a, PsAvh262 localized to the EHM in the absence of co-expression with GmBiP1 (Fig. 5c). Taken together, these results suggest that PsAvh262 and its target GmBiP1 co-localize around the haustoria and so may possibly influence infection.
Figure 5. PsAvh262 and GmBiP1 co-localize around haustoria during infection.
In all panels, proteins were expressed in N. benthamiana through agroinfiltration. The leaves were inoculated with P. infestans zoospores 2 days after agroinfiltration. Images were taken 2 days after inoculation with the relevant P. infestans strain. (a) GFP-SP-GmBiP1-HDEL accumulates around haustoria during infection by P. infestans strain 88069td expressing cytoplasmic tdTomato. Scale bars, 15 μm. (b) GFP-PsAvh262 and SP-GmBiP1-HDEL-RFP co-localized around haustoria following inoculation with untagged P. infestans strain T30-4. White arrows indicate the tips of haustoria. a.u., arbitrary units. Scale bars, 10 μm. (c) GFP-PsAvh262 and RFP-Avrblb2 (RFP attached to the N terminus of AvrBlb2 without its signal peptide) were co-localized around haustoria during infection by untagged P. infestans strain T30-4. In b and c, the fluorescence plots show the relative fluorescence along the dotted lines in the images. Arrowheads point to the tips of haustoria. a.u., arbitrary units. Scale bar, 10 μm.
PsAvh262 stabilizes BiPs in planta
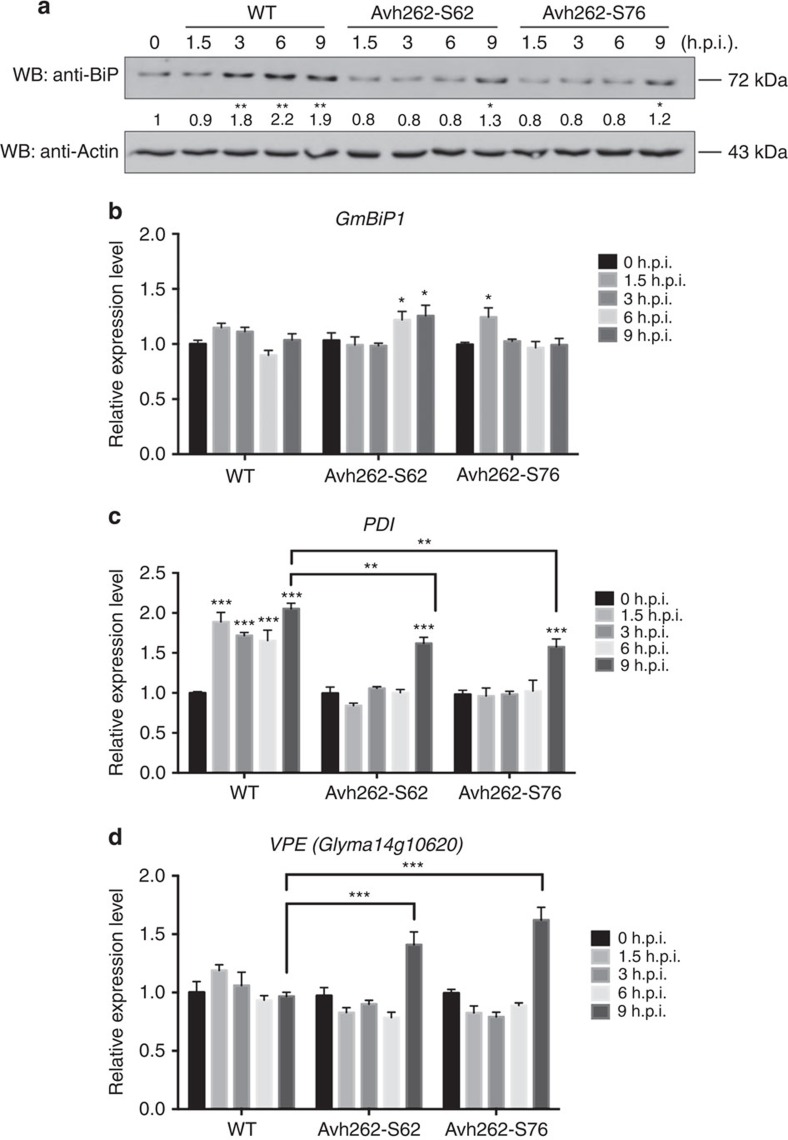
We next investigated whether the abundance of GmBiP1 could be affected by PsAvh262. For this purpose, we used western blots with anti-BiP antibodies to determine the endogenous levels of GmBiPs during P. sojae infection of soybean. The hypocotyls of etiolated soybean seedlings were inoculated with wild-type strain P6497 or with two independent PsAvh262-silenced transformants (Avh262-S62 and Avh262-S76). Total proteins were extracted from the infected tissues at 0, 1.5, 3, 6 and 9 h.p.i. We used 9 h.p.i. as our latest time point because PsAvh262-silenced transformants caused cell death at this time point. Abundances of GmBiPs increased from 3 to 9 h.p.i. in tissues inoculated with wild-type P. sojae, but much less in samples inoculated with the PsAvh262-silenced transformants (Fig. 6a). In addition, we found a small increase in GmBiP levels at 9 h.p.i. during infection by the PsAvh262-silenced transformants. Transcriptome analysis did not show significant differential accumulation of GmBiP transcripts during P. sojae infection (Supplementary Fig. 5). qRT–PCR results confirmed that the transcript levels of GmBiP1 remained almost the same in soybean inoculated by either wild type or PsAvh262-silenced P. sojae, although there were slight fluctuations at some time points (Fig. 6b). These results suggest that PsAvh262 might have increased the stability of GmBiPs proteins during P. sojae infection, though we cannot rule out that stabilization of the GmBiPs is caused indirectly by the greater level infection by the wild-type strain, which results in a higher level of ER stress (see below).
Figure 6. PsAvh262 attenuates endoplasmic reticulum stress-triggered cell death during P. sojae infection.
(a) Accumulations of GmBiPs in soybean after hypocotyls were infected with P. sojae P6497 (wild-type) or two PsAvh262-silenced transformants (S62 and S76) were determined by western blotting using an anti-BiP antibody. Antibody against actin was used as an internal standard. Numbers below the blot indicate relative abundances of GmBiPs. Asterisks (* or **) denote significant differences (P<0.05 or P<0.01, one-way ANOVA, n=3) between samples. (b–d) Transcript abundances of ER-stress-related genes. The soybean housekeeping gene CYP2 (TC224926) was used as an internal standard in each case. GmBiP1 (b), Protein disulfide isomerase (PDI) (c) and vacuolar processing enzyme (VPE) (d) were determined by qRT–PCR in soybeans inoculated with wild-type or PsAvh262-silenced mutants of P. sojae. Error bars represent the mean±s.d.(n=3) and asterisks (*, ** or ***) denote significant differences (P<0.05, P<0.01 or P<0.001, respectively) between samples. The statistical significance of the pairwise differences between PsAvh262-silenced mutants and wild-type strain at 0 d.p.i. was assessed with one-way ANOVA. Similar results were observed in three independent experiments.
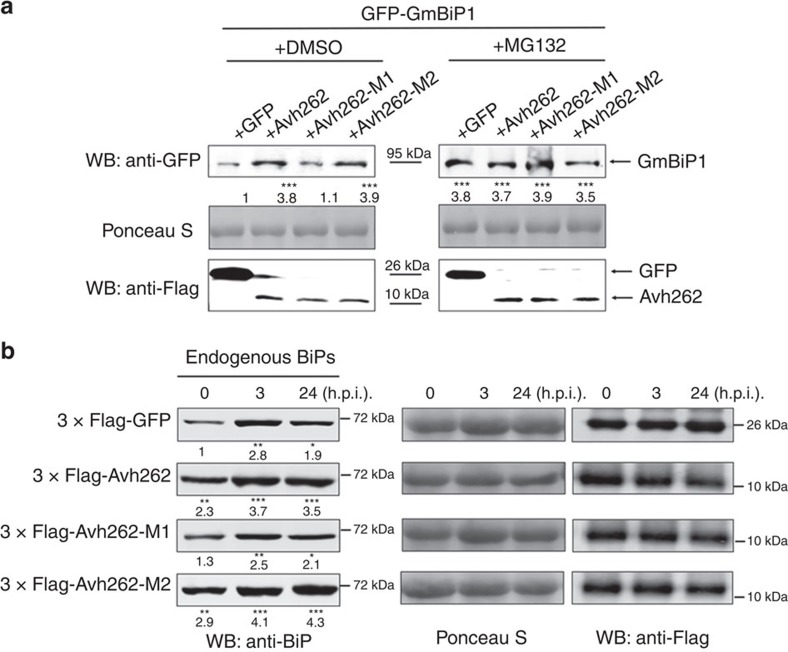
To more directly examine the effect of PsAvh262 on BiP stability, we compared the accumulation of NbBiP5-RFP in N. benthamiana when co-expressed with GFP-PsAvh262 or GFP using Agroinfiltration. As shown in Supplementary Fig. 8a, NbBiP5-RFP accumulated to a much higher level in the presence of GFP-PsAvh262 than when co-expressed with GFP. These results provide evidence that the presence of PsAvh262 can enhance the accumulation of NbBiP5. Plant ER-resident proteins can be efficiently transported out of the ER for degradation38 via the ubiquitin/26S proteasome pathway39. BiPs may also be regulated similarly by this mechanism. It is therefore possible that the interaction with PsAvh262 increases the retention of BiPs in the ER, thereby preventing their degradation. To test this hypothesis, we co-expressed NbBiP5 and GmBiP1 with wild-type PsAvh262 or the PsAvh262 mutants in N. benthamiana, and treated BiP-expressing leaves with the proteasomal inhibitor MG132. Increased accumulations of GmBiP1 (Fig. 7a) and NbBiP5 (Supplementary Fig. 8a) were observed in leaves treated with MG132 or co-expressing PsAvh262. Furthermore, MG132 treatment did not further increase the levels of GmBiP1 produced by Avh262 expression, suggesting that Avh262 and MG132 act via the same process (Fig. 7a). These results suggest that PsAvh262 prevents BiPs from being degraded in planta through the 26S proteasomal pathway. Interestingly, we found that PsAvh262-M1, but not PsAvh262-M2, lost the capacity to stabilize GmBiP1 (Fig. 7a; Supplementary Fig. 8b), indicating that direct PsAvh262-BiP interaction is required for BiP stabilization. PsAvh262-mediated inhibition of BiP degradation is specific because the P. infestans effector Avrblb2, which also associates with NbBiP5 (ref. 13), was unable to stabilize NbBiP5 (Supplementary Fig. 8b). Taken together, these results suggest that PsAvh262 stabilizes BiPs by preventing their degradation.
Figure 7. PsAvh262 stabilizes BiPs in planta.
(a) Immunoblots showing the stabilization of GFP-GmBiP1 by PsAvh262 or the 26S proteasomal inhibitor MG132, but not by PsAvh262-M1. GFP-GmBiP1 (GFP was attached to the N terminus of the signal peptide of GmBiP1) was expressed in N. benthamiana leaves together with wild-type PsAvh262 or its mutants by agroinfiltration. MG132 (100 μM) or DMSO was infiltrated into the leaves at 24 h before protein extraction. Abundances of GFP-GmBiP1 were determined using an anti-GFP antibody. Numbers below the blot indicate relative abundances of GmBiP1. (b) Endogenous NbBiPs were stabilized by PsAvh262, but not by PsAvh262-M1 in N. benthamiana infected with P. capsici. N. benthamiana leaves expressing GFP, PsAvh262, PsAvh262-M1 or PsAvh262-M2 were inoculated with zoospore suspension of P. capsici, and the NbBiP levels were determined at 0, 3 and 24 h.p.i. Abundances of NbBiPs relative to GFP at 0 h were determined using an anti-BiP antibody. Numbers below the blots represent relative abundances of NbBiPs. Asterisks (*, ** or ***) denote significant differences (P<0.05, P<0.01 or P<0.001, respectively, one-way ANOVA, n=3) between samples. Similar results were observed in at least three independent experiments.
We next explored whether PsAvh262 influences the endogenous BiP levels in planta during Phytophthora infection. N. benthamiana leaves expressing GFP, PsAvh262, PsAvh262-M1 or PsAvh262-M2 were inoculated with zoospores of P. capsici, and the endogenous NbBiPs levels were determined at 0, 3 and 24 h.p.i. using anti-BiP antibodies. As shown in Fig. 7b, the accumulation of endogenous NbBiPs was enhanced during P. capsici infection; furthermore, NbBiP protein levels had an overall increase in leaves expressing PsAvh262 or PsAvh262-M2, but not PsAvh262-M1, at all three time points (Fig. 7b). These results support that PsAvh262 stabilizes BiPs during infection, and suggest that P. capsici may also produce effector(s) that play a similar role as PsAvh262.
Avh262 suppresses ER stress-associated cell death
In a previous study, overexpression of GmBiP4 in soybean attenuated N-rich protein (NRP)-mediated cell death signalling by inhibiting the induction of several components in the pathway, such as homologues of the vacuolar processing enzyme (VPE)40. Protein disulfide isomerase (PDI), an ER-resident chaperone involved in mediating ERQC40, was shown to be induced at both the RNA and protein levels by ER stress41. Thus PDI can serve as a marker of ER stress. We therefore examined the transcript levels of PDI and VPE during P. sojae infection to monitor the induction of ER stress and the induction of ER stress-triggered cell death, respectively. qRT–PCR results showed that PDI transcript levels were upregulated nearly two-fold (P<0.01, one-way ANOVA, n=3) within 1.5 h after infection by wild-type P. sojae (Fig. 6c), whereas during infection by the PsAvh262-silenced transformants, minimal changes were observed in the levels of PDI transcripts prior to 9 h.p.i. PDI induction was observed at 9 h.p.i., but the levels were slightly lower than in tissues infected with the wild-type strain (Fig. 6c). These results suggest that the higher virulence of the wild-type strain results in earlier induction of ER stress than in the less virulent-silenced lines. The levels of VPE transcripts were not significantly changed during wild-type infection, however VPE levels were significantly upregulated at 9 h.p.i. during infection by the PsAvh262-silenced transformants (Fig. 6d), at which time cell death symptoms were observed. These results suggest that the PsAvh262-silenced transformants were no longer able to attenuate cell death resulting from the ER stress triggered by infection, even though the level of ER stress imposed by the silenced lines was less than that imposed by the wild type.
BiPs regulate resistance and ER stress-triggered cell death
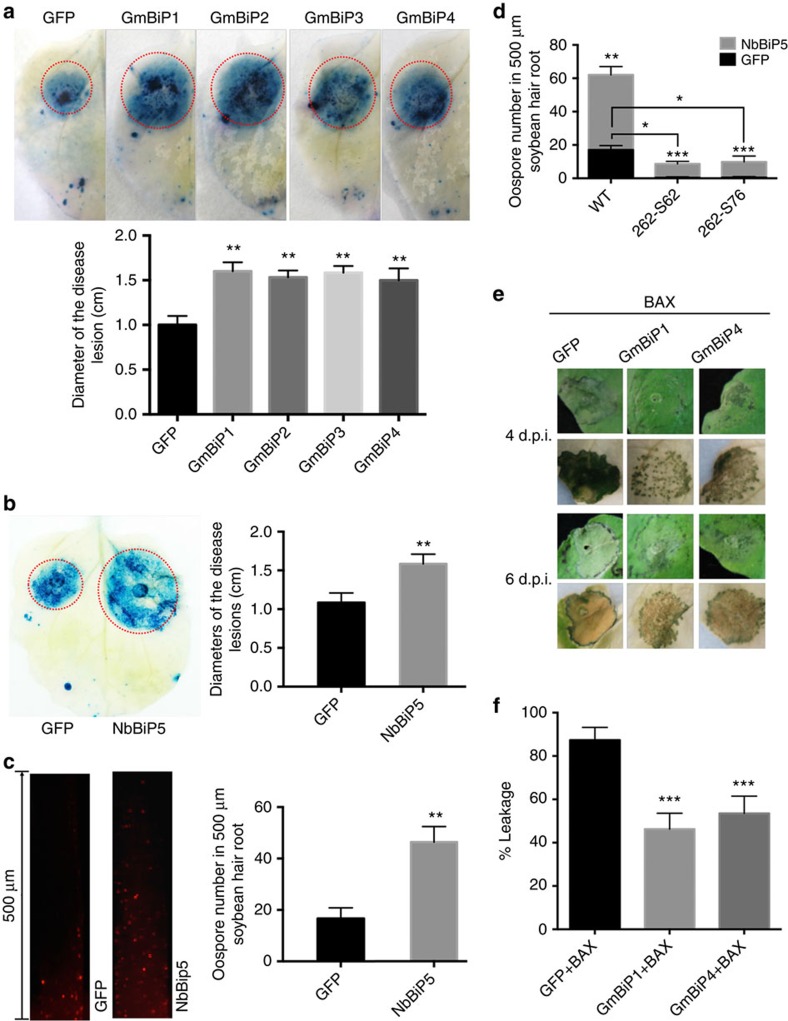
The influence of PsAvh262 on BiP levels during infection suggests that BiPs play a role in plant immunity. To test this, we transiently expressed GmBiPs and NbBiP5 (with GFP fused to the N terminus of the signal peptides) in N. benthamiana via agroinfiltration, and inoculated the leaves with P. capsici at 2 days post agroinfiltration. At 36 h.p.i., larger lesions were observed on leaves expressing each of the four GmBiPs (Fig. 8a) or NbBiP5 (Fig. 8b), indicating that increased accumulation of BiPs promoted P. capsici infection. Consistent with this result, overexpression of GFP-SP-NbBiP5-HDEL in soybean hairy roots also increased their susceptibility to P. sojae (Fig. 8c,d; Supplementary Fig. 9). In addition, the overexpression of GFP-SP-NbBiP5-HDEL could partly restore the susceptibility of the plants to PsAvh262-silenced P. sojae transformants. Taken together, our results suggest that PsAvh262-mediated virulence is affected through host BiPs (Fig. 8d; Supplementary Figs 9 and 10).
Figure 8. BiPs regulate plant resistance to Phytophthora and ER stress-triggered cell death in plants.
(a) Expression of GmBiPs in N. benthamiana led to increased susceptibility to P. capsici. Leaves transiently expressing GFP-GmBiPs (GFP was attached to the N terminus of the signal peptide in each case) or GFP were inoculated with mycelia of P. capsici. Disease lesions were visualized by Trypan blue staining of the inoculated leaves at 36 h.p.i. (upper panel) and the sizes of the lesions were statistically analysed (lower panel). (b) Expression of GFP-NbBiP5 (GFP was attached to the N terminus of the signal peptide of NbBiP5) in N. benthamiana led to increased susceptibility to P. capsici. Disease lesions were visualized by Trypan blue staining at 36 h.p.i. (left panel) and the sizes of the lesions were statistically analysed (right panel). (c) Expression of GFP-SP-NbBiP5-HDEL in soybean hairy roots enhanced the infection by P. sojae strain P6497-RFP. Increased numbers of oospores developed in NbBiP5-expressing roots at 48 h after inoculation as shown by microscopic images (left panel) and statistical analysis (right panel). (d) Overexpression of GFP-SP-NbBiP5-HDEL can partly restore the virulence of PsAvh262-silenced P. sojae transformants in soybean roots. Soybean hairy roots expressing NbBiP5 or GFP were inoculated by wild-type P. sojae or PsAvh262-silenced transformants (262-S62, 262-S76), respectively. Increased number of oospores was developed in roots expressing NbBiP5 at 48 h after infection by PsAvh262-silenced P. sojae transformants. (e) Expression of GmBiP1 or GmBiP4 attenuated cell death triggered by BAX in N. benthamiana. GFP-SP-GmBiP1-HDEL, GFP-SP-GmBiP4-HDEL or GFP were expressed in N. benthamiana through agroinfiltration. Twelve hours later, the leaves were infiltrated again with A. tumefaciens cells carrying HA tagged BAX. Leaves were bleached with ethanol to visualize the cell death symptoms. Immunoblots confirmed the expression of GFP-SP-GmBiP1-HDEL and GFP-SP-GmBiP4-HDEL in N. benthamiana (lower panel). (f) Quantification of electrolyte leakage in N. benthamiana leaves expressing BAX together with GFP-SP-GmBiP1-HDEL, GFP-SP-GmBiP4-HDEL or GFP, 4 days after agroinfiltration. Electrolyte leakage from the infiltrated leaf discs was measured as a percentage of leakage from boiled discs. Error bars represent the mean±s.d. (n=3), and asterisks denote significant differences (*P<0.05; **P<0.01; ***P<0.001, one-way ANOVA). These experiments were repeated three times with similar results.
Silencing of NbBiPs individually in N. benthamiana is difficult due to their highly conserved nucleotide sequences (Supplementary Fig. 11). It has been shown that silencing of NbBiPs caused developmental defects, including curling of leaves and the appearance of many pits on leaves42. However, the lower leaves in NbBiPs-silenced plants could still be used for pathogen inoculation as the developmental defects were only observed in the newly developing plant parts42. We silenced all NbBiPs by PVX-based virus-induced gene silencing (VIGS) to investigate their roles in plant defence during Phytophthora infection. The mRNA levels of NbBiPs were reduced by 80–90% in several independent VIGS plants (Supplementary Fig. 12a). The NbBiPs-silenced plants showed a cell death phenotype as previously described43 (Supplementary Fig. 12b). When infected with P. capsici, the sizes of disease lesions on NbBiPs-silenced leaves were significantly smaller than those on the non-silenced leaves (Supplementary Fig. 12c). Consistent with the smaller lesion size, a greatly reduced biomass of P. capsici was observed in NbBiPs-silenced leaves (Supplementary Fig. 12d). Taken together, these results suggest that BiPs are negative regulators of plant resistance to Phytophthora infection, though we cannot rule out that the physiological changes in the BiP-silenced plants indirectly resulted in resistance.
Since PsAvh262 suppresses BAX-triggered cell death and interacts with all four soybean BiPs (Fig. 3 and Supplementary Figs 1c; 6), we also examined the influence of NbBiP5, GmBiP1 and GmBiP4 on BAX-triggered cell death. ER stress is a component of the mechanism by which BAX triggers cell death44. Our results showed that these three proteins (expressed with GFP attached to the N terminus of the signal peptide) all significantly alleviated BAX-triggered cell death and electrolyte leakage compared with a GFP control when expressed in N. benthamiana (Fig. 8e,f; Supplementary Fig. 13). Thus, these results suggest that BiPs can negatively regulate cell death triggered by ER stress.
Discussion
Hemibiotrophic pathogens initially establish a biotrophic relationship with their hosts, but at later stages of the infection they kill the host cells. During the initial biotrophic phase, the pathogen feeds on viable host tissues for nutrition via haustoria10,45,46; thus, efficient mechanisms must be employed to suppress or evade host defenses at this stage, especially programmed cell death (PCD)47. To achieve this, Phytophthora pathogens deliver a large repertoire of effectors into host cells to suppress immune responses including PCD. PCD is a widespread mechanism for plants to cope with environmental stresses such as attacks by pathogens. Identifying the target host factors of these effectors and their functions will lead to detailed understanding of how infection is established. Using a combination of genetic, cellular and biochemical approaches, we established the P. sojae RxLR effector PsAvh262 as a critical virulence factor for infection in soybean. One function of PsAvh262 is to suppress cell death triggered by PAMPs and effectors. We identified BiPs from soybean as one of the major virulence targets of PsAvh262. PsAvh262 could also interact with N. benthamiana and rice homologues of GmBiPs. Our experiments showed that PsAvh262 stabilizes host BiPs from degradation by an MG132-sensitive mechanism, that is probably the ERAD pathway. Furthermore, overexpression of BiPs could suppress PCD triggered by BAX; BAX relies partly on ER-stress to trigger PCD. Finally, our results showed that PsAvh262-silenced P. sojae lines were ineffective in suppressing PCD associated with infection-induced ER stress. Even when the level of ER stress was reduced by the poor virulence of the PsAvh262-silenced P. sojae lines (as measured by PDI transcript levels; Fig. 6c), the silenced lines were ineffective in suppressing PCD (as measured by VPE transcript levels; Fig. 6d). Together, these results suggest that stabilization of BiPs by PsAvh262 acts to attenuate PCD triggered by infection-associated ER stress (Fig. 9).
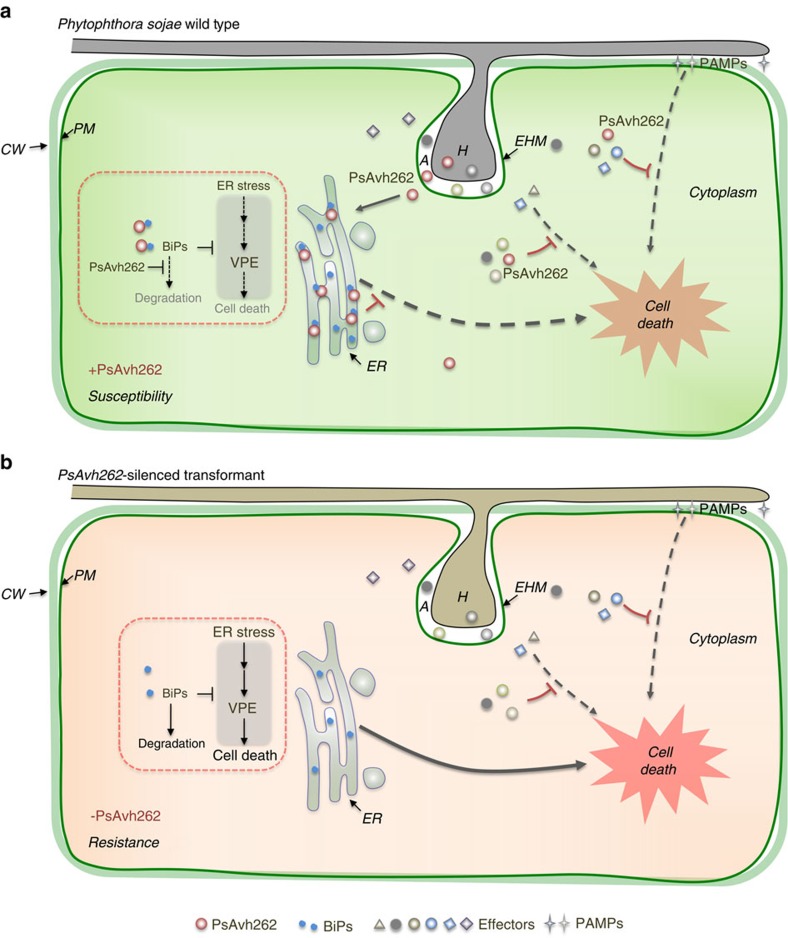
Figure 9. A schematic summary illustrating PsAvh262 suppression of ER stress-mediated immunity by stabilization of plant BiPs during infection.
During infection, P. sojae produces haustoria, through which effectors are secreted and translocated into host cells. In infected ‘haustoriated' cells, the host plasma membrane remains intact but is invaginated by the invading hyphae and becomes the extrahaustorial membrane (EHM). Phytophthora infection can activate the ER stress response in the host plants, which subsequently triggers cell death to block infection. (a) In the initial biotrophic phase, PsAvh262 associates and stabilizes host BiPs in the ER to attenuate the ER stress-triggered cell death. As such, PsAvh262 acts as an essential virulence factor to enhance P. sojae colonization and infection. Avh262 (along with many other RxLR effectors) can also suppress PAMP- and effector-triggered cell death, but it is unknown if this activity of Avh262 is mediated by the BiP interaction. (b) In the absence of PsAvh262, P. sojae is no longer able to attenuate the ER stress-induced cell death, which prevents infection. A, apoplast; CW, cell wall; EHM, extrahaustorial membrane; ER, endoplasmic reticulum; H, haustoria; PM, plasma membrane.
There is growing evidence that different forms of PCD may harm or benefit pathogens depending on the timing and nature of the cell death mechanism, and whether the pathogen has physiologically adapted to benefit from the dying tissue26. Among the various cellular stress responses that induce plant PCD, the ER stress response governs resistance to diverse environmental stresses43,48. BiPs play both broad and specific roles in regulating ER stress-triggered cell death41,49. For example, they act as moderators of PCD and master regulators of the UPR during ER stress27,42,50,51,52. Thus, attenuation of ER stress-triggered PCD by PsAvh262 through its interaction with BiPs represents a novel and effective counter-defence strategy by P. sojae. BiP-targeting is likely of broad significance, given that BiPs function in systemic acquired resistance (SAR)27, pathogen recognition receptor (PRR) biosynthesis31,53 and regulation of cell surface receptor-mediated innate immunity54. Silencing of BiPs in N. benthamiana resulted in delays in PCD induced by Xanthomonas oryzae pv. oryzae, but not by Pseudomonas syringae pv. tomato strain DC3000 (ref. 42). Silencing of ERD2a and/or ERD2b reduced accumulation of BiPs in the ER, resulting in increased sensitivity of plants to ER stress and exacerbation of PCD induced by non-host pathogens42. In contrast, Carvalho et al.41 found that soybean transgenic lines overexpressing GmBiP4 displayed enhanced or accelerated PCD induced by Pst DC300041. In Arabidopsis, transgenic plants overexpressing another ER chaperone, calreticulin 2 (CRT2), were more susceptible to Pst DC300055. Thus, the expression level of ER chaperones may increase or decrease plant susceptibility, depending on the pathogen involved. Our results, combined with the observations of these authors, highlight the ER stress response as a key battleground in the struggle between plants and pathogens to control plant cell death.
We found that PsAvh262 co-localizes with BiPs to the ER in uninfected plant cells, and also to the EHM during infection of N. benthamiana by P. infestans (Fig. 5). Co-localization of BiPs and PsAvh262 to the ER displayed some unexpected features. First, the localization of GmBiP1 to the ER was unaffected by fusion of GFP or RFP to the N terminus of its signal peptide, or to the C terminus of its HDEL ER retention signal (Fig. 4b,c). This suggests that the BiPs may reach the ER by a post-translational pathway56. Second, our BiFC results revealed that PsAvh262 could co-localize with BiP even when BiP was targeted directly to the ER by an unobstructed signal peptide (Fig. 4f,g). This observation suggests that even with an unobstructed signal peptide, BiP passes through the cytoplasm where it may interact with PsAvh262. In the absence of co-expressed GmBiP1, PsAvh262 appeared to display little localization to the ER (Fig. 4a). A small amount of localization to punctate structures was observed; this might represent localization to the ER mediated by endogenous BiPs, however the identity of the punctate structures was not confirmed in our study. Furthermore, the PsAvh262 M1 deletion mutant that does not interact with BiP did not colocalize to the ER (Fig. 4e). These results suggest that cytoplasmic PsAvh262 is dependent on BiP binding to localize to the ER. When PsAvh262 was targeted directly to the ER by the signal peptide of GmBiP1, it could also complex with GmBiP1 as judged by our BiFC results (Fig. 4f,g). Thus PsAvh262 may have the ability to find and bind to BiP whether it is in the cytoplasm or the ER.
The most commonly accepted idea about RxLR effector entry is that it involves endocytosis from the apoplast or extra-haustorial matrix into host cells2,3. If PsAvh262 enters via endocytosis and then is trafficked from the endosomes into the ER, then it would be in the appropriate compartment for interaction with ER-localized BiPs. On the other hand, if PsAvh262 was delivered directly into the host cell cytoplasm, either from endosomes or across the plasma membrane, then it could interact with BiPs in the cytoplasm and from there be targeted to the ER, as shown in Fig. 4. The targeting of PsAvh262 (and BiPs) to the EHM surrounding the pathogen during infection (Fig. 5) may also be relevant to its delivery pathway. PsAvh262 did not require co-expression with BiP to be targeted to the EHM. Thus PsAvh262 might be delivered directly to the EHM during infection, and might rendezvous with BiP at that location prior to delivery to the ER.
Several other oomycete RxLR effectors have been reported to localize to the ER in host cells including nine RxLR effectors from Hyaloperonospora arabidopsidis (Hpa)57 and the P. infestans RxLR effector Pi03192. In particular, Pi03192 interacts with NAC transcription factors in the ER and prevents re-localization of NAC transcription factors from the ER to the Nucleus58. It is unknown how those effectors reach the ER.
PsAvh262 may have additional functions than the binding of BiPs. A substantial fraction of PsAvh262 accumulated in plant nuclei and nucleoli. Furthermore, additional candidate targets were identified in the immuno-purification experiments that revealed the BiPs as targets (Supplementary Table 1). In a broader context, it is notable that many Gram-positive bacteria express surface proteins that bind host immunoglobulins or serum proteins59. As an example of this important function, Peptostreptococcus magnus expresses an albumin-binding surface protein that binds human serum albumin to promote bacterial growth and virulence30. Given that plants do not have immunoglobulins or albumins, our finding that PsAvh262 has a weak match to an IABD domain within the region corresponding to mutant M1 is of interest. It would be of interest to explore effectors containing potential IABD and investigate whether they target yet-to-be-discovered plant proteins to promote infection.
Methods
Plasmid construction
The PsAvh262 gene was cloned using complementary DNA from P. sojae. Full-length BiP genes were cloned from N. benthamiana, soybean cultivar Williams 82 and rice cDNAs using gene-specific primers containing at least 15 bp of a vector sequence available on each side of the cloning site (Supplementary Table 2). The amplified fragments were ligated into pBINGFP2 (a plasmid containing green fluorescent protein, GFP), pCAM1300-Flag and pCAM1300-RFP with the In-Fusion HD Cloning Kit (Clontech, Mountain View, CA, USA). PsAvh262 without a signal peptide and the PsAvh262 deletion mutants were amplified using combinations of primers (Supplementary Table 2). The amplicons were prepared using the appropriate restriction enzymes (Supplementary Table 2) and ligated into pBINGFP2, pCAM1300-Flag, pCAM1300-RFP, pEGX4T-2, PVX (pGR107) and pTOR. The RFP insert was ligated into SalI- and KpnI-digested pCAM1300 to yield pCAM1300-RFP. The fragments used to generate PsAvh262 deletion mutants 3 × Flag-Avh262, 3 × Flag-Avh262-M1, 3 × Flag-Avh262-M2 and 3 × Flag-Avh262-M3 were synthesized by Nanjing Genscript (Nanjing, China) and ligated into pBINGFP2 and pCAM1300 with the In-Fusion HD Cloning Kit. Individual colonies for each construct were tested for inserts by PCR, and selected clones were verified by sequencing.
A. tumefaciens infiltration assays and plant growth
Constructs were introduced into Agrobacterium tumefaciens (A. tumefaciens) strain GV3101 by electroporation. The transformed bacterial cells were grown on LB agar plates supplemented with appropriate antibiotics. Individual colonies were verified by PCR using both vector- and gene-specific primers. For agroinfiltration assays, recombinant A. tumefaciens strains were grown at 28 °C in a shaking incubator at 200 r.p.m. After 36–48 h, bacterial cells were spun down by centrifugation, and resuspended in MES buffer (10 mM MgCl2 and 1 mM MES, pH 5.6). The resuspended A. tumefaciens cells were diluted and mixed with P19 silencing suppressor60 in a 1:1 or 1:1:1 ratio (depending on the number of constructs) to a final OD600=0.3 for each construct. For stabilization assays, MG132 or DMSO (a control) was mixed or individually infiltrated into plant leaves after the agroinfiltration for 24 h. N. benthamiana plants were grown in a greenhouse for 4–6 weeks at 25 or 20 °C under a 16 h/8 h day/night photoperiod. For VIGS, the two largest leaves of four-leaf-stage N. benthamiana plants were pressure-infiltrated with A. tumefaciens strain GV3101 containing either the VIGS construct PVX-NbBiPs or the PVX-GFP control with a concentration of OD600=1.0. Three weeks after agroinfiltration, plants were used for infection assays or for analysis of gene silencing efficiency using qRT–PCR.
Immunopurification of transiently expressed proteins
Leaves of 4–6-week-old N. benthamiana plants were agroinfiltrated with pBINGFP2-PsAvh262 (and pBINGFP2 as the control) and the P19 silencing suppressor in a 1:1 ratio at a final OD600=0.3 for each construct. Two days after agroinfiltration, the leaves were frozen in liquid nitrogen and ground to a fine powder using a mortar and pestle. Proteins were extracted using lysis buffer (10 mM Tris-Cl (pH 7.5), 150 mM NaCl, 0.5 mM ethylenediaminetetraacetic acid (EDTA), 0.5% NP-40) plus 1 mM phenylmethylsulfonyl fluoride and a protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA) by mixing 1 g leaf tissue with 2 ml of lysis buffer. The samples were centrifuged at 4 °C for 15 min at 14,000g and the supernatant was transferred to a new tube. For mass spectrometry analysis of proteins co-purified with the PsAvh262 proteins, 5 ml of total protein extract was incubated at 4 °C for 3 h with 30 μl of GFP-Trap_A beads (Chromotek, ABIN509397, Planegg-Martinsried, Germany). For the other immunopurification experiments, 2 ml of total protein extract was incubated at 4 °C for 2 h with 20 μl of GFP-Trap_A beads. The beads were then collected by centrifugation at 2,500g and washed five times in 1 ml of washing buffer (10 mM Tris-Cl (pH 7.5), 150 mM NaCl and 0.5 mM EDTA). Bound proteins were eluted by adding 50 μl of 0.2 M glycine (pH=2.5) for 30 s with constant mixing, followed by centrifugation. The supernatant was transferred to a fresh tube, and 5 μl of 1 M Tris base (pH=10.4) was added for neutralization. To increase elution efficiency, this step was repeated. The resuspended beads were boiled for 10 min at 95 °C to dissociate the immunocomplexes from the beads. The beads were then collected by centrifugation at 2,500g for 2 min at 4 °C and SDS–PAGE (SDS–polyacrylamide gel electrophoresis) was performed with the supernatant.
GST pull-down
PsAvh262 was inserted into the vector pGEX4T-2 (GE Healthcare Life Science, Little Chalfont, Buckinghamshire, UK) and expressed in E. coli strain BL21. Soluble total proteins (GST-Avh262) were incubated with 50 μl glutathione-agarose beads (GE Healthcare Life Science) at 4 °C for 2 h, supernatant was removed and the beads were washed three times with ice-cold phosphate-buffered saline (PBS) buffer. Next, GmBiP1-RFP protein extracted from GmBiP1-RFP-expressing N. benthamiana plants was added to the above beads for the incubation at 4 °C for 2 h. After six times of washing with 1 × PBST, the SDS sample loading buffer was added to beads and heated to 100 °C for 5 min for the immunoblot analysis.
Western blotting
Proteins from the sample lysate were fractionated by SDS–PAGE. The separated proteins were transferred from the gel to an Immobilon-PSQ polyvinylidene difluoride membrane (pretreated with methanol for 15 s; Millipore) using a transfer buffer (20 mM Tris, 150 mM glycine). The membrane was then blocked using PBS(pH 7.4) containing 3% non-fat dry milk for 30 min at room temperature with 50 r.p.m. shaking, followed by one wash with PBST (PBS with 0.1% Tween 20). Anti-Flag (1:2,000; #F3165; Sigma-Aldrich) and anti-BiP (1:1,000; #sc-33757; Santa Cruz Biotechnology), anti-GFP (1:2,000; #M20004; Abmart), anti-RFP (1:1,000; #5f8; Chromotek), anti-HA (1:2,000; #M20003; Abmart), anti-myc (1:2,000; #M20002; Abmart) and anti-actin (1:2,000; #M20009; Abmart) antibodies were added to PBSTM (PBS with 0.1% Tween 20 and 3% non-fat dry milk) and incubated at room temperature for 90 min, followed by three washes (5 min each) with PBST. The membrane was then incubated with a goat anti-mouse IRDye 800CW antibody (Odyssey, no. 926-32210; Li-Cor) at a ratio of 1:10,000 in PBSTM at room temperature for 30 min with 50 r.p.m. shaking. The membrane was washed three times (5 min each) with PBST, once for 5 min with PBS, and then visualized by excitation at 780 and 800 nm. Full-size images are presented in Supplementary Fig. 14.
P. sojae transformation and characterization
P. sojae strain P6497 (race 2) was routinely grown and maintained on V8 agar. Stable transformation was performed as the following protocol12. Two or three-day-old mycelia, cultured in pea broth medium, were washed in 0.8 M mannitol, then placed in enzyme solution (0.4 M mannitol, 20 mM KCl, 20 mM MES, pH 5.7, 10 mM CaCl2, 10 mg ml−1 β-1.3 glucanase and 5 mg ml−1 cellulysin, and incubated for 35–45 min at 25 °C with ∼100 r.p.m. shaking. The protoplasts were harvested by centrifugation at 1,500 r.p.m. for 3–5 min and resuspended in W5 solution (5 mM KCl, 125 mM CaCl2, 154 mM NaCl and 31 mg ml−1 glucose) at a concentration of 2 × 106 protoplasts per ml or higher. After 30 min, the protoplasts were centrifuged at 1,200g for 5 min and resuspended in an equal volume of MMg solution (0.4 M mannitol, 15 mM MgCl2 and 4 mM MES, pH 5.7) to allow protoplasts to swell. To each of 1 ml MMg solution, 25 μg transforming DNA was added and incubated for 10 min on ice. Then, three aliquots of 580 ml each of freshly made polyethylene glycol solution (40% (v/v) polyethylene glycol 4,000, 0.3 M mannitol and 0.15 M CaCl2) were slowly pipetted into the protoplast suspension and gently mixed. After 20 min incubation on ice, 10 ml pea broth containing 0.5 M mannitol was added, and the protoplasts were incubated overnight to regenerate. The regenerated protoplasts were suspended in liquid pea agar containing 0.5 M mannitol and 25 μg ml−1 G418 and plated. The visible colonies could be observed after 2–3 days incubation at 25 °C. All transformants were propagated on V8 agar with 50 μg ml−1 G418 at 25 °C.
For DNA or RNA extraction, mycelia of P. sojae transformants were cultured in V8 liquid, harvested and ground into a powder in liquid nitrogen. Genomic DNA was isolated from mycelia using the Multisource Genomic DNA miniprep kit (Axygen, Corning, NY, USA) following the manufacturer's protocol. To screen PsAvh262-silenced transformants, total RNA was extracted from the mycelia and used to generate cDNA. The resultant cDNA was used as template in the quantitative real-time PCR assays to determine the expression levels of PsAvh262.
The virulence of P. sojae transformants was determined by inoculation of etiolated soybean seedlings (Chinese susceptible cv. HF47). Stationary mycelial cultures were grown in liquid V8 broth in 90 mm Petri dishes at 25 °C in the dark for 2 days. The hyphae were repeatedly washed with sterile distilled water and then incubated in the dark at 25 °C for 4 to 8 h until sporangia developed on most of the hyphae and zoospores were released. Soybean seedlings were grown in the dark for 4 days at 25 °C (16 h per day) and 16 °C (8 h per day). Hypocotyls of etiolated soybean seedlings (9–12 per assay) were inoculated 2–3 cm from the base of the cotyledon with 200 zoospores and then incubated in the dark at 25 °C. Tissue samples from five seedlings were pooled and used to quantify oospores.
Analysis of transcription profiles
Transcription data of the four GmBiP genes were obtained from RNA-seq for infected soybean roots (cultivar: Williams 82) by P. sojae (P6497) zoospores (NCBI Sequence Read Archive (SRA) accession: SRP073278). Except 0 h.p.i., using only soybean roots, the others (0.5, 3, 6 and 12 h.p.i.) were mixed samples of soybean roots and P. sojae. RNA was extracted using the EZNA total RNA kit I (Omega Bio-Tek, USA) and used to make RNA-seq libraries with the Illumina TruSeq RNA Sample Preparation Kit v2 following the manufacturer's recommendations. These libraries were sequenced using Illumina HiSeq 2000 in paired-end mode with a read length of 90 bp. The sequence reads were mapped to the soybean Williams 82 genome (Phytozome database, https://phytozome.jgi.doe.gov/; Glycine max Wm82.a2.v1), using TopHat 2.1.0 (http://ccb.jhu.edu/software/tophat/index.shtml). The gene expression levels were calculated using Cufflinks 2.2.1 (http://cole-trapnell-lab.github.io/cufflinks).
SYBR green qRT–PCR
The RNA was quantified using a spectrophotometer (ND-1000; NanoDrop, Wilmington, DE, USA). To remove contaminating genomic DNA in the RNA samples, 3 μg of total RNA was treated with two units of RNase-free DNase I (Takara Bio Inc., Otsu, Japan) at 37 °C for 30 min. First-strand cDNA was synthesized using Moloney murine leukemia virus reverse transcriptase (RNase-free) and an oligo (dT) 18 primer (Invitrogen, Carlsbad, CA, USA). qPCR was performed in 20 μl reactions containing 20 ng of cDNA, 0.2 mM gene-specific primer or the reference actin gene, 10 μl of SYBR Premix ExTaq (TaKaRa) and 6.8 μl of deionized water. PCR was performed on an ABI Prism 7500 Fast Real-Time PCR System (Applied Biosystems Inc., Foster City, CA, USA) under the following conditions: 95 °C for 30 s and 40 cycles at 95 °C for 5 s and 60 °C for 34 s, followed by a dissociation step, that is, 95 °C for 15 s, 60 °C for 1 min and 95 °C for 15 s.
Phytophthora infection assays in N. benthamiana
N. benthamiana leaves were collected 36 or 48 h after agroinfiltration and maintained on half-strength Murashige and Skoog (MS) medium in a Petri dish. Infiltrated regions were inoculated with 2.5% V8 juice agar plugs (diameter=0.5 cm) infected with fresh P. capsici LT263 mycelia as previously described61. For assays of P. capsici virulence in NbBiP5-silenced and non-silenced leaves, zoospores were used as inoculum and prepared as follows. Mycelia of P. capsici were grown on 20% V8 juice agar in 9-cm disposable Petri dishes for 2 days at 25 °C. The mycelia were rinsed twice with sterile distilled water, flooded with sterile distilled water to cover the mycelia, and then kept overnight at 25 °C to release zoospores. Zoospore concentration was determined by counting the number of zoospores under a microscope. N. benthamiana leaves were inoculated with 1,500 zoospores and incubated in a sealed Petri dish for 36 h at 25 °C in the dark. After that, the inoculated leaves were stained by Trypan blue as previously described62. Stained leaves were photographed and the diameters of the lesions were photographed and measured. Total DNA was extracted from P. capsici-infected regions (diameter=4 cm) was isolated at 36 h.p.i. and used for real-time PCR to quantify the ratio of host-to-pathogen biomass, using primers specific for N. benthamiana and P. capsici actin genes (Supplementary Table 2). Three independent biological replicates were included. The P. infestans strain 88069td or T30-4 was inoculated in the leave of N. benthamiana for the microscopical observation as previously described13.
Transformation of soybean and P. sojae infection assays
Soybean cotyledons were inoculated with A. rhizogenes K599 carrying pBINGFP2, pBINGFP2-PsAvh262, or pBINGFP2-NbBiP5 (ref. 63). Individual cotyledons were collected from 10-day-old soybean seedlings. Detached cotyledons were surface-sterilized with 70% ethanol before a small roughly circular (diameter=∼0.4 cm) cut was made in each cotyledon ∼0.3 cm from the petiole end. The wounded cotyledons were then transferred to a sterile Petri plate containing 0.8% agar. A. rhizogenes cells grown in LB medium supplemented with kanamycin were washed and resuspended in 10 mM MgCl2 to a final concentration of OD600=0.3. Twenty microlitres of the cell suspension was directly spotted onto the wound site of each cotyledon. The inoculated cotyledons were incubated in a growth chamber at 22 °C under high humidity with a 16 h/8 h light/dark regime. Hairy roots usually started to generate from the wound sites at ∼3 weeks post inoculation. GFP-tagged PsAvh262 and NbBiP5 or GFP were expressed under the control of the CaMV 35S promoter. Fluorescence microscopy was used to select GFP-, GFP-PsAvh262 or GFP-NbBiP5 expressing roots. Green fluorescence was detected in hairy roots using a fluorescence stereomicroscope (Leica MZ FLIII, Wetzlar, Germany) with a GFP2 filter (excitation 480/40 nm, emission 510 nm). Expression of GFP-PsAvh262, or GFP-NbBiP5 proteins in hair roots was confirmed by western blotting. Four-week-old hairy roots were infected by P. sojae-RFP mycelia, and the number of oospores was determined by microscopy at 48 h.p.i.
Electrolyte leakage assay
Cell death was assayed by measuring ion leakage from leaf discs61. For each sample, five leaf discs (1 cm diameter) were floated on 5 ml distilled water for 3 h at room temperature. Then the conductivity of the bathing solution was measured with a conductivity metre (Con 700; Consort, Tutnhout, Belgium) to give ‘value A'. The leaf discs were then returned to the bathing solution and boiled in sealed tubes for 25 min. After cooling the solution to room temperature, the conductivity was measured again to obtain ‘value B'. For each measurement, ion leakage was expressed as per cent leakage, that is (value A/value B × 100). All assays were repeated three times.
Confocal microscopy
Patches of agroinfiltrated N. benthamiana leaves were cut and mounted in water and analysed using an LSM 710 laser scanning microscope with a × 20, × 40 or × 60 objective lens (Carl Zeiss, Jena, Germany). GFP or RFP fluorescence was observed at excitations of 488 or 561 nm, respectively. Bright field in soybean epidermal cells in Supplementary Fig. 1b was detected using a × 20 objective lens (IX71; Olympus, Tokyo, Japan).
Protein structure analysis
Protein secondary structures and protein surface accessibility were predicted using NetSurfP 1.1 (http://www.cbs.dtu.dk/services/NetSurfP). The protein secondary structure class (alpha-helix, beta-strand and coil) predicted to have the greatest probability was considered as the result. Surface accessibility results were obtained directly from the default output. The putative IABD domain was identified using Phyre2 (http://www.sbg.bio.ic.ac.uk/phyre2)29.
Data availability
The sequences data (PsAvh262, GmBiPs and NbBiP5) that support the finding of this study have been deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank/), Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html) and Sol Genomics Network (http://solgenomics.net/), respectively. The primary accession codes are JN254253.1 (PsAvh262, GenBank); Glyma08g02940 (GmBiP1), Glyma08g02960 (GmBiP2), Glyma05g36600 (GmBiP3) and Glyma05g36620 (GmBiP4) in Phytozome; NbS00040865g0006.1 (NbBiP5, Sol Genomics Network). RNA-seq data described in this study has been deposited in the NCBI sequence read archive under accession code SRP073278. The authors declare that all other data supporting the findings of this study are available from the corresponding author on request.
Additional information
How to cite this article: Jing, M. et al. A Phytophthora sojae effector suppresses endoplasmic reticulum stress-mediated immunity by stabilizing plant Binding immunoglobulin Proteins. Nat. Commun. 7:11685 doi: 10.1038/ncomms11685 (2016).
Supplementary Material
Supplementary Figures 1-14 and Supplementary Tables 1-2
Acknowledgments
We thank Dr Biao Ding (Ohio State University, USA) for editing the article. We thank Drs Zhengguang Zhang and Hongwei Zhao (Nanjing Agricultural University, China) for helpful suggestions. Dr Sophien Kamoun (The Sainsbury Laboratory, UK) kindly provided P. infestans strain 88069td, RFP-Avrblb2 and GFP-Avrblb2 vectors. Drs Wenhua Zhang and Daolong Dou (Nanjing Agricultural University, China) provided the vectors pCAM1300 and pBINGFP2, respectively. This work is supported by the key program of National Natural Science Foundation Grant No. 31430073 and National Science Fund for Distinguished Young Scholars Grant No. 31225022 of China to Y.-C.W. from National Natural Science Foundation.
Footnotes
Author contributions Y.-C.W., W.M., B.T. and M.-F.J. designed the research; M.-F.J., B.-D.G., H.-Y.L., B.Y., H.-N.W. G.-H.K and H.-W.X performed the research; S.-M.D., Y.W., W.-W.Y. and Y.-L.Q. contributed new tools; Y.-C.W., M.-F.J., B.T., S.-M.D. and B.-D.G. analysed the data; M.-F.J., W.M., B.T. and Y.-C.W. wrote the paper; all authors commented on the article before submission.
References
- Jones J. D. G. & Dangl J. L. The plant immune system. Nature 444, 323–329 (2006). [DOI] [PubMed] [Google Scholar]
- Kale S. D. et al. External lipid PI3P mediates entry of eukaryotic pathogen effectors into plant and animal host cells. Cell 142, 284–295 (2010). [DOI] [PubMed] [Google Scholar]
- Dou D. et al. RXLR-mediated entry of Phytophthora sojae effector Avr1b into soybean cells does not require pathogen-encoded machinery. Plant Cell 20, 1930–1947 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petre B. & Kamoun S. How do filamentous pathogens deliver effector proteins into plant cells. PLoS Biol. 12, e1001801 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou D. & Zhou J.-M. Phytopathogen effectors subverting host immunity: different foes, similar battleground. Cell Host Microbe 12, 484–495 (2012). [DOI] [PubMed] [Google Scholar]
- Wawra S. et al. Secretion, delivery and function of oomycete effector proteins. Curr. Opin. Microbiol. 15, 685–691 (2012). [DOI] [PubMed] [Google Scholar]
- Jiang R. H. Y., Tripathy S., Govers F. & Tyler B. M. RXLR effector reservoir in two Phytophthora species is dominated by a single rapidly evolving superfamily with more than 700 members. Proc. Natl Acad. Sci. USA 105, 4874–4879 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B. M. et al. Phytophthora genome sequences uncover evolutionary origins and mechanisms of pathogenesis. Science 313, 1261–1266 (2006). [DOI] [PubMed] [Google Scholar]
- Haas B. J. et al. Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature 461, 393–398 (2009). [DOI] [PubMed] [Google Scholar]
- Baxter L. et al. Signatures of adaptation to obligate biotrophy in the Hyaloperonospora arabidopsidis genome. Science 330, 1549–1551 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamour K. H. et al. Genome sequencing and mapping reveal loss of heterozygosity as a mechanism for rapid adaptation in the vegetable pathogen Phytophthora capsici. Mol. Plant Microbe Interact. 25, 1350–1360 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. et al. Transcriptional programming and functional interactions within the Phytophthora sojae RXLR effector repertoire. Plant Cell 23, 2064–2086 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt T. O. et al. Phytophthora infestans effector AVRblb2 prevents secretion of a plant immune protease at the haustorial interface. Proc. Natl Acad. Sci. USA 108, 20832–20837 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S. et al. Phytophthora sojae avirulence effector Avr3b is a secreted NADH and ADP-ribose pyrophosphorylase that modulates plant immunity. PLoS Pathog. 7, e1002353 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y. L. et al. Oomycete pathogens encode RNA silencing suppressors. Nat. Genet. 45, 330–333 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I., Xu W. & Reed J. C. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 7, 1013–1030 (2008). [DOI] [PubMed] [Google Scholar]
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell. Biol. 13, 89–102 (2012). [DOI] [PubMed] [Google Scholar]
- Ellgaard L. & Helenius A. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 4, 181–191 (2003). [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S. & Weissman A. M. Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu. Rev. Cell Dev. Biol. 14, 19–57 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo Y. ER quality control of immune receptors and regulators in plants. Cell Microbiol. 12, 716–724 (2010). [DOI] [PubMed] [Google Scholar]
- Bertolotti A., Zhang Y., Hendershot L. M., Harding H. P. & Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2, 326–332 (2000). [DOI] [PubMed] [Google Scholar]
- Shen J., Chen X., Hendershot L. & Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 3, 99–111 (2002). [DOI] [PubMed] [Google Scholar]
- Qiang X., Zechmann B., Reitz M. U., Kogel K. H. & Schafer P. The mutualistic fungus Piriformospora indica colonizes Arabidopsis roots by inducing an endoplasmic reticulum stress-triggered caspase-dependent cell death. Plant Cell 24, 794–809 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. et al. When supply does not meet demand-ER stress and plant programmed cell death. Front. Plant Sci. 5, 211 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente M. A. et al. The ER luminal binding protein (BiP) mediates an increase in drought tolerance in soybean and delays drought-induced leaf senescence in soybean and tobacco. J. Exp. Bot. 60, 533–546 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbage M., Williams B. & Dickman M. B. Cell death control: the interplay of apoptosis and autophagy in the pathogenicity of Sclerotinia sclerotiorum. PLoS Pathog. 9, e1003287 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Weaver N. D., Kesarwani M. & Dong X. N. Induction of protein secretory pathway is required for systemic acquired resistance. Science 308, 1036–1040 (2005). [DOI] [PubMed] [Google Scholar]
- Ye C., Dickman M. B., Whitham S. A., Payton M. & Verchot J. The unfolded protein response is triggered by a plant viral movement protein. Plant Physiol. 156, 741–755 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley L. A., Mezulis S., Yates C. M., Wass M. N. & Sternberg M. J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chateau M., Holst E. & Bjorck L. Protein PAB, an albumin-binding bacterial surface protein promoting growth and virulence. J. Biol. Chem. 271, 26609–26615 (1996). [DOI] [PubMed] [Google Scholar]
- Liebrand T. W. H. et al. Endoplasmic reticulum-quality control chaperones facilitate the biogenesis of Cf receptor-like proteins involved in pathogen resistance of tomato. Plant Physiol. 159, 1819–1833 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascardo J. C. et al. The phosphorylation state and expression of soybean BiP isoforms are differentially regulated following abiotic stresses. J. Biol. Chem. 275, 14494–14500 (2000). [DOI] [PubMed] [Google Scholar]
- Cheong M. S. et al. AvrBsT acetylates Arabidopsis ACIP1, a protein that associates with microtubules and is required for immunity. PLoS Pathog. 10, e1003952 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas I. G. & Wabl M. Immunoglobulin heavy chain binding protein. Nature 306, 387–389 (1983). [DOI] [PubMed] [Google Scholar]
- Hendershot L. M. The ER chaperone BiP is a master regulator of ER function. Mt Sinai J. Med. 71, 289–297 (2004). [PubMed] [Google Scholar]
- Hendershot L. M., Valentine V. A., Lee A. S., Morris S. W. & Shapiro D. N. Localization of the gene encoding human Bip/Grp78, the endoplasmic-reticulum cognate of the Hsp70 family, to chromosome-9q34. Genomics 20, 281–284 (1994). [DOI] [PubMed] [Google Scholar]
- Bozkurt T. O. et al. The plant membrane-associated REMORIN1.3 accumulates in discrete perihaustorial domains and enhances susceptibility to Phytophthora infestans. Plant Physiol. 165, 1005–1018 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Yamada K., Shimada T. & Hara-Nishimura I. Endoplasmic reticulum-resident proteins are constitutively transported to vacuoles for degradation. Plant J. 39, 393–402 (2004). [DOI] [PubMed] [Google Scholar]
- Smith M. H., Ploegh H. L. & Weissman J. S. Road to ruin: targeting proteins for degradation in the endoplasmic reticulum. Science 334, 1086–1090 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura I., Hatsugai N., Nakaune S., Kuroyanagi M. & Nishimura M. Vacuolar processing enzyme: an executor of plant cell death. Curr. Opin. Plant. Biol. 8, 404–408 (2005). [DOI] [PubMed] [Google Scholar]
- Carvalho H. H. et al. The endoplasmic reticulum binding protein BiP displays dual function in modulating cell death events. Plant Physiol. 164, 654–670 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G. et al. Plant ERD2-like proteins function as endoplasmic reticulum luminal protein receptors and participate in programmed cell death during innate immunity. Plant J. 72, 57–69 (2012). [DOI] [PubMed] [Google Scholar]
- Cai Y.-M., Yu J. & Gallois P. Endoplasmic reticulum stress-induced PCD and caspase-like activities involved. Front. Plant Sci. 5, 41 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong W. X. et al. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J. Cell Biol. 162, 59–69 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendgen K. & Hahn M. Plant infection and the establishment of fungal biotrophy. Trends. Plant Sci. 7, 352–356 (2002). [DOI] [PubMed] [Google Scholar]
- Duplessis S. et al. Obligate biotrophy features unraveled by the genomic analysis of rust fungi. Proc. Natl Acad. Sci. USA 108, 9166–9171 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horbach R., Navarro-Quesada A. R., Knogge W. & Deising H. B. When and how to kill a plant cell: infection strategies of plant pathogenic fungi. J. Plant Physiol. 168, 51–62 (2011). [DOI] [PubMed] [Google Scholar]
- Moreno A. A. et al. IRE1/bZIP60-mediated unfolded protein response plays distinct roles in plant immunity and abiotic stress responses. PLoS ONE 7, e31944 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis P. A. & Fontes E. P. N-rich protein (NRP)-mediated cell death signaling: a new branch of the ER stress response with implications for plant biotechnology. Plant Signal Behav. 7, 628–632 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Narendra S. & Fedoroff N. Heterotrimeric G protein signaling in the Arabidopsis unfolded protein response. Proc. Natl Acad. Sci. USA 104, 3817–3822 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis P. A. A. et al. The binding protein BiP attenuates stress-induced cell death in soybean via modulation of the N-rich protein-mediated signaling pathway. Plant Physiol. 157, 1853–1865 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R., Deng Y., Shah S., Rao A. G. & Howell S. H. BINDING PROTEIN is a master regulator of the endoplasmic reticulum stress sensor/transducer bZIP28 in Arabidopsis. Plant Cell 25, 1416–1429 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekrasov V. et al. Control of the pattern-recognition receptor EFR by an ER protein complex in plant immunity. EMBO J. 28, 3428–3438 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.-J. et al. Overexpression of the endoplasmic reticulum chaperone BiP3 regulates XA21-mediated innate immunity in rice. PLoS ONE 5, e9262 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Xi J., Du L., Roje S. & Poovaiah B. W. A dual regulatory role of Arabidopsis calreticulin-2 in plant innate immunity. Plant J. 69, 489–500 (2012). [DOI] [PubMed] [Google Scholar]
- Johnson N. et al. The signal sequence influences post-translational ER translocation at distinct stages. PLoS ONE 8, e75394 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillaud M. C. et al. Subcellular localization of the Hpa RxLR effector repertoire identifies a tonoplast-associated protein HaRxL17 that confers enhanced plant susceptibility. Plant J. 69, 252–265 (2012). [DOI] [PubMed] [Google Scholar]
- McLellan H. et al. An RxLR effector from Phytophthora infestans prevents re-localisation of two plant NAC transcription factors from the endoplasmic reticulum to the nucleus. PLoS Pathog. 9, e1003670 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarre W. W. & Schneewind O. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63, 174–229 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet O., Rivas S., Mestre P. & Baulcombe D. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 33, 949–956 (2003). [DOI] [PubMed] [Google Scholar]
- Yu X. et al. The RxLR effector Avh241 from Phytophthora sojae requires plasma membrane localization to induce plant cell death. New Phytol. 196, 247–260 (2012). [DOI] [PubMed] [Google Scholar]
- Dong S., Zhang Z., Zheng X. & Wang Y. Mammalian pro-apoptotic bax gene enhances tobacco resistance to pathogens. Plant Cell Rep. 27, 1559–1569 (2008). [DOI] [PubMed] [Google Scholar]
- Kereszt A. et al. Agrobacterium rhizogenes-mediated transformation of soybean to study root biology. Nat. Protoc. 2, 948–952 (2007). [DOI] [PubMed] [Google Scholar]
- King S. R. et al. Phytophthora infestans RXLR effector PexRD2 interacts with host MAPKKK epsilon to suppress plant immune signaling. Plant Cell 26, 1345–1359 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-14 and Supplementary Tables 1-2
Data Availability Statement
The sequences data (PsAvh262, GmBiPs and NbBiP5) that support the finding of this study have been deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank/), Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html) and Sol Genomics Network (http://solgenomics.net/), respectively. The primary accession codes are JN254253.1 (PsAvh262, GenBank); Glyma08g02940 (GmBiP1), Glyma08g02960 (GmBiP2), Glyma05g36600 (GmBiP3) and Glyma05g36620 (GmBiP4) in Phytozome; NbS00040865g0006.1 (NbBiP5, Sol Genomics Network). RNA-seq data described in this study has been deposited in the NCBI sequence read archive under accession code SRP073278. The authors declare that all other data supporting the findings of this study are available from the corresponding author on request.