This is the first study investigating changes in endothelium-dependent dilation in parenchymal arterioles from a model of chronic cerebral hypoperfusion. Our findings correlate changes in the dilatory pathways of arterioles with cognitive deficits observed after chronic cerebral hypoperfusion.
Keywords: cerebral microcirculation, vascular remodeling, endothelium-dependent dilation, parenchymal arterioles, posterior communicating artery, epoxyeicosatrienoic acid
Abstract
Chronic cerebral hypoperfusion is a risk factor for cognitive impairment. Reduced blood flow through the common carotid arteries induced by bilateral carotid artery stenosis (BCAS) is a physiologically relevant model of chronic cerebral hypoperfusion. We hypothesized that BCAS in 20-wk-old Wistar-Kyoto (WKY) rats would impair cognitive function and lead to reduced endothelium-dependent dilation and outward remodeling in the parenchymal arterioles (PAs). After 8 wk of BCAS, both short-term memory and spatial discrimination abilities were impaired. In vivo assessment of cerebrovascular reserve capacity showed a severe impairment after BCAS. PA endothelial function and structure were assessed by pressure myography. BCAS impaired endothelial function in PAs, as evidenced by reduced dilation to carbachol. Addition of nitric oxide synthase and cyclooxygenase inhibitors did not change carbachol-mediated dilation in either group. Inhibiting CYP epoxygenase, the enzyme that produces epoxyeicosatrienoic acid (EETs), a key determinant of endothelium-derived hyperpolarizing factor (EDHF)-mediated dilation, abolished dilation in PAs from Sham rats, but had no effect in PAs from BCAS rats. Expression of TRPV4 channels, a target for EETs, was decreased and maximal dilation to a TRPV4 agonist was attenuated after BCAS. Together these data suggest that EET-mediated dilation is impaired in PAs after BCAS. Thus impaired endothelium-dependent dilation in the PAs may be one of the contributing factors to the cognitive impairment observed after BCAS.
NEW & NOTEWORTHY
This is the first study investigating changes in endothelium-dependent dilation in parenchymal arterioles from a model of chronic cerebral hypoperfusion. Our findings correlate changes in the dilatory pathways of arterioles with cognitive deficits observed after chronic cerebral hypoperfusion.
chronic cerebral hypoperfusion has been implicated in dementias ranging from vascular cognitive impairment (20) to Alzheimer's disease (1, 12). Animal models of chronic cerebral hypoperfusion exhibit neuronal loss, white matter lesions, and increased levels of reactive astrocytes (10, 28, 29). Few studies have investigated the effect of chronic cerebral hypoperfusion on the parenchymal arterioles (PAs) (27, 49). However, none have reported changes in endothelial function in PAs or studied the effects of prolonged hypoperfusion.
PAs arise from the pial arteries, perfuse the parenchyma, and eventually branch into the capillaries. These capillaries are in intimate contact with neurons, astrocytes, and pericytes and together these cell types form the neurovascular unit. Maintaining a constant and controlled flow of blood to the brain parenchyma is important for its homeostasis. PAs act as a bottleneck to parenchymal perfusion, and are responsible for modulating nutrient and oxygen delivery to the neurovascular unit (38). Despite studies that suggest that PA dysfunction can cause and exacerbate cerebrovascular disorders, a knowledge gap exists regarding endothelial function in these arterioles during chronic hypoperfusion.
Endothelium-dependent dilation is mediated by nitric oxide (NO), prostacyclin, and endothelium-dependent hyperpolarizing factors (EDHF). EDH is more prominent in smaller resistance arteries and arterioles compared with larger conduit arteries (4, 15, 48). In this study we sought to determine the effect of chronic cerebral hypoperfusion on EDH-mediated dilation in the PAs in a model of hypoperfusion-induced cognitive impairment. We investigated the contribution of EDHF epoxyeicosatrienoic acids (EETs) (5) to dilation after cognitive dysfunction was established. EETs are important regulators of parenchymal blood flow and neurovascular coupling (23) and studies suggest that cognitive impairment in humans is linked to dysfunctional cerebral eicosanoid signaling (35). For comparative analysis of the effect of BCAS on arterioles and the major collateral artery between the posterior and anterior cerebral circulation, we also studied structural and mechanical changes in the PAs as well as posterior communicating arteries (PComAs) and evaluated cerebrovascular reserve capacity, a critical prognostic factor for cognitive impairment (17). The aim of this study was to evaluate changes in the PAs and collaterals such as the PComAs in a model of cognitive impairment to understand the mechanism by which cerebral perfusion and brain functions are linked.
METHODS
Animals and surgery.
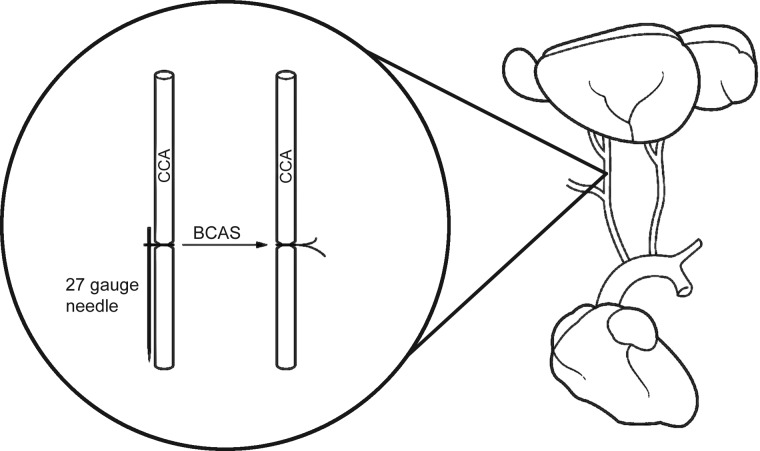
Twenty-week-old male WKY rats, purchased from Harlan Sprague Dawley (Indianapolis, IN), were randomized into two groups: one group underwent the Sham surgery and the other group had bilateral common carotid artery stenosis (BCAS). Rats were anesthetized using isoflurane and were placed in a supine position and the skin over the common carotid arteries was shaved and cleaned with alcohol. An incision was made in the skin, the right common carotid artery was separated from adherent connective tissue, and a 27-gauge blunt needle was placed next to the vessel. Two 6-0 silk sutures were used to firmly tie the carotid artery and the needle together. After the ties were in place, the needle was carefully removed (29). In this manner the needle was used as a guide to induce partial occlusion or stenosis of the artery. The procedure was then repeated on the left common carotid artery (Fig. 1). Ketoprofen (5 mg·kg−1·day−1) and Combi-pen 48 (22,000 units/day) were administered subcutaneously 3 and 2 days postsurgery, respectively. The stenosis surgery had 100% survival rate and rats were monitored until they were completely awake and moving around freely. The rats were singly housed for the duration of the experimental period. The experimental protocol was approved by the Michigan State University Institutional Animal Care and Use Committee and was in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals (2011).
Fig. 1.
Bilateral common carotid artery stenosis (BCAS). Diameters of common carotid arteries (CCAs) were reduced using a blunted 27-gauge needle as a guide.
Novel objection recognition test.
An open box with opaque walls was utilized for the novel object recognition test (24). Rats were acclimatized to the box for 15 min/day for 3 days. On the test day, during the familiarization phase the rats were removed from the box after they explored the two identical objects for 30 s. Ninety minutes after the end of the familiarization phase, during the acquisition phase, a novel object of similar size and texture replaced one of the familiar objects in the box. The time spent exploring the novel object was recorded along with the total exploration time. For both the familiarization and acquisition phase, exploration took place when the rat pawed at, sniffed or whisked with its snout directed at the object from a distance of under ∼1 cm (24). The positions of the familiar and novel objects were alternated to prevent bias for a particular location. After each trial, 10% alcohol was used to clean the objects and the box to remove any olfactory cues. Novel exploration quotient was measured as a ratio of the time spent exploring the novel object to the total exploration time (34).
Morris water maze.
A circular tank filled with water (30°C), was positioned in a room with external cues visible to the swimming rat. During the training phase, rats were placed in the tank from all four possible directions (north, south, west and east), and they learned to escape by locating a platform located 1 cm above the water level. The test phase started 4 wk after BCAS. The test was carried out in trials of 2 from all four directions per session and was performed 5 wk after the surgery, for 3 wk. The protocol for the test was the same as training with the exception that the platform was hidden 1 in. below the surface of water colored opaque with nontoxic tempera paint. To evaluate the rat's spatial retention ability, latency (time required to reach the platform) was measured (52).
Cerebral tissue perfusion.
A scanning laser Doppler (PeriScan PIM 3, Perimed, Stockholm, Sweden) was used to measure cerebral tissue perfusion, while the rat was under anesthesia. The scanning laser Doppler was positioned ∼18 cm above the exposed and cleaned skull, and cerebral tissue perfusion was recorded. The wavelength of the laser light was 670–690 nm with a penetration depth of 0.5–1 mm. A total of 4 consecutive scans were performed immediately after BCAS, and 8 wk after BCAS. Mean perfusion was analyzed using the LDPIwin 3.1 software (Perimed). Results were expressed as mean perfusion units.
Acetazolamide challenge.
Changes in cerebral perfusion following administration of acetazolamide, a potent cerebral artery vasodilator, were used to estimate cerebrovascular reserve capacity (26). Cerebrovascular reserve capacity was calculated as the percentage increase in cerebral tissue perfusion after acetazolamide relative to baseline perfusion (26). Rats were anesthetized and placed on a heated platform in a supine position while acetazolamide (0.2 mg/g) was injected into the tail vein. Scanning laser Doppler was used to assess cerebral tissue perfusion as described above. Perfusion was measured every 5 min for 30 min.
PA isolation and cannulation.
After 8 wk of BCAS, rats were anesthetized using isoflurane. After thoracotomy, blood was collected by cardiac puncture, the heart was removed, and the rat was decapitated. The brain was placed in ice-cold Ca2+-free physiological saline solution (PSS, in mM: 140 NaCl, 5 KCl, 1 MgCl2·7H2O, 10 HEPES, 10 dextrose) for isolation of the PAs. To dissect out the PAs, a section of brain tissue containing the middle cerebral artery was removed and placed in Ca2+-free PSS at 4°C, with 10% bovine serum albumin. PAs branching from the middle cerebral artery were carefully dissected and transferred to a cannulation chamber using a Wiretrol II positive displacement pipette (Drummond, PA). PAs were cannulated between two glass micropipettes (<40 μm) bent to a 45° angle and mounted on a small, 3-axis micromanipulator (MT-XYZ, Newport, Irvine, CA) such that the tips of the pipettes could be adjusted in three dimensions (6). Cannulated PAs were bathed in warm (37°C) PSS containing 1.8 mM Ca2+, and pressurized at 60 mmHg until spontaneous myogenic tone developed. The perfusion chamber was positioned on the stage of an inverted microscope (Leica DMIL, Wetzlar, Germany) and was measured using a 20× objective (Leitz Wetzlar objective, numerical aperture: 0.3). PA outer and lumen diameters were constantly tracked and recorded using MyoView 2.0 software (Danish Myo Technology, Aarhus, Denmark). To assess endothelium-dependent dilation, PAs were incubated with increasing concentrations of the muscarinic receptor agonist carbachol (1 nM to 100 μM) in the bath. Carbachol dilates cerebral vessels via production of NO, cyclooxygenase (COX) metabolites, and EDHF (8). To assess the role played by EDHF in the carbachol-induced dilation, PAs were incubated with the nitric oxide synthase (NOS) inhibitor NG-nitro-l-arginine methyl ester (l-NAME, 100 μM) and the COX inhibitor indomethacin (Indo, 10 μM) for 30 min, prior to pressurization and development of myogenic tone and prior to carrying out the concentration-response protocols. To evaluate the contribution of epoxyeicosatrienoic acids (EETs) in endothelium-dependent dilation, PAs were incubated in a similar fashion with CYP450 epoxygenase inhibitor N-methylsulfonyl-6-(2-propargyloxyphenyl) hexanamide (MS-PPOH; 10 μM). GSK1016790A, a TRPV4 channel agonist, was used to assess downstream mediators of EET-mediated dilation. Endothelium-independent dilation was studied by incubating PAs with sodium nitroprusside (SNP: 1 nM to 100 μM). Only one concentration-response experiment was performed on each cannulated PA. Baseline tone was calculated using the following formula: [1 − (active external diameter/passive external diameter)] × 100. % Dilation was calculated using the formula: [(external diameter at drug concentration − baseline external diameter)/(passive external diameter − baseline external diameter)]×100.
Assessment of the structural and mechanical properties in PAs.
After the end of the vasoreactivity studies the PAs were bathed in Ca2+-free PSS containing 2 mM EGTA + 100 μM SNP to assess the passive structure of the vessels as described previously (3). A CCD camera (Hitachi Kokusai Electric, Japan), with number of effective pixels 768 (H) × 494 (V), was connected to a video dimension analyzer (Living Systems Instrumentation, Burlington, VT); a final magnification of ×1,100 was used. The video dimension analyzer operates on the relative optical density changes of wall structures at the chosen level of a preselected scan line. It was calibrated using a stage micrometer according to the manufacturer's protocol. Lumen diameter and wall thickness were measured after 5 min at each pressure step and intraluminal pressure was increased from 3 to 180 mmHg in 20-mmHg increments. Outer diameter was calculated as lumen diameter + left wall thickness and right wall thickness. The wall-to-lumen ratio and circumferential wall stress were calculated as described previously, as was the passive distensibility (3).
Cannulation and assessment of the structural and mechanical properties of PComAs.
The PComA was carefully dissected from the brain and transferred to a pressure myograph chamber. A branchless segment of the PComA was cannulated between two glass micropipettes as previously described (45). The structural and mechanical properties of PComAs were assessed and calculated as described for the PAs.
Semiquantitative RT-PCR to assess changes in EETs dilatory pathway.
Total mRNA was isolated from the brain region around the middle cerebral arteries from which the PAs were dissected, and a Qiagen RNeasy Lipid Tissue Mini Kit (Qiagen Sciences) was used to extract RNA. Total mRNA was also extracted obtained from the dissected middle cerebral arteries and the PAs that were used for the vasoreactivity studies. The vessel samples were homogenized in Trizol reagent (Life Technologies, Gaithersburg, MD) and total RNA was extracted from the tissue according to the manufacturer's suggested protocol. Total mRNA collected from the tissues was reverse-transcribed using a qScript cDNA Synthesis Kit (Quanta Biosciences, Gaithersburg, MD). PCR was then performed using Taqman primer and probe sets in a 7,500 real time PCR system (Applied Biosystem, Foster City, CA). Fold changes in expression from Sham group were calculated using the 2−ΔΔCT method (30) with β-2-microglobulin used as endogenous control (44).
Statistical analyses.
Novel object recognition test, myogenic tone, and resting lumen diameter data were analyzed by Student's t-test or a nonparametric alternative if the data were not normally distributed. Cerebrovascular reactivity, endothelium-dependent dilation, passive and mechanical properties were analyzed by two-way ANOVA followed by Sidak correction for multiple comparisons, or a nonparametric alternative. Analyses were performed using the software GraphPad Prizm 6.0 (La Jolla, CA).
Chemicals and reagents.
MS-PPOH was purchased from Cayman Chemical (Ann Arbor, MI, USA). Acetazolamide was purchased from X-Gen (Big Flats, NY). All other chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO).
RESULTS
BCAS impairs short-term memory and spatial discrimination abilities and abolishes cerebrovascular reactivity.
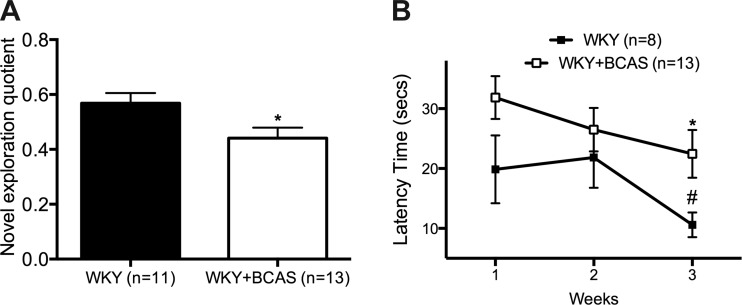
Eight weeks of BCAS reduced the novel exploration quotient (Fig. 2A). BCAS rats also showed reduced spatial discrimination abilities as evidenced by an increased time to find the platform in the Morris water maze test (Fig. 2B). While Sham rats showed significant improvement in learning abilities at the 3rd wk, BCAS rats did not.
Fig. 2.
BCAS impairs short-term memory and spatial discrimination abilities in WKY rats. A: BCAS rats spend a smaller portion of the time exploring the novel object as depicted by reduced novel exploration quotient. B: in the 3rd week of Morris Water Maze (MWM), BCAS rats showed a significantly increased time to find the hidden platform, where Sham rats found the hidden platform in a significantly shorter time compared with 2nd week after the training. Each data point is mean ± SE. *Data different from Sham rats (P < 0.05). #Data different from Sham rats in the 2nd week (P < 0.05).
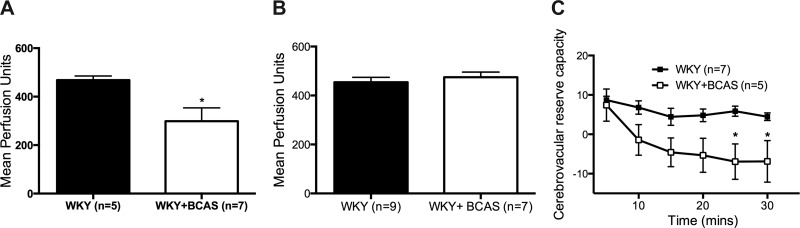
A scanning laser-Doppler system was used to measure cerebral tissue perfusion immediately after surgery and then after 8 wk of BCAS. While perfusion fell immediately in BCAS rats compared with Sham rats (Fig. 3A), there was no difference in baseline mean perfusion between Sham and BCAS rats at the end of 8 wk (Fig. 3B). Acetazolamide, a potent carbonic anhydrase inhibitor, was used to measure cerebrovascular reserve capacity. The initial dilatory response between Sham and BCAS did not differ; however, tissue perfusion in BCAS rats was not sustained and perfusion rapidly diminished in this group (Fig. 3C).
Fig. 3.
Eight weeks after BCAS baseline perfusion was restored; however cerebrovascular reserve capacity was impaired. A: immediately after BCAS, mean perfusion in BCAS rats was reduced. B: however, 8 wk after the surgery there was no different in cerebral perfusion between Sham rats and BCAS rats. C: cerebrovascular reserve capacity, measured during carbonic anhydrous inhibitor acetazolamide, quickly diminished in BCAS rats; *P < 0.05, different from Sham rats.
BCAS impairs endothelium-dependent dilation in PAs.
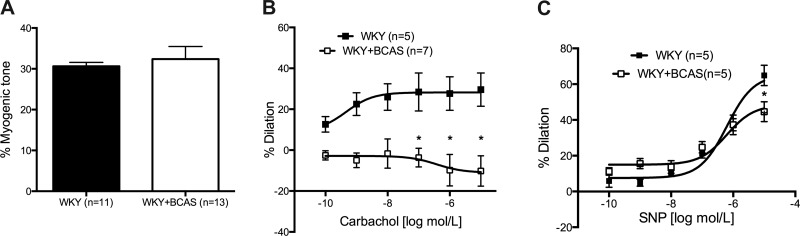
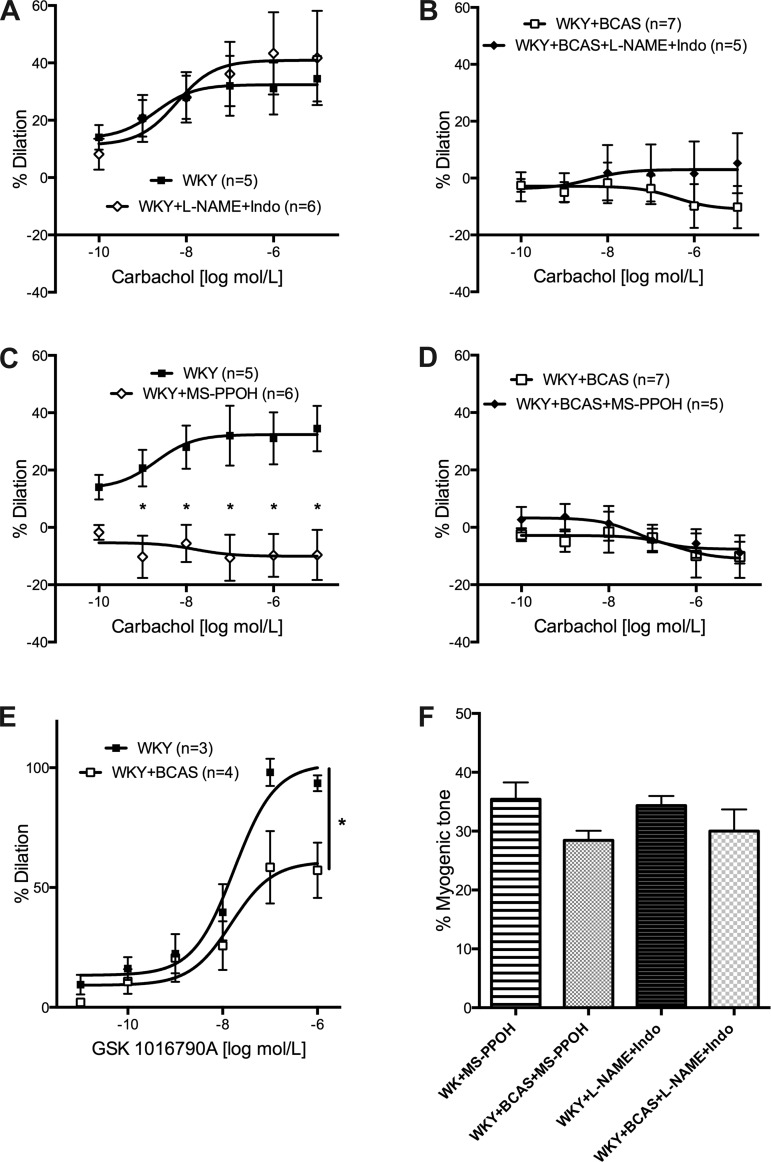
There was no difference in myogenic tone generation between PAs from Sham and BCAS rats (Fig. 4A). Endothelium-dependent dilation, assessed using carbachol, was impaired in PAs from BCAS rats compared with Sham rats (Fig. 4B). Carbachol induced negligible dilation in PAs from BCAS rats. Endothelium-independent dilation to the NO-donor SNP was unaltered between PAs from Sham and BCAS rats at the lower concentrations; however, at the highest concentration dilation in PAs from BCAS rats was impaired (Fig. 4C). EC50 for the SNP-mediated dilation did not differ between the groups.
Fig. 4.
Both endothelium-dependent and -independent dilation were impaired in PAs from BCAS rats. A: myogenic tone generation in the PAs was not affected by BCAS. PAs with at least 20 percent tone was used for further studies. B: endothelium-dependent dilation assessed using carbachol was abolished in PAs from BCAS rats. C: at the highest concentration of SNP endothelium-independent dilation in PAs from BCAS rats was reduced. At 10−5 M of SNP dilation was impaired in PAs from BCAS rats. *P < 0.05, different from Sham rats.
Endothelium-dependent dilation in PAs is NO and prostacyclin independent.
Residual dilation after inhibition of NO and COX is considered to be EDHF mediated (5). To assess whether EDHF is upregulated as reported after cerebrovascular insults (19, 32, 33), l-NAME (10−4 M) and Indo (10−5 M) were added to the bath before pressurization and the development of myogenic tone. Concentration-response curves to carbachol were generated to assess the contribution of the NO and the COX dilatory pathways to dilation. In PAs from Sham rats, carbachol-mediated dilation was unaltered in the presence of l-NAME and Indo (Fig. 5A). There was very little dilation in PAs from BCAS rats with or without the inhibitors (Fig. 5B).
Fig. 5.
EETs-mediated dilation is impaired in PAs from BCAS rats. A: PAs from Sham rats were incubated with l-NAME and Indo before the generation of tone. Dilation to carbachol remained unaltered in the presence of the inhibitors in PAs from Sham rats. B: inhibition of production of NO and prostacyclin had no effect on dilation in PAs from BCAS rats. C: inhibition of EETs production with MS-PPOH abolished dilation in PAs from Sham rats. D: incubating with MS-PPOH had no effect on dilation in PAs from BCAS rats. E: dilation to TRPV4 agonist GSK 1016790A was reduced in PAs from BCAS rats. F: no difference in myogenic tone generation with addition of the inhibitors; n = 4–6. *P < 0.05, different from Sham rats.
EETs production is an important determinant of carbachol-mediated dilation in PAs from sham rats.
MS-PPOH (10−5 M), an inhibitor of CYP450 epoxygenases, was added to the bath before pressurization and development of myogenic tone, to inhibit EETs production. Dilation of PAs from Sham rats was abolished when EETs production was inhibited (Fig. 5C). There was no change in percent dilation in PAs from BCAS rats with inhibition of the CYP450 epoxygenase (Fig. 5D). Dilation induced by EETs may involve the activation of TRPV4 channels (13). Therefore, we also assessed the effects of BCAS on dilation induced by the TRPV4 agonist, GSK1016790A. We found that dilation induced by GSK1016790A was significantly impaired in PAs isolated from BCAS rats (Fig. 5E). Myogenic tone was unaltered in both the groups with the addition of the MS-PPOH (Fig. 5F).
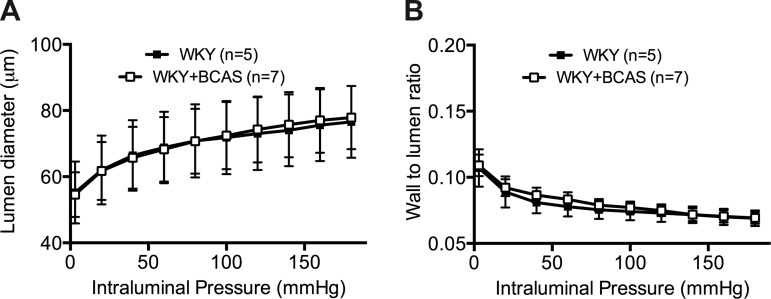
BCAS did not induce remodeling the PAs.
There was no difference in PA lumen diameter between BCAS and Sham rats (Fig. 6A). There was also no difference in wall-to-lumen ratio between the groups (Fig. 6B) or any changes in any of the mechanical parameters such as distensibility between the groups.
Fig. 6.
There was no difference between the structural and mechanical properties of PAs between the two groups. A: lumen diameters of the PAs were measured after the PAs were equilibrated in Ca2+ free PSS with EGTA and SNP. There was no difference between the lumen diameter of PAs from Sham rats and BCAS rats. B: wall-to-lumen ratio was not different between the two groups.
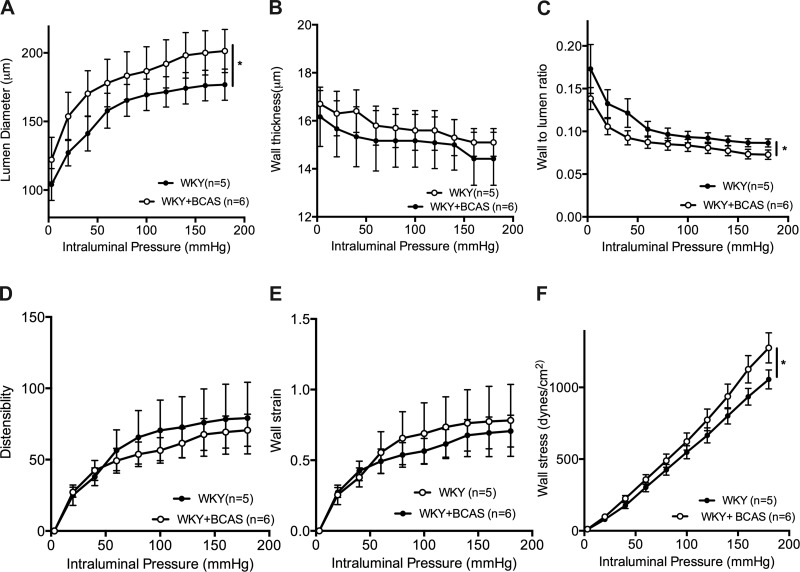
PComAs from BCAS rats exhibited structural remodeling.
Passive structure and mechanics of the PComA were carried out for a comparative analysis of the effect of BCAS on arteries and arterioles. The lumen diameter of PComAs from BCAS rats was increased compared with Sham rats (Fig. 7A). BCAS had no effect on wall thickness (Fig. 7B) but the wall-to-lumen ratio was reduced in PComAs from BCAS rats (Fig. 7C). There was no change in distensibility (Fig. 7D) or strain (Fig. 7E) between the groups. PComAs from BCAS rats had increased wall stress compared with PAs from Sham rats (Fig. 7F).
Fig. 7.
Outward remodeling was observed in the PComAs from BCAS rats. A: data from pressure myograph shows the lumen diameter increased in the PComAs from BCAS rats. B: BCAS did not change wall thickness in PAs. C: BCAS reduced wall to lumen ratio in the PComAs. D: BCAS did not induce changes in distensibility in the PComAs. E: wall strain was not different between the groups. F: wall stress was increased in PComAs from WKY rats with BCAS. *P < 0.05, different from Sham rats.
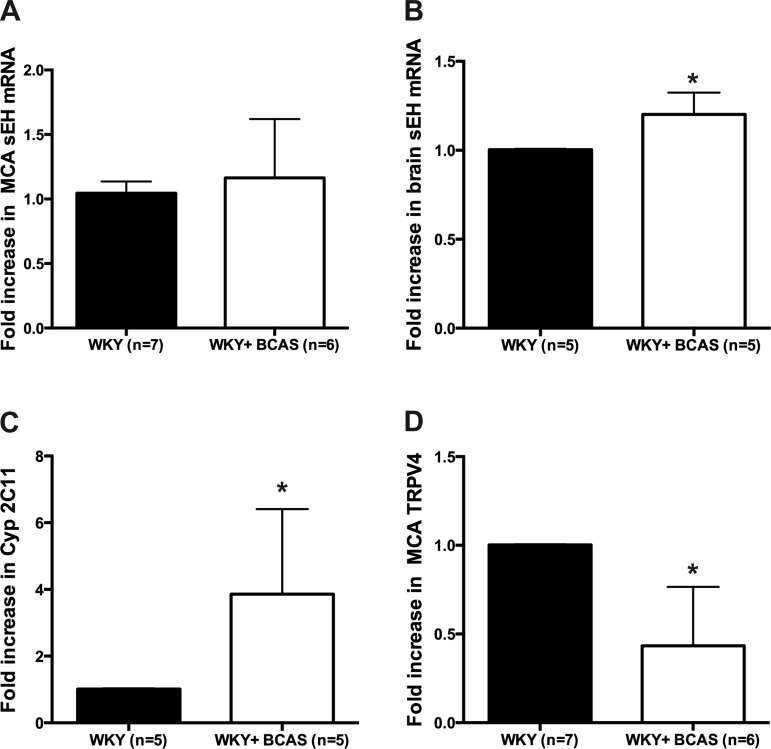
BCAS changed mRNA levels of key mediators in EETs dilatory pathway.
To assess possible changes in EETs metabolism we measured the mRNA levels of soluble epoxide hydrolase, the enzyme that converts EETs into less active DHETs. We also assessed the mRNA expression of the transient receptor potential cation channel vanilloid 4 (TRPV4) which has been suggested to be the downstream mediator of EETs-mediated dilation (13). Soluble epoxide hydrolase mRNA expression was unchanged in the middle cerebral artery and arterioles after chronic hypoperfusion (Fig. 8A). However, mRNA for this enzyme was upregulated in the brain region surrounding the PAs from BCAS rats (Fig. 8B). Expression levels of the mRNA for CYP2C11, the epoxygenase that forms EETs from arachidonic acid, were also increased by BCAS (Fig. 8C), whereas expression levels of TRPV4 were reduced in the cerebral arteries and arterioles from BCAS rats (Fig. 8D).
Fig. 8.
Altered expression of markers in EETs mediated signaling in BCAS rats. A: there was no change in mRNA levels of soluble epoxide hydrolase (sEH) in middle cerebral arteries (MCAs) and PAs after chronic cerebral hypoperfusion. B: however, there was increased expression of sEH in the brain from BCAS rats. C: increased expression of sEH was coupled with increased expression of Cyp2C11 in the brain region around the PAs from BCAS rats. D: mRNA level of TRPV4 was reduced in MCAs and PAs from BCAS rats. *P < 0.05, different from Sham rats.
DISCUSSION
The novel finding of this study is that impaired dilation in the PAs is associated with the cognitive impairment resulting from cerebral hypoperfusion. Although tissue perfusion was restored 8 wk after the stenosis, dilatory reserve was impaired and PA endothelium-dependent dilation was essentially abolished. This impairment appears to be the result of reduced EETs-mediated dilation. Together, these results provide evidence that dysfunction in the EETs dilatory pathway in PAs may be associated with the cognitive impairment observed with chronic hypoperfusion.
BCAS impairs short-term memory and spatial discrimination abilities and abolishes cerebrovascular reactivity.
After 8 wk of chronic cerebral hypoperfusion, spatial discrimination and learning assessed using Morris water maze was impaired in BCAS rats. Carotid artery occlusion causes a similar impaired short-term memory evident just 2 wk after chronic hypoperfusion and learning deficits that persisted at least 3 wk after cerebral hypoperfusion (29, 36). In the current study BCAS rats also had an impaired ability to recognize novel objects using a test that evaluates nonspatial short-term memory (14, 24) related to frontal-subcortical circuits.
In models of cerebral hypoperfusion vascular adaptations occur that restore resting cerebral tissue perfusion to normal levels several weeks after the initial insult (16) as was the case in the current study. However, despite the restoration of cerebral perfusion the cerebrovascular reserve capacity was impaired in the BCAS rats 7 wk after the stenosis. Insufficient collateral supply after chronic cerebral hypoperfusion causes dilation of the arterial circulation and reduced response to administered vasodilator. Acetazolamide induces dilation by extracellular and intracellular acidosis and is frequently used to assess cerebrovascular reserve capacity (18), which is an indicator of the severity of cognitive impairment (31). The initial response to acetazolamide in the BCAS rats was not different from Sham rats but the cerebral vasculature appears to have lost its ability to maintain the dilatory response. Prolonged cerebral hypoperfusion may have exaggerated the steal phenomenon, with an increase in flow velocity in the outward remodeled arteries in the circle of Willis and a flow reduction in the pial arteries and the arterioles measured with the scanning laser Doppler. Alternatively, increased oxidative metabolism in the brain after chronic hypoperfusion (2) could enhance constrictor activity (11, 43) and impair cerebrovascular reserve capacity. Impaired response to acetazolamide has been observed immediately postocclusion in WKY rats with common carotid artery occlusion and this persisted for 4 wk (50).
BCAS attenuated endothelium-mediated dilation in the PAs.
Normalization of resting cerebral tissue perfusion with persistent cognitive impairment hinted at dysfunction at the level of parenchymal perfusion. In our study endothelium-dependent dilation to carbachol was abolished and the response to the highest concentration of the endothelium-independent dilator SNP was reduced in PAs from BCAS rats. As there have been no other studies assessing the temporal effects of hypoperfusion on the endothelium of the PAs, it is difficult to surmise whether this impairment occurs during the acute or chronic phase of BCAS. Studies in the literature suggest this may be an acute response. In vivo assessment of the PAs 30 min after occlusion of carotid arteries showed a reduction in their resting diameter (27), suggesting possible impaired dilation. Regardless of the onset, impaired dilation could play a role in the development of the cognitive deficits observed in our model. Cognitive impairment is observed after occlusion in a single PA (38). This may be due to functional changes in PAs, which may alter homeostasis in the neurovascular unit. Impaired dilation in a single PA may worsen the severity of hypoperfusion in the brain parenchyma since there is little collateral flow in the PA network (37).
We observed no difference in myogenic tone in the PAs between the groups. This is in contrast to the results obtained in a study using 4 wk of unilateral common carotid occlusion in 14-wk-old WKY rats where myogenic tone in the PAs was reduced (49). The duration and severity of the hypoperfusion, as well as the age of the WKY rats used in our study, may have negated this compensatory decrease in myogenic tone.
BCAS impaired EETs-mediated dilation in the PAs.
We observed that endothelium-dependent dilation in the PAs was NO and COX independent in the Sham rats. This suggests that EDHF, a dilator pathway that is more prominent in smaller resistance arteries and arterioles compared with larger conduit arteries (4, 15, 48), is a major contributor of endothelium-dependent dilation in PAs from 28-wk-old WKY rats.
CYP450 epoxygenase pathways metabolize arachidonic acid to produce EETs (46). EETs act on TRPV4 channels to induce Ca2+ flux that causes NO synthase and cyclooxygenase-independent dilation by membrane hyperpolarization (13). In PAs from Sham rats dilation was abolished by MS-PPOH, an inhibitor of CYP450 epoxygenases, primarily CYP2C11 enzymes (23). The lack of dilation in PAs from the BCAS group and attenuated dilation with the inhibition of EETs in PAs from Sham group suggests that BCAS impairs EETs-mediated dilation. Coupled with the observed impaired dilation to TRPV4 agonist GSK1016790A and reduced expression of TRPV4 mRNA, impaired dilation in PAs from BCAS rats may be due to reduced number of TRPV4 channels that can be activated by EETs. We also observed increased levels of soluble epoxide hydrolase in the brain region around the PAs from BCAS rats, suggesting that after prolonged hypoperfusion there might be increased degradation of EETs into less vasoactive products. This increased activity of soluble epoxide hydrolase may also have caused a compensatory increase in expression of CYP2C11 mRNA, the epoxygenase that produces EETs in the brain.
Evidence suggests that cognitive impairment in humans is linked to dysfunctional cerebral eicosanoid signaling (35). In our model of cognitive impairment, despite restoration of cerebral perfusion (39, 40), impaired PA dilation suggests that the arterioles may be less responsive to vasodilatory signals from astrocytes and may exhibit impaired neurovascular coupling. Inhibition of EETs production reduces coupling of blood flow to neural activation (42), suggesting that impaired EETs-mediation dilation after BCAS may worsen cognitive outcomes. Disruption of the spatial and temporal relationship between neural activity and cerebral blow flow is observed during Alzheimer's disease and after ischemic stroke (21), a major risk factor for vascular cognitive impairment.
Heterogeneous remodeling of the PAs and PComAs after BCAS.
Structural changes in the PAs after 8 wk of BCAS were not observed. However, arteries in the vertebrobasilar circulation have increased lumen diameters (7, 16) suggesting that in chronic hypoperfuson models remodeling depends on the artery type studied. Following the stenosis, redistribution of flow results in increased intravascular shear forces. As a result, in our study PComAs from BCAS rats exhibited an increase in lumen diameter of ∼14% accompanied by reduced wall-to-lumen ratio and increased wall stress. This remodeling is essential for restoration of cerebral perfusion. Lack of a fully formed PComA in gerbils exacerbates the effects of chronic hypoperfusion (16), whereas in rats a complete circle of Willis allows for restoration of perfusion (7, 10).
Normalization of cerebral perfusion, observed in the current study, may be associated with outward remodeling of collaterals such as the PComA. However, one of the characteristic outcomes of reducing blood flow through the carotid arteries is sustained impairment in cognitive function (9, 51), suggesting that cerebrovascular adaptations after BCAS are not enough to reverse cognitive impairment. Cognitive deficits after BCAS may be an outcome of a synchronized dysfunction in the cerebral microcirculation and the neurons. However, there is a growing body of evidence that cerebrovascular dysfunction may precede measurable cognitive deficits (22, 25, 41). Our study emphasizes the contribution of impaired PA dilation that may worsen cognitive end points. Investigating drugable targets that restore the dilatory properties of PAs may allow us to intervene even before cognitive impairment is observed. Therapeutic agents that enhance EETs mediated dilatory pathway may be a credible option for reducing microcirculatory dysfunction and improving associated cognitive functions during vascular cognitive impairment.
Limitations.
A few limitations and caveats of our study should be acknowledged. First, since we used a scanning laser-Doppler flowmetry, we did not perform quantitative measurements of cerebral blood flow. Even though it measures perfusion in arbitrary units (perfusion units), calibration of the laser detector is not changed between subjects. Thus, it still allows for comparative analyses between experimental groups. Second, we did not assess anxiety levels in the rats and that could affect the tests used to evaluate cognitive deficits. Third, we did not measure the contribution of other mediators in the EDHF consortium, since MS-PPOH abolished dilation in the Sham rats. Fourth, we only studied PAs from male 28-wk-old WKY rats and cannot confirm that similar findings can be expected in female rats or in rats of a different age. Finally, the conclusions of this study are supported by associative evidence rather than direct links. Due to the intricate nature of the neurodegeneration and vascular dysfunction, it is difficult to elucidate direct cause-and-effect links between vascular dysfunction and measurable cognitive impairment.
GRANTS
This study was supported by American Heart Association (AHA) Grants AHA-13-GRNT1721000 (A. M. Dorrance) and AHA-14PRE19890001 (to N. Matin) and National Heart, Lung, and Blood Institute Grant PO1-HL-070687 (to W. F. Jackson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.M. and A.M.D. conception and design of research; N.M. and C.F. performed experiments; N.M. analyzed data; N.M. and A.M.D. interpreted results of experiments; N.M. prepared figures; N.M. drafted manuscript; N.M., W.F.J., and A.M.D. edited and revised manuscript; N.M., C.F., W.F.J., and A.M.D. approved final version of manuscript.
REFERENCES
- 1.Aliev G, Smith MA, Obrenovich ME, de la Torre JC, Perry G. Role of vascular hypoperfusion-induced oxidative stress and mitochondria failure in the pathogenesis of Alzheimer disease. Neurotox Res 5: 491–504, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Aytac E, Seymen HO, Uzun H, Dikmen G, Altug T. Effects of iloprost on visual evoked potentials and brain tissue oxidative stress after bilateral common carotid artery occlusion. Prostaglandins Leukot Essent Fatty Acids 74: 373–378, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Baumbach GL, Hajdu MA. Mechanics and composition of cerebral arterioles in renal and spontaneously hypertensive rats. Hypertension 21: 816–826, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Brandes RP, Schmitz-Winnenthal FH, Feletou M, Godecke A, Huang PL, Vanhoutte PM, Fleming I, Busse R. An endothelium-derived hyperpolarizing factor distinct from NO and prostacyclin is a major endothelium-dependent vasodilator in resistance vessels of wild-type and endothelial NO synthase knockout mice. Proc Natl Acad Sci USA 97: 9747–9752, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryan RM Jr, You J, Golding EM, Marrelli SP. Endothelium-derived hyperpolarizing factor: a cousin to nitric oxide and prostacyclin. Anesthesiology 102: 1261–1277, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Burns WR, Cohen KD, Jackson WF. K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation 11: 279–293, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busch HJ, Buschmann IR, Mies G, Bode C, Hossmann KA. Arteriogenesis in hypoperfused rat brain. J Cereb Blood Flow Metab 23: 621–628, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Cechetti F, Pagnussat AS, Worm PV, Elsner VR, Ben J, da Costa MS, Mestriner R, Weis SN, Netto CA. Chronic brain hypoperfusion causes early glial activation and neuronal death, and subsequent long-term memory impairment. Brain Res Bull 87: 109–116, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Choy M, Ganesan V, Thomas DL, Thornton JS, Proctor E, King MD, van der Weerd L, Gadian DG, Lythgoe MF. The chronic vascular and haemodynamic response after permanent bilateral common carotid occlusion in newborn and adult rats. J Cereb Blood Flow Metab 26: 1066–1075, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Chrissobolis S, Faraci FM. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol Med 14: 495–502, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Torre JC. Cerebral hypoperfusion, capillary degeneration, and development of Alzheimer disease. Alzheimer Dis Assoc Disord 14, Suppl 1: S72–S81, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Earley S. Endothelium-dependent cerebral artery dilation mediated by transient receptor potential and Ca2+-activated K+ channels. J Cardiovasc Pharmacol 57: 148–153, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res 31: 47–59, 1988. [DOI] [PubMed] [Google Scholar]
- 15.Faraci FM, Lynch C, Lamping KG. Responses of cerebral arterioles to ADP: eNOS-dependent and eNOS-independent mechanisms. Am J Physiol Heart Circ Physiol 287: H2871–H2876, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Farkas E, Luiten PG, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev 54: 162–180, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Fearn SJ, Hutchinson S, Riding G, Hill-Wilson G, Wesnes K, McCollum CN. Carotid endarterectomy improves cognitive function in patients with exhausted cerebrovascular reserve. Eur J Vasc Endovasc Surg 26: 529–536, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Fierstra J, Sobczyk O, Battisti-Charbonney A, Mandell DM, Poublanc J, Crawley AP, Mikulis DJ, Duffin J, Fisher JA. Measuring cerebrovascular reactivity: what stimulus to use? J Physiol 591: 5809–5821, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golding EM, You J, Robertson CS, Bryan RM Jr. Potentiated endothelium-derived hyperpolarizing factor-mediated dilations in cerebral arteries following mild head injury. J Neurotrauma 18: 691–697, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42: 2672–2713, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci 5: 347–360, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol 120: 287–296, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iliff JJ, Close LN, Selden NR, Alkayed NJ. A novel role for P450 eicosanoids in the neurogenic control of cerebral blood flow in the rat. Exp Physiol 92: 653–658, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Jablonski SA, Schreiber WB, Westbrook SR, Brennan LE, Stanton ME. Determinants of novel object and location recognition during development. Behav Brain Res 256: 140–150, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ 322: 1447–1451, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuroda S, Kamiyama H, Abe H, Houkin K, Isobe M, Mitsumori K. Acetazolamide test in detecting reduced cerebral perfusion reserve and predicting long-term prognosis in patients with internal carotid artery occlusion. Neurosurgery 32: 912–918; discussion 918–919, 1993. [DOI] [PubMed] [Google Scholar]
- 27.Lapi D, Vagnani S, Pignataro G, Esposito E, Paterni M, Colantuoni A. Protective effects of quercetin on rat pial microvascular changes during transient bilateral common carotid artery occlusion and reperfusion. Front Physiol 3: 32, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li N, Gu Z, Li Y, Fu X, Wang J, Bai H. A modified bilateral carotid artery stenosis procedure to develop a chronic cerebral hypoperfusion rat model with an increased survival rate. J Neurosci Methods 255: 115–121, 2015. [DOI] [PubMed] [Google Scholar]
- 29.Li N, Gu Z, Li Y, Fu X, Wang J, Bai H. A modified bilateral carotid artery stenosis procedure to develop a chronic cerebral hypoperfusion rat model with an increased survival rate. J Neurosci Methods 255: 115–121, 2015. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta CT) Method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 124: 457–467, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Marrelli SP. Altered endothelial Ca2+ regulation after ischemia/reperfusion produces potentiated endothelium-derived hyperpolarizing factor-mediated dilations. Stroke 33: 2285–2291, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Marrelli SP, Khorovets A, Johnson TD, Childres WF, Bryan RM Jr. P2 purinoceptor-mediated dilations in the rat middle cerebral artery after ischemia-reperfusion. Am J Physiol Heart Circ Physiol 276: H33–H41, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem 9: 49–57, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson JW, Young JM, Borkar RN, Woltjer RL, Quinn JF, Silbert LC, Grafe MR, Alkayed NJ. Role of soluble epoxide hydrolase in age-related vascular cognitive decline. Prostaglandins Other Lipid Mediat 113–115: 30–37, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ni J, Ohta H, Matsumoto K, Watanabe H. Progressive cognitive impairment following chronic cerebral hypoperfusion induced by permanent occlusion of bilateral carotid arteries in rats. Brain Res 653: 231–236, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura N, Rosidi NL, Iadecola C, Schaffer CB. Limitations of collateral flow after occlusion of a single cortical penetrating arteriole. J Cereb Blood Flow Metab 30: 1914–1927, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci USA 104: 365–370, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohta H, Nishikawa H, Kimura H, Anayama H, Miyamoto M. Chronic cerebral hypoperfusion by permanent internal carotid ligation produces learning impairment without brain damage in rats. Neuroscience 79: 1039–1050, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Otori T, Katsumata T, Muramatsu H, Kashiwagi F, Katayama Y, Terashi A. Long-term measurement of cerebral blood flow and metabolism in a rat chronic hypoperfusion model. Clin Exp Pharmacol Physiol 30: 266–272, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Panza F, D'Introno A, Colacicco AM, Capurso C, Parigi AD, Capurso SA, Caselli RJ, Pilotto A, Scafato E, Capurso A, Solfrizzi V. Cognitive frailty: Predementia syndrome and vascular risk factors. Neurobiol Aging 27: 933–940, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Peng X, Carhuapoma JR, Bhardwaj A, Alkayed NJ, Falck JR, Harder DR, Traystman RJ, Koehler RC. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol Heart Circ Physiol 283: H2029–H2037, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Phillips SA, Sylvester FA, Frisbee JC. Oxidant stress and constrictor reactivity impair cerebral artery dilation in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 288: R522–R530, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Pires PW, Girgla SS, Moreno G, McClain JL, Dorrance AM. Tumor necrosis factor-alpha inhibition attenuates middle cerebral artery remodeling but increases cerebral ischemic damage in hypertensive rats. Am J Physiol Heart Circ Physiol 307: H658–H669, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pires PW, Jackson WF, Dorrance AM. Regulation of myogenic tone and structure of parenchymal arterioles by hypertension and the mineralocorticoid receptor. Am J Physiol Heart Circ Physiol 309: H127–H136, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosolowsky M, Campbell WB. Role of PGI2 and epoxyeicosatrienoic acids in relaxation of bovine coronary arteries to arachidonic acid. Am J Physiol Heart Circ Physiol 264: H327–H335, 1993. [DOI] [PubMed] [Google Scholar]
- 47.Sehba FA, Schwartz AY, Chereshnev I, Bederson JB. Acute decrease in cerebral nitric oxide levels after subarachnoid hemorrhage. J Cereb Blood Flow Metab 20: 604–611, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol 28: 703–711, 1996. [DOI] [PubMed] [Google Scholar]
- 49.Sweet JG, Chan SL, Cipolla MJ. Effect of hypertension and carotid occlusion on brain parenchymal arteriole structure and reactivity. J Appl Physiol (1985) 119: 817–823, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ulrich PT, Kroppenstedt S, Heimann A, Kempski O. Laser-Doppler scanning of local cerebral blood flow and reserve capacity and testing of motor and memory functions in a chronic 2-vessel occlusion model in rats. Stroke 29: 2412–2420, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Vicente E, Degerone D, Bohn L, Scornavaca F, Pimentel A, Leite MC, Swarowsky A, Rodrigues L, Nardin P, de Almeida LM, Gottfried C, Souza DO, Netto CA, Goncalves CA. Astroglial and cognitive effects of chronic cerebral hypoperfusion in the rat. Brain Res 1251: 204–212, 2009. [DOI] [PubMed] [Google Scholar]
- 52.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1: 848–858, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]