The major findings of this study are that reactive vagus nerve stimulation targets autonomic (intrinsic) cardiac neurons to mitigate the sympathoexcitation accompanying pressure overload to alter myocyte energetics and prevent the associated adverse myocardial remodeling.
Keywords: autonomic regulation therapy, cardiac hypertrophy, guinea pig, intrinsic cardiac nervous system, pressure overload, vagus nerve stimulation
Abstract
Our objective was to determine whether chronic vagus nerve stimulation (VNS) mitigates pressure overload (PO)-induced remodeling of the cardioneural interface. Guinea pigs (n = 48) were randomized to right or left cervical vagus (RCV or LCV) implant. After 2 wk, chronic left ventricular PO was induced by partial (15–20%) aortic constriction. Of the 31 animals surviving PO induction, 10 were randomized to RCV VNS, 9 to LCV VNS, and 12 to sham VNS. VNS was delivered at 20 Hz and 1.14 ± 0.03 mA at a 22% duty cycle. VNS commenced 10 days after PO induction and was maintained for 40 days. Time-matched controls (n = 9) were evaluated concurrently. Echocardiograms were obtained before and 50 days after PO. At termination, intracellular current-clamp recordings of intrinsic cardiac (IC) neurons were studied in vitro to determine effects of therapy on soma characteristics. Ventricular cardiomyocyte sizes were assessed with histology along with immunoblot analysis of selected proteins in myocardial tissue extracts. In sham-treated animals, PO increased cardiac output (34%, P < 0.004), as well as systolic (114%, P < 0.04) and diastolic (49%, P < 0.002) left ventricular volumes, a hemodynamic response prevented by VNS. PO-induced enhancements of IC synaptic efficacy and muscarinic sensitivity of IC neurons were mitigated by chronic VNS. Increased myocyte size, which doubled in PO (P < 0.05), was mitigated by RCV. PO hypertrophic myocardium displayed decreased glycogen synthase (GS) protein levels and accumulation of the phosphorylated (inactive) form of GS. These PO-induced changes in GS were moderated by left VNS. Chronic VNS targets IC neurons accompanying PO to obtund associated adverse cardiomyocyte remodeling.
NEW & NOTEWORTHY
The major findings of this study are that reactive vagus nerve stimulation targets autonomic (intrinsic) cardiac neurons to mitigate the sympathoexcitation accompanying pressure overload to alter myocyte energetics and prevent the associated adverse myocardial remodeling.
autonomic control of the heart is mediated by complex interactions throughout the peripheral and central components of the cardiac neuraxis (11, 27, 62). Neural activity within the intrathoracic autonomic ganglia is influenced by afferent and preganglionic efferent inputs as processed by local circuit neurons therein, with subsequent modulation of postganglionic efferent outflows to all regions of the heart (11, 14). As a consequence, the cardiac neuraxis can be considered a potential target for bioelectric neuromodulation strategies to treat select cardiac pathologies (19, 21, 23, 49, 61). We have demonstrated that select pathologies are associated with time-dependent changes in its intrinsic cardiac (IC) component (30, 32, 37). For example, for states of chronic pressure overload (PO), myocyte hypertrophy results in altered neural control associated with enhanced numbers of nitric oxide synthase-immunoreactive IC neurons, along with increased density of mast cells in the IC nervous system (ICNS) (30). The neurohumoral remodeling associated with PO is further indicated by the effects of chronic β-blockade (e.g., timolol), which inhibits the synergism between angiotensin II and norepinephrine (NE) to increase IC neuronal excitability and to likewise reduce synaptic efficacy within the IC neuronal network (34). The therapeutic efficiency of pharmacological (β-adrenergic receptor blockers, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers) and bioelectric manipulations of the ICNS is pronounced, especially during dynamic states of disease progression (15, 33, 34).
Alterations in sensory transduction are central to the reflex adaptations in the cardiac nervous system in response to cardiac pathology (3, 13, 58). Alterations in sensory inputs arising from cardiac and cardiovascular afferent neurons transducing PO are reflected in central-mediated reflex sympathoexcitation coupled with a corresponding reduction in central neuronal drive of cardiac parasympathetic efferent neurons (38, 62). Bioelectric therapy directed at the cervical vagus has the potential to impact multiple levels of the hierarchy for cardiac control via its activation of ascending (afferent) and descending (efferent) axons (8, 43, 59). Bioelectric activation of afferent fibers impacts central drive for sympathetic and parasympathetic nervous systems (5, 8). Electrical activation of preganglionic parasympathetic fibers can impact sympathetic-parasympathetic interactions mediated within IC ganglia (14, 28, 46, 51) and at the end effectors of the heart (43, 44, 53). It is the dynamic interplay between central and peripheral reflex control that ultimately determines functional neural regulation of regional cardiac function (11).
Since central parasympathetic outflow is reduced as a compensatory response to chronic cardiac insults (25, 27, 62), we hypothesize that restoration of biomimetic levels of “central” parasympathetic drive via cervical vagus nerve stimulation (VNS) mitigates adverse remodeling of the ICNS evoked in reflex response to PO. We propose that cardiomyocytes are rendered “stress-resistant” due, in part, to attenuation of excessive sympathetic drive to IC neurons and cardiac end effectors. We further propose that VNS will stabilize peripheral reflex processing by limiting adverse remodeling of synaptic efficacy within the ICNS. Finally, we propose that VNS imparts changes in myocyte metabolic function in response to alterations in neural inputs. To mechanistically evaluate this hypothesis, we studied IC neuronal and cardiac function in response to chronic PO of the left ventricle (LV) to determine how VNS impacts 1) passive and active membrane properties of the ICNS, such that 2) cardiac hemodynamics are maintained, even in the face of a sustained afterload stressor.
MATERIALS AND METHODS
All procedures were approved by the Institutional Animal Care and Use Committee of East Tennessee State University and were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (8th ed., National Academy Press, Washington, DC, 2010).
Implantation of VNS Systems
Male Hartley guinea pigs (n = 48, 500–650 g body wt, 9 wk of age; Charles River) were implanted with bipolar VNS electrodes connected to a pulse generator. Under aseptic conditions, animals were pretreated with atropine (0.1 mg/kg sc) and ketamine (80 mg/kg ip). Thereafter, anesthesia was induced with 3% isoflurane via an induction chamber (VetEquip, Pleasanton, CA). Upon removal of the animals from the induction chamber, 2.5% isoflurane was delivered via a conical nose cone (VetEquip) until responses to hindlimb toe pinch stimuli were absent. After endotracheal intubation, mechanical ventilation was initiated and maintained with a positive-pressure ventilator (model SAR-830/P, IITC Life Science, Woodland Hills, CA) using 100% O2. Anesthesia was maintained with isoflurane (1–3%). Core body temperature was maintained at 38.5°C via a circulating-water heating pad. Buprenorphine (0.05 mg/kg sc) was administered preoperatively.
After anesthesia induction, a midline incision was made in the ventral neck. The right or left vagus nerve and the adjacent carotid arteries were identified and isolated, and a bipolar VNS electrode (PerennialFlex, Cyberonics) was positioned around that artery-nerve complex. The leads were secured in place and tunneled to a subcutaneous pocket created over the dorsal aspect of the back, where the implantable VNS pulse generator (Demipulse, model 103, Cyberonics) was positioned. The incisions were closed in layers. Subsequent postoperative care included buprenorphine (0.05 mg/kg sc as needed) and cefazolin (30 mg/kg im) administered for 7 days. The pulse generator remained inactive during the recovery period (∼2 wk duration).
Animal Identification
At the time of VNS system implantation, a 12-gauge needle was used to place a microchip (AVID MicroChip ID Systems, Folsom, LA) into the interscapular subcutaneous space. A MiniTracker (AVID MicroChip ID Systems) scanner was passed over the implant site to detect the identification number assigned to each animal.
Induction of Chronic PO
PO was induced 2 wk after implantation of the VNS stimulator. The anesthetic regimen described above for VNS implantation under aseptic surgical techniques was used to perform a left thoracotomy in the second-third intercostal space to expose the descending thoracic aorta. A 3-0 surgical ligature tied around a metal tube (1–2 mm external diameter and ∼1 cm long, made from an 18-gauge needle) placed adjacent to the descending aorta was used to produce uniform constriction of the thoracic aorta. After suture placement to produce the aortic constriction, the metal tube was removed. After placement of a flexible chest tube into the chest cavity and closure of the rib space, local musculature and subcutaneous tissues were closed with absorbable sutures; the skin was closed with nonabsorbable sutures. Once the chest was closed, residual air was withdrawn via the chest tube, the chest tube was removed, and spontaneous ventilation was reinstituted. Postoperative care included administration of buprenorphine (0.05 mg/kg sc) as needed and cefazolin (30 mg/kg im) once per day for the next 7 days. Animals were maintained for (on average) 50 days after PO induction. In this group of PO animals, 17 with PO induction demonstrated clinical signs of pulmonary congestion within a few days; these animals were euthanized within 0–2 days of PO onset and were not included in subsequent data accumulation.
Neuromodulation Therapy
In 19 of these animals, active VNS therapy was initiated 10 days following PO induction: 10 were treated with right-sided [right cervical vagus (RCV)] VNS and 9 with left-sided [left cervical vagus (LCV)] VNS. These groups are designated RCV-PO and LCV-PO, respectively. The parameters chosen for VNS therapy were close to the neural fulcrum, where we previously demonstrated that any effects on heart rate (HR) are minimized by the combined effects on VNS on afferent and efferent axonal stimulation within the cervical vagosympathetic complex (8, 15). Continuous cyclic VNS therapy was delivered at a pulse frequency of 20 Hz, 250-μs pulse duration, and 22.5% duty cycle (14 s on-phase and 48 s off-phase). The average current intensity was 1.13 ± 0.04 mA for RCV and 1.17 ± 0.06 mA for LCV. The intensity of stimulation elicited by VNS therapy was limited in the guinea pig over time by its effects on water and/or food intake. In those animals that did not exhibit bradycardia, attempts to further increase stimulus intensity resulted in loss of body weight. In 12 of the animals the VNS system implant remained inactive throughout the 50-day chronic PO induction (sham treatment control group). Time-matched controls (n = 9) were also evaluated concurrently with the PO models.
Cardiac Indexes
After sedation with isoflurane (1–2% via nose cone), short-axis echocardiograms were used to determine LV internal diameter at end systole and end diastole, along with estimated LV volume, such that stroke volume could be estimated. These data, along with HR data, were used to derive cardiac output for each animal in the initial and final stages of each experiment. As such, these indexes were determined prior to PO and/or VNS implant, as well as at 50 days after PO just prior to the terminal experiment.
After completion of the echocardiogram, the isoflurane dose was increased to 2.5% until responses to hindlimb toe pinch stimuli were absent. After endotracheal intubation, mechanical ventilation was initiated and maintained with a positive-pressure ventilator (model SAR-830/P, IITC Life Science) using 100% O2. The right carotid artery was isolated, and a 2-Fr pressure-volume catheter connected to a pressure-volume loop single-segment system (MPVS, Millar Instruments, Houston, TX) was inserted into it and advanced to the LV. From this catheter, indexes of LV performance, including LV systolic pressure, LV end-diastolic pressure, and rate of change of LV developed pressure (LV +dp/dt and LV −dp/dt) were determined, along with basal HR.
Terminal Experiments
After echocardiographic and LV hemodynamic analyses, animals were euthanized via CO2 inhalation. The heart and lungs were removed rapidly and placed into ice-cold Krebs-Ringer solution (mM: 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 8 glucose) aerated with 95% O2-5% CO2 to achieve pH 7.4. Hearts were weighed, and lungs were dried at 37°C and weighed (dry lungs). The IC nerve plexus, located in the epicardium of dorsal atrial walls, was dissected free of other tissues and placed in a tissue bath, so that the tissues could be continuously superfused (6–8 ml/min) with 35–37°C Krebs-Ringer solution.
Preparation of Guinea Pig Heart Homogenates and Western Blots
The ventricles from time-matched control, PO, LCV-PO, and RCV-PO guinea pigs were removed and briefly washed in ice-cold PBS to remove blood, and the LV was removed, flash-frozen, and ground into a fine powder using a liquid nitrogen-jacketed mortar and pestle. The frozen heart powder was homogenized in RIPA buffer [50 mM Tris·HCl, pH 7.4 (Calbiochem, Darmstadt, Germany), 1% Triton X-100 (Fisher, Fair Lawn, NJ), 1% (wt/vol) sodium deoxycholate (Fisher), 0.1% (wt/vol) SDS (EMD, Billerica, MA), and 1 mM EDTA (Fisher)] with 1:100 (vol/vol) protease inhibitor cocktail mix (Sigma, St. Louis, MO). These homogenates were incubated on ice for 1 h and then centrifuged at 12,000 rpm at 4°C for 10 min. The supernatant was collected so that the following assays of tissues could be performed.
Protein quantification of lysates was performed on ventricular homogenates using the Pierce bicinchoninic acid protein assay kit (Thermo Scientific, Rockford, IL), according to the manufacturer's protocol. Protein samples were subjected to SDS-PAGE using Pierce Tris-HEPES-SDS precast 4–20% polyacrylamide mini gels (Thermo Scientific). Proteins were transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA), and Ponceau S (Sigma) staining was used to ensure complete transfer and equal protein loading. Membranes were blocked in 5% nonfat dry milk (Bio-Rad Laboratories) in Tris-buffered saline (TBS) with 0.1% Tween 20 (TBS-T) for 1 h at room temperature.
Phosphorylated Bcl-2-associated death promoter (pBAD) and BAD were exposed to ventricular tissues incubated with rabbit monoclonal primary antibodies diluted 1:1,000 in TBS-T (Cell Signaling Technology, Danvers, MA): Bcl-2-associated X (BAX), Bcl-xL, and phosphorylated Akt (pAkt). Glycogen synthase (GS) and phosphorylated GS (pGS) were incubated with rabbit polyclonal primary antibodies diluted 1:1,000 in TBS-T (Cell Signaling Technology). Membranes were incubated in primary antibody at 4°C overnight. After incubation in primary antibody, the membranes were washed three times for 10 min each in TBS-T before incubation with 1:3,000 goat anti-rabbit horseradish peroxidase-conjugated secondary antibodies (EMD Millipore, Temecula, CA) for all the primary antibodies as described above at room temperature for 1 h. Membranes were washed three times in TBS-T for 10 min. Pierce SuperSignal West Pico chemiluminescence substrate (Thermo Scientific) was used for signal detection in the G:BOX imaging system (Syngene, Frederick, MD). ImageJ software (National Institutes of Health, Bethesda, MD) was used for densitometry of the protein bands.
Terminal Deoxynucleotide Transferase-Mediated Nick-End Labeling Assay
Ventricular tissue sections were deparaffinized gradually in xylene and ethanol and then fixed in 4% paraformaldehyde and embedded in paraffin (Fisher). Apoptotic guinea pig cardiomyocytes in ventricular tissues were assayed by terminal deoxynucleotide transferase-mediated nick-end labeling (TUNEL) using CardioTACS in situ detection kit (R & D Systems, Minneapolis, MN) according to the manufacturer's instructions. Thereafter, TUNEL-positive cardiomyocytes were counted throughout random fields of tissue (×20; Nikon Eclipse TE2000s). ImageJ software was used for myocyte size determinations of paraffin sections stained with Masson's trichrome using standard procedures.
Neuronal Electrophysiological Methods
Neuronal transmembrane properties.
Intracellular voltage recordings from IC neurons derived from explanted IC ganglia placed in 35–37°C Krebs-Ringer solution were obtained by impaling cells with 3 M KCl-filled glass micropipettes (40- to 80-MΩ resistance) using an Axoclamp 2B amplifier (Molecular Devices). Data were collected, digitized, and analyzed using pClamp 10.2 (Molecular Devices). Individual neurons were used for data analysis if their resting membrane potential (RMP) was −40 mV or less and produced action potentials (APs) with an overshoot of ≥20 mV. Input resistance was determined using 0.1- and 0.2-nA pulses (500 ms). Neuronal soma excitability was monitored by observing the response to a series of long depolarizing current pulses (0.1–0.6 nA, 500 ms). The number of evoked APs vs. stimulus intensity was determined to assess relative changes in excitability. Afterhyperpolarization (AHP) durations were analyzed to determine the time to reach 50% of the amplitude from the peak of the AHP to the RMP.
For each cell, after characterization of the basic electrophysiological properties, induced changes in the number of evoked APs by depolarizing pulses were again assessed immediately following a 1- to 2-s application of NE (10−3 M; Sigma) or bethanechol (a muscarinic agonist, 10−3 M; Sigma). Drugs were applied by local pressure ejection (6–9 psi; Picospritzer, General Valve) through small-tip-diameter (5–10 μm) glass micropipettes positioned 50–100 μm from the individual neuron. For multiple tests of responses in the same cell, the cells were allowed to remain in the circulating Krebs solution for several minutes between applications, until the responses returned to control levels.
Neuronal synaptic efficacy.
To activate synaptic inputs to investigated neurons, a bipolar concentric electrode was placed on nerve bundles connected to the ganglion containing the neuron of interest. Orthodromic responses to fiber tract stimulation (0.1–10 V, 1-ms duration) were assessed by studying 1) the ability of axonal activation to generate an excitatory postsynaptic potential and/or 2) the presence of a time delay between the stimulus artifact and a neuronal response. Suprathreshold stimuli leading to APs were then applied in 2-s trains at varying frequencies (5, 10, 20, and 30 Hz). The number of APs produced by the neuron of interest at each stimulus frequency was assessed.
Statistical Analysis
Cardiac indexes recorded in the control, PO, and different therapy states were analyzed via ANOVA to compare changes induced among different animal groups compared with baseline conditions, as well as among groups. The Holm-Sidak method was used for all pair-wise post hoc multiple comparisons. As neuronal activity was not normally distributed when analyzed using a Shapiro-Wilk test, a nonparametric Friedman's test was utilized at the ordinal level followed by post hoc Wilcoxon's signed-rank tests with Bonferroni's correction to determine differences in neural data obtained in the different study groups. A Shapiro-Wilk test showed data depicting HR and LV pressure indexes (Table 1) and tissue weights (Table 2), along with neuronal transmembrane properties (Table 3) and synaptic properties (see Fig. 3), as well as myocyte structure (see Fig. 5) and function (see Figs. 6–8), to be continuous and normally distributed. These data were analyzed using a simple or a mixed-model ANOVA followed by a Newman-Keuls post hoc analysis. P < 0.05 was considered statistically significant. Statistical analyses were conducted using SigmaPlot 12 software.
Table 1.
Cardiac hemodynamics at termination: among-group comparisons
| Chronic PO Treatment | n | LVSP, mmHg | LVEDP, mmHg | LV +dp/dt, mmHg/s | LV −dp/dt, mmHg/s | HR, beats/min |
|---|---|---|---|---|---|---|
| Sham VNS | 12 | 49.3 ± 3.4 | 3.1 ± 1.2 | 1,211 ± 100 | −1,265 ± 126 | 198.0 ± 7.2 |
| LCV VNS | 9 | 61.1 ± 4.6# | 0.9 ± 1.1 | 1,912 ± 206*# | −1,839 ± 164*# | 215.7 ± 8.8# |
| RCV VNS | 8 | 44.2 ± 2.7 | 2.5 ± 0.5 | 1,058 ± 187 | −1,054 ± 133 | 177.1 ± 12.9 |
Values are means ± SE; n, number of animals.
PO, pressure overload; VNS, vagus nerve stimulation; LCV and RCV, left and right cervical vagus; LVSP, left ventricular (LV) systolic pressure; LVEDP, LV end-diastolic pressure; LV +dp/dt and LV −dp/dt, rate of change of LV developed pressure; HR, heart rate. #P ≤ 0.05 vs. RCV;
P ≤ 0.05 vs. sham VNS.
Table 2.
Analysis of heart and lung weight in controls and PO, LCV-PO, and RCV-PO animals
| Controls (n = 8) | PO (n = 12) | LCV-PO (n = 9) | RCV-PO (n = 10) | |
|---|---|---|---|---|
| Age at termination, wk | 28.2 ± 1.1 | 27.3 ± 1.8 | 26.5 ± 2.8 | 25.6 ± 1.9 |
| Postoperative recovery, wk | 7.3 ± 0.5 | 7.3 ± 0.3 | 7.5 ± 0.5 | |
| Body wt, g | 1011 ± 56 | 1030 ± 77 | 969 ± 94 | 946 ± 86 |
| Heart wt, %body wt | 0.65 ± 0.08 | 0.57 ± 0.05 | 0.59 ± 0.08 | 0.60 ± 0.07 |
| Wet lung wt, %body wt | 0.50 ± 0.08 | 0.41 ± 0.07 | 0.48 ± 0.17 | 0.42 ± 0.05 |
| Dry lung wt, %body wt | 0.09 ± 0.01 | 0.08 ± 0.03 | 0.09 ± 0.05 | 0.09 ± 0.01 |
Values are means ± SD; n, number of animals. No significant effect (P < 0.05) was found between groups (by ANOVA).
Table 3.
Soma properties of intrinsic cardiac neurons derived from controls and PO, RCV-PO, and LCV-PO animals
| Controls (n = 66) | PO (n = 131) | RCV-PO (n = 113) | LCV-PO (n = 78) | |
|---|---|---|---|---|
| RMP, mV | −49.64 ± 0.83 | −49.82 ± 0.68 | −56.58 ± 0.80* | −52.23 ± 0.81 |
| Input resistance, MΩ | 73.5 ± 6.0 | 67.9 ± 5.0 | 73.1 ± 6.6 | 65.3 ± 5.2 |
| AHP amplitude, mV | 15.65 ± 0.58 | 17.46 ± 0.45 | 16.56 ± 0.49 | 16.11 ± 0.53 |
| AHP half-decay time, ms | 119.49 ± 6.15 | 99.46 ± 5.77 | 141.4 ± 6.97* | 109.9 ± 8.37 |
Values are means ± SE; n, number of neurons.
RMP, resting membrane potential; AHP, afterhyperpolarization.
Significant effect (P < 0.05) vs. other groups (by ANOVA).
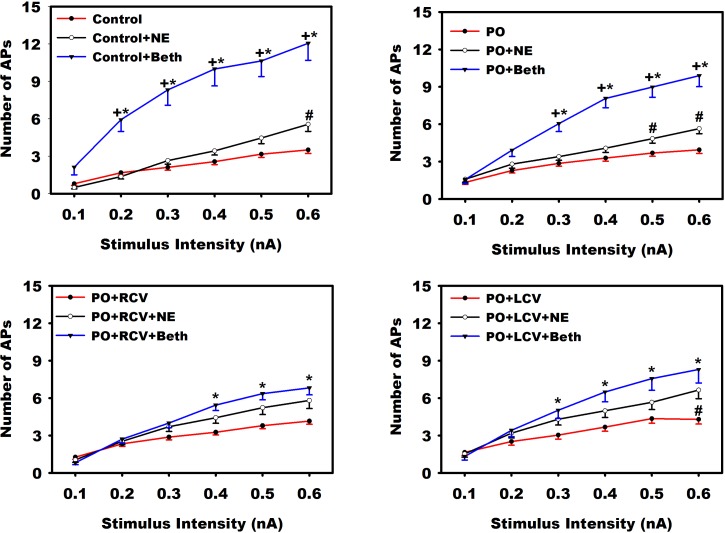
Fig. 3.
Muscarinic enhancement of neuronal excitability is mitigated with VNS. Evoked action potential (AP) frequencies in response to increasing intracellular stimulus intensities were evaluated concurrently with brief (1-s) local exposure to exogenous norepinephrine (NE) or bethanechol (Beth) in intrinsic cardiac (IC) somata derived from control animals and animals subjected to PO with and without chronic VNS (RCV or LCV). Animals were evaluated 50 days after PO induction. RCV or LCV was initiated 10 days after PO induction and was maintained to termination. Values are means ± SE from ∼60 cells for each condition. A nonparametric Friedman's test was used to evaluate difference among groups followed by Wilcoxon's signed-rank post hoc tests using Bonferroni's correction. *P < 0.05, baseline (control, PO, PO + RCV, or PO + LCV) vs. Beth. +P < 0.05, NE vs. Beth. #P < 0.05, baseline (control, PO, PO + RCV, or PO + LCV) vs. NE.
Fig. 5.
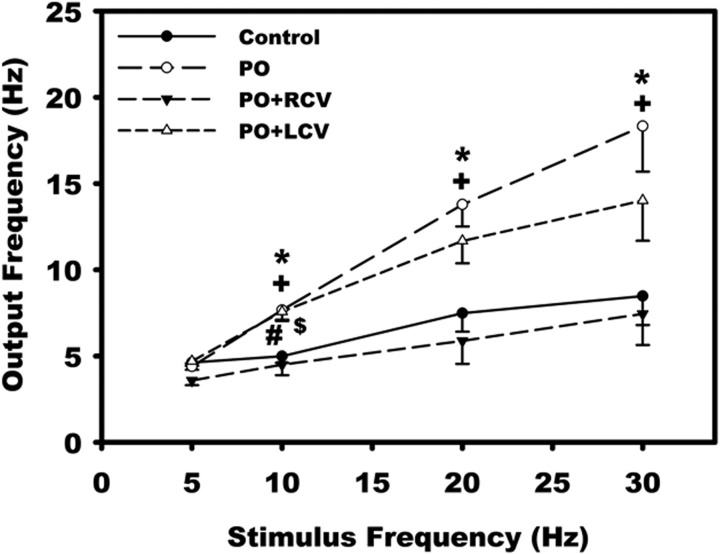
Chronic VNS reduces synaptic efficacy of IC neurons. Nerve fibers synapsing with the IC neurons were stimulated via an extracellular concentric electrode (0.1–10 V, 2 ms) for 2 s at frequencies of 5, 10, 20, and 30 Hz. Values are means ± SE from ∼30 cells for each condition. *P < 0.05, control vs. PO; #P < 0.05, control vs. PO + LCV; +P < 0.05, PO vs. PO + RCV; $P < 0.05, PO + LCV vs. PO + RCV (by ANOVA followed by Newman-Keuls post hoc analysis).
Fig. 6.
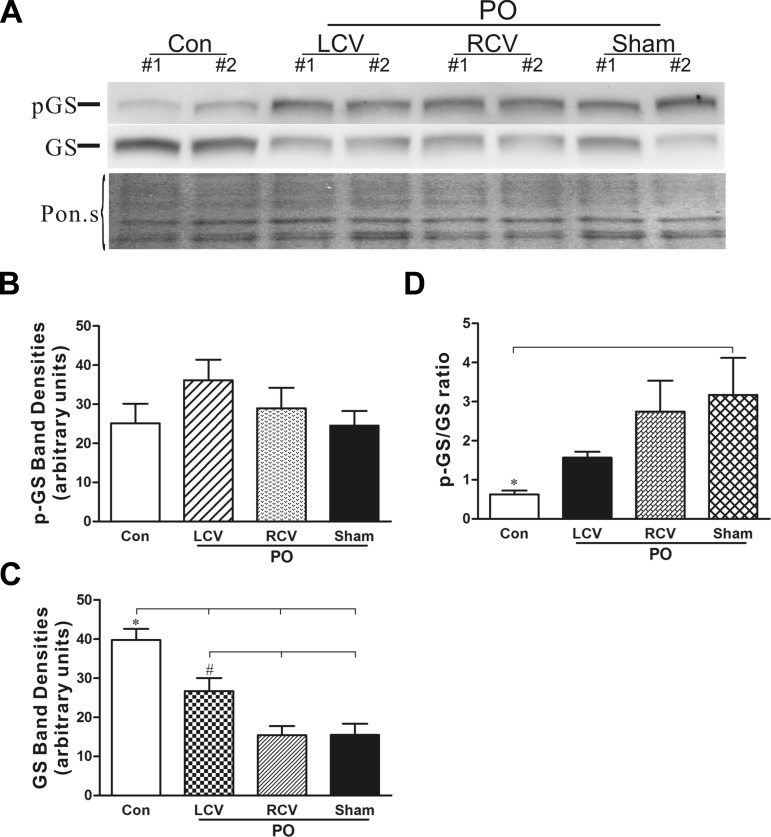
Glycogen synthase (GS) protein levels are significantly reduced and shifted to the inactive phosphorylated form (pGS) in PO; these effects are opposed by left vagus stimulation. A: representative Western blots showing pGS and total GS protein levels in control (n = 7), PO (n = 7), PO with right vagus stimulation (PO-RCV, n = 7), and PO with left vagus stimulation (PO-LCV, n = 3) heart extracts. Blot stained with Ponceau S (Pon.s) is shown as a protein-loading control. B and C: densitometry analysis of pGS and total GS protein band intensity for all Western blots. *P < 0.05, Con vs. all PO; #P < 0.05, PO + LCV vs. PO-RCV and PO + sham (by ANOVA followed by Newman-Keuls post hoc analysis). D: ratio of pGS to GS band intensities. *P < 0.05, Con vs. all PO (by ANOVA followed by Newman-Keuls post hoc analysis).
Fig. 8.
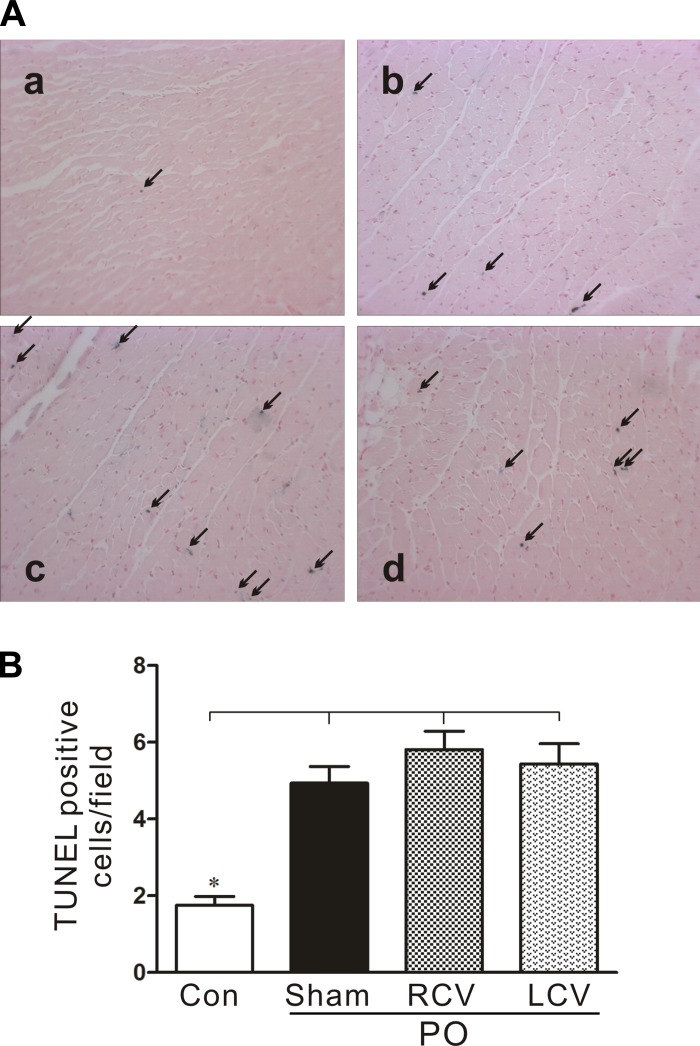
Moderate increases in the number of apoptotic myocytes with PO are unaffected by RCV or LCV therapies. A: representative terminal deoxynucleotide transferase-mediated nick-end labeling (TUNEL) in CardioTACS-stained sections of LV tissue from control (a) and PO-treated [PO + sham (b), PO + RCV (c), and PO + LCV (d)] hearts. Arrows indicate blue-stained nuclei, indicative of DNA fragmentation, a hallmark of apoptosis. B: quantification of apoptotic cells in experimental groups shown in A. *P < 0.05 vs. all PO.
RESULTS
Hemodynamic Indexes
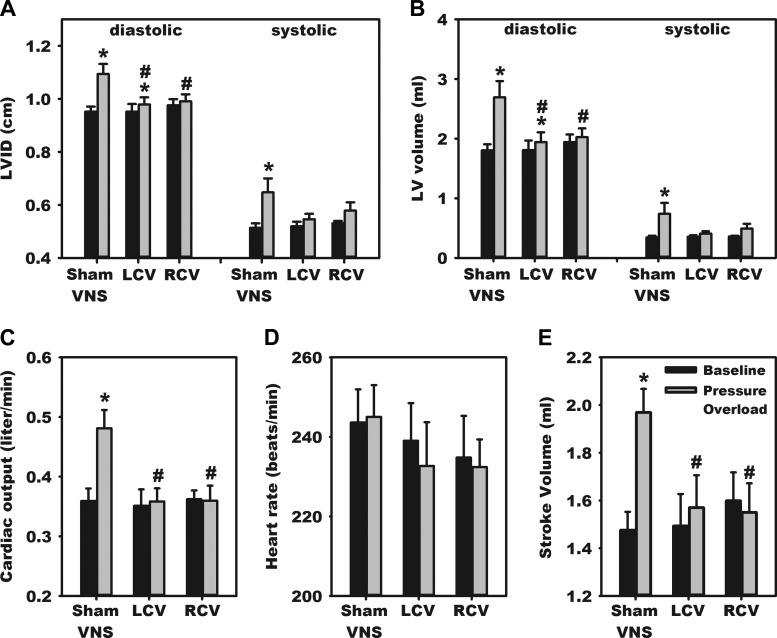
Paired echocardiographic assessments, from baseline vs. time of termination (51.5 ± 0.5 days after PO induction), demonstrated that LV diameters and volumes (systolic and diastolic) increased significantly in the untreated PO states (Fig. 1). PO likewise was associated with significantly increased LV stroke volume and cardiac output. These PO-induced cardiac changes were minimized by application of chronic VNS, either RCV or LCV. In these three chronic PO groups, LV pressure measurement at termination further indicted that LCV differentially increased LV chamber systolic pressure and ±dp/dt relative to sham VNS or RCV (Table 1).
Fig. 1.
Vagal nerve stimulation (VNS) mitigates pressure overload (PO)-induced hypertrophy and hyperdynamic cardiac behavior. Echocardiographic indexes were determined via a short-axis view at baseline (before) and again at 50 days after PO induction. Treatment groups are as follows: animals with VNS implant, but without active stimulation (sham VNS) and animals in which right cervical vagus (RCV) or left cervical vagus (LCV) stimulation was initiated at 10 days after PO induction and maintained until termination. Cardiac indexes evaluated included left ventricular (LV) internal diameter (LVID; A), LV volume (B), cardiac output (C), heart rate (D), and stroke volume (E). *P < 0.05 vs. baseline; #P < 0.05 vs. sham VNS.
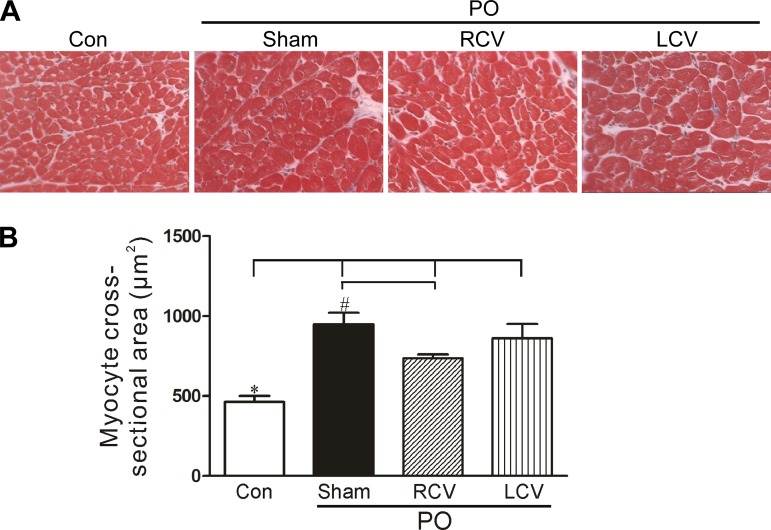
In support of echocardiographic data, measurement of LV myocyte cross-sectional area confirmed the PO-induced hypertrophy (Fig. 2). Note the doubling in myocyte size in the sham VNS group, a response that was mitigated by RCV, but not by LCV. In contrast to the myocyte cross-sectional area, no significant differences were found in heart (wet) and lung (wet and dry) weights as a percentage of body weight among treated groups (Table 2).
Fig. 2.
Myocyte hypertrophy associated with PO is significantly reduced by RCV. A: representative Masson's trichrome-stained sections of LV tissue from control and PO hearts. B: histomorphometric quantification of myocyte cross-sectional areas of experimental tissues. PO leads to greater myocyte cross-sectional area (*P < 0.05). Hypertrophy was significantly reduced (#P < 0.05) in RCV-treated PO tissue compared with sham VNS PO-treated tissue. Con, control.
IC Neuronal Transmembrane Properties
The transmembrane potentials of IC neurons derived from controls and PO animals, as well as animals subjected to right (RCV-PO) vs. left (LCV-PO) VNS [including time-matched sham VNS (PO)] are summarized in Table 3. No significant differences in the amplitude of AHP or neuronal input resistances were identified among groups. However, cellular RMPs increased (became more negative) in neurons derived from PO animals subjected to RCV compared with controls or animals subjected to PO alone. AHP half-decay time also increased with RCV in the presence of PO compared with the other groups.
Functional excitability of somata, as assessed by measurement of the number of APs evoked in response to intracellular depolarizing current injection steps, was not significantly altered by PO alone or in response to chronic VNS (Fig. 3). Across all groups, changes in IC neuronal sensitivity elicited by local NE application was less than that elicited by bethanecol. Soma excitability to muscarinic agonists was blunted by chronic RCV or LCV (Fig. 3, bottom).
IC Neuronal Synaptic Efficacy
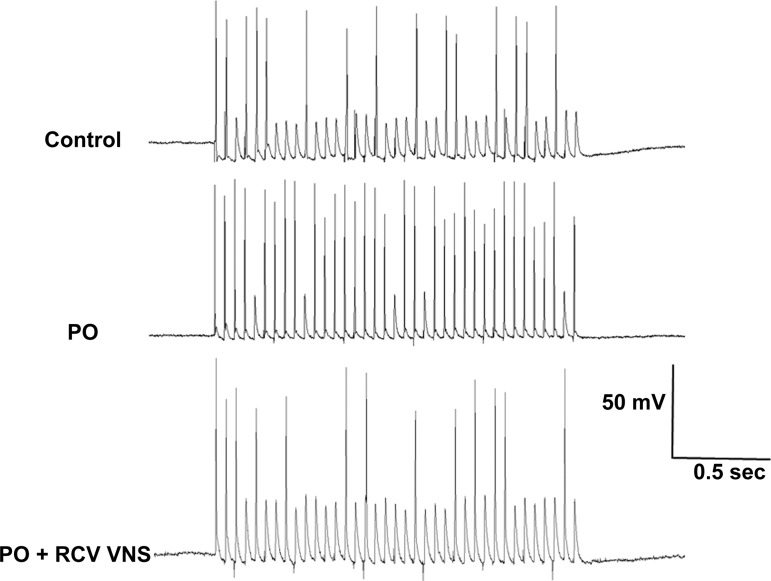
Input synaptic efficacy was evaluated by measuring IC neuronal responsiveness during stimulation of axon bundles associated with the ganglia containing these neurons of interest (Fig. 4). Suprathreshold trains of stimuli (delivered for 2 s at 5, 10, 20, and 30 Hz) resulted in significantly greater output frequencies of neurons derived from PO animals than controls (Fig. 5, control). While RCV restored this index to control values (Fig. 5, PO + RCV), LCV only showed a tendency to reduce the index.
Fig. 4.
Representative responses of IC neurons to local bioelectric stimulation of primary nerve inputs. Neurons were derived from control, PO, and PO + RCV VNS animal models. Nerve fibers were stimulated at 20 Hz for 2 s.
Cardiomyocytes
Chronic PO alters cardiomyocyte structure and function (Figs. 1 and 2). One aspect of this remodeling can involve changes in energy utilization (54). GS protein levels were significantly depressed in PO tissue (Fig. 6). Moreover, the ratio of the inactive pGS to the unphosphorylated GS was increased with PO. These changes are consistent with greater mobilization/utilization of glucose in the PO tissue. The changes in GS expression, induced by PO, were significantly mitigated by LCV, but not by RCV.
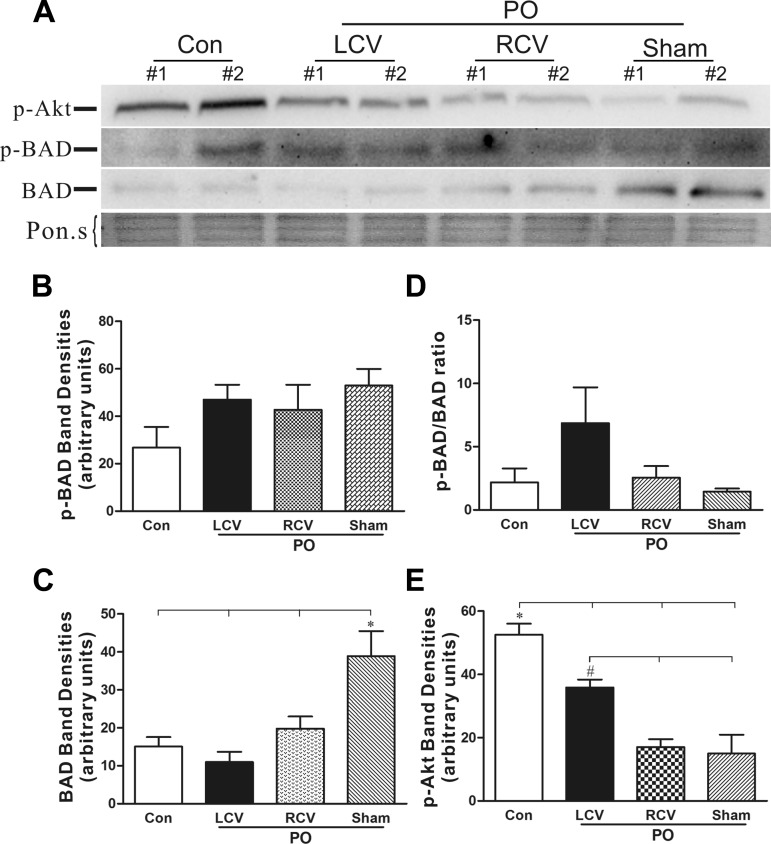
Apoptosis contributes to the transition from hyperdynamic hypertrophied myocardium to chronic heart failure and the potential for sudden cardiac death (27, 38). The levels of pAkt, the active antiapoptotic form of the kinase, were significantly reduced in PO tissue (Fig. 7E). Chronic LCV significantly blunted this effect of PO on pAkt levels compared with PO-sham VNS. BAX and Bcl-xL levels did not change significantly among the experimental groups (not shown). BAD protein levels were elevated in PO tissue. BAD phosphorylation status did not significantly differ among the experimental groups (Fig. 7B). However, VNS led to significant reductions in gross BAD protein levels (Fig. 7C). While circumstantial, these findings suggest that VNS may exert antiapoptotic effects on the stressed myocardium. This led us to evaluate the occurrence of apoptosis within the myocardium. Significantly more apoptotic nuclei were found in PO tissue than in control unstressed tissue (Fig. 8). However, VNS had no significant effect on the numbers of myocytes undergoing apoptosis in response to PO stress.
Fig. 7.
Phosphorylated Akt (pAkt, active form) was decreased and proapoptotic Bcl-2-associated death promoter (BAD) protein level was significantly elevated in the PO heart. LCV partially restored pAkt and BAD protein levels to control values. A: representative Western blots probed for pAkt and pBAD proteins in control (Con), PO + LCV, PO + RCV, and PO + sham (n = 5) heart extracts. Total BAD protein levels are also shown. Ponceau S (Pon.s) staining is shown as a protein-loading control. B and C: densitometry analysis of pBAD and total BAD protein band intensity for all Western blots. *P < 0.05, PO + sham vs. Con, PO + LCV, and PO + RCV (by ANOVA followed by Newman-Keuls post hoc analysis). D: ratio of pBAD to BAD. No significant difference was observed. E: densitometry analysis of pAkt protein level (n = 4). *P < 0.05, Con vs. all PO; #P < 0.05, PO + LCV vs. PO + RCV and PO + sham.
DISCUSSION
A critical benchmark for any interventional therapy applied in progressive cardiovascular pathology is ultimately its efficacy to preserve cardiac function, often in the presence of a sustained stressor. Aortic banding provides a model of chronic PO stress that remains throughout (30, 38). From an echocardiographic perspective, the time point that was evaluated here reflected a hyperdynamic state characterized by a 34% increase in cardiac output with a corresponding increase in systolic and diastolic LV volumes. From a histomorphometric perspective, myocyte cross-sectional area doubled with PO. From an autonomic perspective, withdrawal of central parasympathetic drive coupled with reflex-mediated sympathoexcitation and concurrent activation of angiotensin II contributed to the adverse remodeling (24, 33, 34, 38). This neurohumoral interplay represents an emerging target for therapeutics (19, 24, 25). This study demonstrated that chronic VNS therapy directly targets the ICNS when appropriately applied, such that LV functional deterioration during the evolution of chronic LV PO is mitigated. Our data further indicate that mitigation of adverse PO-induced remodeling involves both myocyte- and neural-dependent mechanisms.
VNS and Cardioprotection
PO-induced heart failure is accompanied by changes in the ventricular metabolic profile, affecting, among other things, a shift to greater reliance on glucose that is associated with downregulation of fatty acid oxidation (54). We found an increase in the ratio of inactive pGS to unphosphorylated GS during the evolution of chronic PO. To our knowledge, this is the first report of a change in GS expression and phosphorylation status by PO. These changes are consistent with greater mobilization/utilization of glucose in the PO ventricle (54). Furthermore, changes in ventricular GS expression were mitigated by LCV, but not by RCV. Autonomic neural regulation of glucose and fatty acid metabolism is widely appreciated in liver and skeletal muscle (47) in the context of “rest and digest.” For instance, VNS causes a large increase in the activity of liver GS (52). Direct neural sympathetic effects include stimulation of glycogenolysis in skeletal muscle and liver (47). It is thus surprising that an almost total dearth of information exists concerning autonomic effects on myocardial metabolism. Our data indicate that direct neural control of heart metabolism may be profound and that VNS therapy holds the promise of exploiting metabolic regulation to effect better outcomes in intractable pathologies. While further investigation of this issue is warranted, findings concerning ventricular GS changes indicate a reordering of myocardial metabolism in response to VNS, such that the heart becomes more resistant to the pathological stress associated with PO.
Apoptosis and matrix reorganization contribute to the transition from hyperdynamic hypertrophied myocardium to heart failure (24, 29, 38). We recently reported the efficacy of VNS to minimize the proapoptotic BAX in guinea pigs with chronic myocardial infarction (15). By analogy, in the PO model reported here, the levels of pAkt, the active antiapoptotic form of the kinase, were significantly reduced. Importantly, chronic LCV significantly blunted this effect of PO on pAkt levels compared with PO-sham VNS. However, while chronic PO was associated with significantly more apoptotic than normal nuclei, VNS did not reduce this maladaptive response to PO stress. The difference in part may reflect differences mediated by an eccentric ventricular stressor (e.g., myocardial infarction) compared with the concentric stress imposed by PO (29, 38, 62). It should be further recognized that many of the proteins evaluated subserve dual roles in both apoptotic and hypertrophic cardiac responses (45). Taken together, these data suggest that modulation of cardiomyocyte proteins by VNS relates primarily to the hypertrophic response, rather than being dependent on programmed cell death.
VNS and the Autonomic Neuraxis-Cardiac Interface
Neural control of regional cardiac function is dependent on the dynamic interplay between peripheral and central reflexes (11, 12). The peripheral reflexes involve those contained within the ICNS and within extracardiac autonomic ganglia, including the mediastinal, middle cervical, and stellate ganglia (11, 12). Central reflex components of the cardiac nervous system include the spinal cord, brainstem, and higher centers (5, 22, 35). Each of these processing nodes contain afferent, efferent, and neural processing neurons, the later referred to as local circuit neurons (11). Coordination within these networks allows for effective control of regional cardiac function and the distribution of blood flow throughout the body at baseline and in response to stress (11, 39, 41). Stressors that lead to imbalances within these autonomic networks can lead to disruptions in autonomic outflows, which, in turn, can contribute to adverse remodeling of heart mechanical function and the potential for arrhythmias, including sudden cardiac death (25, 27, 40). The autonomic imbalances, primarily afferent-driven, in turn, are associated with adverse neural remodeling in neural circuits from those on the heart up to and including higher centers up to the insular cortex (3, 30, 37, 42, 50). Autonomic regulation therapy, of which VNS is one modality, is predicated on targeting specific processing centers to stabilize excessive reflex responses and, thereby, moderate efferent outputs (7, 19, 26, 56).
The ICNS is the most proximal reflex processor of the cardiac nervous system (11). It is primarily associated with short-loop coordination of regional cardiac electrical and mechanical function (11). It consists of aggregates on ganglionated plexi that have specific spheres of influence (6, 20). The separate aggregates maintain a degree of coordination imposed by local circuit intra- and interganglionic projections, common shared afferent inputs, and descending efferent projections (6, 13, 14, 57). These efferent projections include sympathetic and parasympathetic efferent axons, with 1) direct connections to postganglionic somata and 2) multisynaptic inputs onto the local circuit (processing) neurons of the ICNS (14, 55, 57). It is recognized that major interactions between sympathetic and parasympathetic efferent neuronal control are exerted at the level of the ICNS and at the end terminus of efferent projections to the heart (28, 44, 46, 51). At least in larger animals, vagus projections to the ventricles are widespread and bilateral (9, 60). In contrast, the sympathetic projections tend to be more unilateral (2, 10). This difference in efferent distribution may explain in part the different efficacy of right vs. left VNS to impact the cardioneural remodeling induced by PO. Regardless, the antiadrenergic effects of VNS are likely a major contributor to the preservation of cardiac function in the setting of ischemic and nonischemic cardiac pathologies.
Cardiac pathologies remodel multiple levels of the neural hierarchy for cardiac control. With respect to heart failure, autonomic regulation is deranged, as usually reflected in sympathoexcitation with a corresponding decrease in central parasympathetic drive (25, 62). Alterations in neurotransmitter interactions at IC somata in conjunction with alterations in synaptic processing within the ICNS are a reflection of these adaptations (17, 30, 33, 34). This results in changes in passive and active membrane properties that underlie overall network function. The restoration of synaptic efficacy of IC neurons to “normal” is a reflection of the restraining effects that VNS can exert in peripheral networks. Several potential ionic mechanisms could underlie these neuronal responses. Indeed, several muscarinic receptor-mediated changes in ion currents, including inhibition of the M current, regulation of the delayed rectifier potassium current, inhibition of calcium currents, and enhanced intracellular calcium release, have been described in IC neurons (1, 4, 16, 48). The downward shift in the modulator effects on IC excitability exerted by muscarinic receptors may reflect some of these changes. The specific neuromediators and neuromodulators involved in cardiac disease-induced neural remodeling and, mechanistically, how these are impacted by autonomic regulation therapy remain largely undefined and represent a critical area for future studies.
It is also critical to note that the majority of axons in a cervical vagus are afferent in nature, projecting directly to neurons in the nucleus tractus solitarius of the medulla (5, 18). By activating such afferent axons with VNS therapy, centrally mediated reflexes target both the sympathetic and parasympathetic efferent neurons controlling the heart (8, 59). Recent data indicate that low-level VNS can exert afferent-mediated withdrawal of centrally derived parasympathetic efferent activity (59). Further increases in stimulus intensity recruit parasympathetic preganglionic axons with the expected suppression of regional cardiac electrical and mechanical indexes (8, 44). We propose that the optimum therapeutic parameters for cervical VNS therapy are at the point at which afferent and efferent fibers are activated in a balanced manner, that is, when afferent-mediated decreases in central-mediated parasympathetic drive are counteracted by direct activation of the cardiac parasympathetic efferent projections to the ICNS and heart. At this point, the net result is a null HR response. We have defined this as the neural fulcrum (8), and the studies presented here utilized this concept to establish the adequacy of the VNS protocol.
Limitations
While we were able to demonstrate functional manifestations of VNS-mediated changes in ICNS function, we did not attempt to histologically detect changes to neuronal somata. Furthermore, we did not characterize the phenotype of the IC neurons from which we recorded, nor by the techniques utilized could we distinguish between postganglionic somata and interneurons contained within the ICNS (14, 36, 48, 50). At least in the guinea pig model, the IC neurons recorded with sharp electrodes are known to be primarily cholinergic in nature (31). Finally, we evaluated IC neural function in explanted ganglia. These peripheral neural networks no longer have endogenous central drive or afferent feedback. Nevertheless, as demonstrated here, important aspects of active and passive membrane properties can be unraveled with such approaches. Future studies should consider the possible contribution of cardiac disease-induced changes in IC neural phenotypes and network interactions that contribute to autonomic dysautonomia associated with ischemic and nonischemic heart disease.
With respect to VNS therapy, we evaluated only one frequency (20 Hz) at a stimulus intensity where afferent- and efferent-evoked responses balanced each other, such that little or no change in HR was evoked (8). VNS was able to engage the cardiac nervous system while minimizing the potential for rebound effects during the off-phase that accompanies an intermittent VNS protocol. Future studies should evaluate other VNS stimulus paradigms (duty cycle, frequency, intensity, and pulse width). VNS, as applied here, nonselectively activates vagal afferent fibers arising from thoracic and visceral structures (8, 18, 19). Future studies should consider the effects of cardiac- vs. non-cardiac-related afferents in mediating central reflex effects. Finally, VNS was delivered early in the disease process. Future studies should also evaluate the efficacy of late-onset VNS and the potential of VNS to reverse-remodel an already established hypertrophic state and in even later stages when the hyperdynamic compensated state is transitioning to decompensated heart failure.
Significance and Perspectives
VNS represents an emerging neuromodulation therapy for treating heart failure. Electrical stimulation of the cervical vagosympathetic truck activates ascending and descending axonal projections therein, thus having the potential to impact both central and peripheral aspects of the cardiac neuraxis to modulate cardiomyocytes. The results of this study indicate that, in animal models, the deleterious consequences of long-term PO on cardiac structure/function can be attenuated by chronic VNS therapy. This therapy acts, in part, by directly and reflexly targeting IC neurons to modify their autonomic outflow and, specifically, to counteract the sympathoexcitation induced by PO. VNS, via modulation of the neural-myocyte interface, likewise can render a state of cardioprotection in the stressed heart. This protection, in part, likely reflects induced changes in cardiomyocyte energy pathways.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-71830 (J. L. Ardell) and by Cyberonics, Inc. (J. L. Ardell, Principal Investigator).
DISCLOSURES
J. L. Ardell and J. A. Armour serve as scientific advisors to Cyberonics. B. H. KenKnight is an employee of Cyberonics.
AUTHOR CONTRIBUTIONS
E.B., G.L.W., and J.L.A. developed the concept and designed the research; E.B., G.L.W., E.M.S., and Y.L. performed the experiments; E.B., G.L.W., E.M.S., Y.L., R.W.C., and J.L.A. analyzed the data; E.B., G.L.W., E.M.S., R.W.C., B.H.K., J.A.A., and J.L.A. interpreted the results of the experiments; E.B., G.L.W., and J.L.A. prepared the figures; E.B., G.L.W., J.A.A., and J.L.A. drafted the manuscript; E.B., G.L.W., B.H.K., J.A.A., and J.L.A. edited and revised the manuscript; E.B., G.L.W., E.M.S., Y.L., R.W.C., B.H.K., J.A.A., and J.L.A. approved the final version of the manuscript.
REFERENCES
- 1.Adams DJ, Cuevas J. Electrophysiological properties of intrinsic cardiac neurons. In: Basic and Clinical Neurocardiology, edited by Armour JA, Ardell JL. New York: Oxford University Press, 2004, p. 1–60. [Google Scholar]
- 2.Ajijola OA, Vaseghi M, Zhou W, Yamakawa K, Benharash P, Hadaya J, Lux RL, Mahajan A, Shivkumar K. Functional differences between junctional and extrajunctional adrenergic receptor activation in mammalian ventricle. Am J Physiol Heart Circ Physiol 304: H579–H588, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajijola OA, Yagishita D, Reddy NK, Yamakawa K, Vaseghi M, Downs AM, Hoover DB, Ardell JL, Shivkumar K. Remodeling of stellate ganglion neurons after spatially targeted myocardial infarction: neuropeptide and morphologic changes. Heart Rhythm 12: 1027–1035, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen TG, Burnstock G. M1 and M2 muscarinic receptors mediate excitation and inhibition of guinea-pig intracardiac neurones in culture. J Physiol 422: 463–480, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andresen MC, Kunze DL, Mendelowitz D. Central nervous system regulation of the heart. In: Basic and Clinical Neurocardiology, edited by Armour JA, Ardell JL. New York: Oxford University Press, 2004, p. 187–219. [Google Scholar]
- 6.Ardell JL. Intrathoracic neuronal regulation of cardiac function In: Basic and Clinical Neurocardiology, edited by Armour JA, Ardell JL. New York: Oxford University Press, 2004, p. 118–152. [Google Scholar]
- 7.Ardell JL, Cardinal R, Vermeulen M, Armour JA. Dorsal spinal cord stimulation obtunds the capacity of intrathoracic extracardiac neurons to transduce myocardial ischemia. Am J Physiol Regul Integr Comp Physiol 297: R470–R477, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ardell JL, Rajendran PS, Nier HA, KenKnight BH, Armour JA. Central-peripheral neural network interactions evoked by vagus nerve stimulation: functional consequences on control of cardiac function. Am J Physiol Heart Circ Physiol 309: H1740–H1752, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ardell JL, Randall WC. Selective vagal innervation of sinoatrial and atrioventricular nodes in canine heart. Am J Physiol Heart Circ Physiol 251: H764–H773, 1986. [DOI] [PubMed] [Google Scholar]
- 10.Ardell JL, Randall WC, Cannon WJ, Schmacht DC, Tasdemiroglu E. Differential sympathetic regulation of automatic, conductile, and contractile tissue in dog heart. Am J Physiol Heart Circ Physiol 255: H1050–H1059, 1988. [DOI] [PubMed] [Google Scholar]
- 11.Armour JA. Potential clinical relevance of the “little brain” on the mammalian heart. Exp Physiol 93: 165–176, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Armour JA, Ardell JL. Basic and Clinical Neurocardiology. New York: Oxford University Press, 2004. [Google Scholar]
- 13.Armour JA, Kember G. Cardiac sensory neurons. In: Basic and Clinical Neurocardiology, edited by Armour JA, Ardell JL. New York: Oxford University Press, 2004, p. 79–117. [Google Scholar]
- 14.Beaumont E, Salavatian S, Southerland EM, Vinet A, Jacquemet V, Armour JA, Ardell JL. Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J Physiol 591: 4515–4533, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaumont E, Southerland EM, Hardwick JC, Wright GL, Ryan S, Li Y, KenKnight BH, Armour JA, Ardell JL. Vagus nerve stimulation mitigates intrinsic cardiac neuronal and adverse myocyte remodeling postmyocardial infarction. Am J Physiol Heart Circ Physiol 309: H1198–H1206, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beker F, Weber M, Fink RH, Adams DJ. Muscarinic and nicotinic ACh receptor activation differentially mobilize Ca2+ in rat intracardiac ganglion neurons. J Neurophysiol 90: 1956–1964, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Bibevski S, Dunlap ME. Evidence for impaired vagus nerve activity in heart failure. Heart Fail Rev 16: 129–135, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Bonaz B, Picq C, Sinniger V, Mayol JF, Clarencon D. Vagus nerve stimulation: from epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol Motil 25: 208–221, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Buckley U, Shivkumar K, Ardell JL. Autonomic regulation therapy in heart failure. Curr Heart Fail Rep 12: 284–293, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardinal R, Page P, Vermeulen M, Ardell JL, Armour JA. Spatially divergent cardiac responses to nicotinic stimulation of ganglionated plexus neurons in the canine heart. Auton Neurosci 145: 55–62, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee NA, Singh JP. Novel interventional therapies to modulate the autonomic tone in heart failure. JACC Heart Fail 3: 786–802, 2015. [DOI] [PubMed] [Google Scholar]
- 22.Coote JH. Myths and realities of the cardiac vagus. J Physiol 591: 4073–4085, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Ferrari GM, Crijns HJ, Borggrefe M, Milasinovic G, Smid J, Zabel M, Gavazzi A, Sanzo A, Dennert R, Kuschyk J, Raspopovic S, Klein H, Swedberg K, Schwartz PJ, CardioFit Multicenter Trial I. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J 32: 847–855, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Dell'Italia LJ. Translational success stories: angiotensin receptor 1 antagonists in heart failure. Circ Res 109: 437–452, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res 114: 1815–1826, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Foreman RD, Linderoth B, Ardell JL, Barron KW, Chandler MJ, Hull SS Jr, TerHorst GJ, DeJongste MJ, Armour JA. Modulation of intrinsic cardiac neurons by spinal cord stimulation: implications for its therapeutic use in angina pectoris. Cardiovasc Res 47: 367–375, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda K, Kanazawa H, Aizawa Y, Ardell JL, Shivkumar K. Cardiac innervation and sudden cardiac death. Circ Res 116: 2005–2019, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furukawa Y, Hoyano Y, Chiba S. Parasympathetic inhibition of sympathetic effects on sinus rate in anesthetized dogs. Am J Physiol Heart Circ Physiol 271: H44–H50, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Gladden JD, Linke WA, Redfield MM. Heart failure with preserved ejection fraction. Pflügers Arch 466: 1037–1053, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardwick JC, Baran CN, Southerland EM, Ardell JL. Remodeling of the guinea pig intrinsic cardiac plexus with chronic pressure overload. Am J Physiol Regul Integr Comp Physiol 297: R859–R866, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardwick JC, Mawe GM, Parsons RL. Evidence for afferent fiber innervation of parasympathetic neurons of the guinea-pig cardiac ganglion. J Auton Nerv Syst 53: 166–174, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Hardwick JC, Ryan SE, Beaumont E, Ardell JL, Southerland EM. Dynamic remodeling of the guinea pig intrinsic cardiac plexus induced by chronic myocardial infarction. Auton Neurosci 181: 4–12, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardwick JC, Ryan SE, Powers EN, Southerland EM, Ardell JL. Angiotensin receptors alter myocardial infarction-induced remodeling of the guinea pig cardiac plexus. Am J Physiol Regul Integr Comp Physiol 309: R179–R188, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardwick JC, Southerland EM, Girasole AE, Ryan SE, Negrotto S, Ardell JL. Remodeling of intrinsic cardiac neurons: effects of β-adrenergic receptor blockade in guinea pig models of chronic heart disease. Am J Physiol Regul Integr Comp Physiol 303: R950–R958, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harper RM, Kumar R, Macey PM, Ogren JA, Richardson HL. Functional neuroanatomy and sleep-disordered breathing: implications for autonomic regulation. Anat Rec 295: 1385–1395, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Hoover DB, Isaacs ER, Jacques F, Hoard JL, Page P, Armour JA. Localization of multiple neurotransmitters in surgically derived specimens of human atrial ganglia. Neuroscience 164: 1170–1179, 2009. [DOI] [PubMed] [Google Scholar]
- 37.Hopkins DA, Macdonald SE, Murphy DA, Armour JA. Pathology of intrinsic cardiac neurons from ischemic human hearts. Anat Rec 259: 424–436, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS, Prabhu SD, Rockman HA, Kass DA, Molkentin JD, Sussman MA, Koch WJ, American Heart Association Council on Basic Cardiovascular Sciences, Council on Clinical Cardiology, Council on Functional Genomics and Translational Biology. Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res 111: 131–150, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Kember G, Armour JA, Zamir M. Dynamic neural networking as a basis for plasticity in the control of heart rate. J Theor Biol 317: 39–46, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Kember G, Armour JA, Zamir M. Neural control hierarchy of the heart has not evolved to deal with myocardial ischemia. Physiol Genomics 45: 638–644, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Kember G, Armour JA, Zamir M. Neural control of heart rate: the role of neuronal networking. J Theor Biol 277: 41–47, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Kumar R, Nguyen HD, Ogren JA, Macey PM, Thompson PM, Fonarow GC, Hamilton MA, Harper RM, Woo MA. Global and regional putamen volume loss in patients with heart failure. Eur J Heart Fail 13: 651–655, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levy MN. Sympathetic-parasympathetic interactions in the heart. Circ Res 29: 437–445, 1971. [DOI] [PubMed] [Google Scholar]
- 44.Levy MN, Martin PJ. Neural control of the heart. In: Handbook of Physiology. The Cardiovascular System. The Heart. Bethesda, MD: Am. Physiol. Soc, 1979, sect. 2, vol. I, p. 581–620. [Google Scholar]
- 45.Matsui T, Nagoshi T, Rosenzweig A. Akt and PI 3-kinase signaling in cardiomyocyte hypertrophy and survival. Cell Cycle 2: 220–223, 2003. [PubMed] [Google Scholar]
- 46.McGuirt AS, Schmacht DC, Ardell JL. Autonomic interactions for control of atrial rate are maintained after SA nodal parasympathectomy. Am J Physiol Heart Circ Physiol 272: H2525–H2533, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia 43: 533–549, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Parsons RL. Mammalian cardiac ganglia as local integration centers: histochemical and electrophysiological evidence. In: Neural Mechanisms in Cardiovascular Regulation, edited by Dun NJ, Machado BH, Pilowsky PM. Boston: Kluwer Academic, 2004, p. 335–356. [Google Scholar]
- 49.Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, DiCarlo LA, Ardell JL, Rector TS, Amurthur B, KenKnight BH, Anand IS. Extended follow-up of patients with heart failure receiving autonomic regulation therapy in the ANTHEM-HF Study. J Card Fail. In press. [DOI] [PubMed] [Google Scholar]
- 50.Rajendran PS, Nakamura K, Ajijola OA, Vaseghi M, Armour JA, Ardell JL, Shivkumar K. Myocardial infarction induces structural and functional remodelling of the intrinsic cardiac nervous system. J Physiol 594: 321–341, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Randall DC, Brown DR, McGuirt AS, Thompson GW, Armour JA, Ardell JL. Interactions within the intrinsic cardiac nervous system contribute to chronotropic regulation. Am J Physiol Regul Integr Comp Physiol 285: R1066–R1075, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Shimazu T. Innervation of the liver and glucoregulation: roles of the hypothalamus and autonomic nerves. Nutrition 12: 65–66, 1996. [DOI] [PubMed] [Google Scholar]
- 53.Shinlapawittayatorn K, Chinda K, Palee S, Surinkaew S, Thunsiri K, Weerateerangkul P, Chattipakorn S, KenKnight BH, Chattipakorn N. Low-amplitude, left vagus nerve stimulation significantly attenuates ventricular dysfunction and infarct size through prevention of mitochondrial dysfunction during acute ischemia-reperfusion injury. Heart Rhythm 10: 1700–1707, 2013. [DOI] [PubMed] [Google Scholar]
- 54.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85: 1093–1129, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Thompson GW, Collier K, Ardell JL, Kember G, Armour JA. Functional interdependence of neurons in a single canine intrinsic cardiac ganglionated plexus. J Physiol 528: 561–571, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaseghi M, Gima J, Kanaan C, Ajijola OA, Marmureanu A, Mahajan A, Shivkumar K. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and long-term follow-up. Heart Rhythm 11: 360–366, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waldmann M, Thompson GW, Kember GC, Ardell JL, Armour JA. Stochastic behavior of atrial and ventricular intrinsic cardiac neurons. J Appl Physiol 101: 413–419, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Wang HJ, Wang W, Cornish KG, Rozanski GJ, Zucker IH. Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension 64: 745–755, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamakawa K, Rajendran PS, Takamiya T, Yagishita D, So EL, Mahajan A, Shivkumar K, Vaseghi M. Vagal nerve stimulation activates vagal afferent fibers that reduce cardiac efferent parasympathetic effects. Am J Physiol Heart Circ Physiol 309: H1579–H1590, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamakawa K, So EL, Rajendran PS, Hoang JD, Makkar N, Mahajan A, Shivkumar K, Vaseghi M. Electrophysiological effects of right and left vagal nerve stimulation on the ventricular myocardium. Am J Physiol Heart Circ Physiol 307: H722–H731, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zipes DP. Antiarrhythmic therapy in 2014: contemporary approaches to treating arrhythmias. Nat Rev Cardiol 12: 68–69, 2015. [DOI] [PubMed] [Google Scholar]
- 62.Zucker IH, Patel KP, Schultz HD. Neurohumoral stimulation. Heart Fail Clin 8: 87–99, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]