Abstract
Intestinal inflammation has been recently characterized by the dysregulation of lipids as metabolic and energy sources, revealing a novel feature of its pathophysiology. Because intracellular lipids, stored in dynamic lipid droplets (LDs), provide energy for cellular needs, we investigated whether they play a role in intestinal inflammation. In the inflamed intestine of mice, elevated LDs were found in colonic and infiltrating immune cells as shown by staining for the LD coat protein PLIN2 and for lipids with BODIPY. In colonic cells, TNF stimulated LD increases by receptor signaling rely on phosphatidylinositol 3-kinase activation. Downstream, TNF triggered a negative regulatory loop between LDs and the transcription factor FOXO3. This was shown in the colon of Foxo3-deficient mice, where elevation in PLIN2 and lipids were further facilitated by inflammation and were more prominent relative to wild-type, whereas, in colonic cells, inhibition of lipogenesis blocked the TNF-mediated loss of FOXO3. Furthermore, blockade of PGE2 synthesis abrogated TNF-stimulated increases in LDs and FOXO3 inactivation. We found in colonic tissue of Foxo3-deficient mice higher levels of cyclooxygenase-2, a mediator of prostaglandin E2 (PGE2) synthesis, supporting involvement of PGE2 in the LD-FOXO3 regulatory loop. Ultimately, TNF-stimulated lipogenesis leading to elevated LDs facilitated NF-κB-mediated increases in IL-8 protein, which is associated with the surface of LDs found in the lumina of the endoplasmic reticulum and Golgi apparatus. This novel immunometabolic mechanism of colonic inflammation involving elevated LDs could provide opportunities for new treatment options.
Keywords: inflammation, lipid droplet, Foxo3, prostaglandin 2
inflammatory bowel disease (IBD) is characterized by immune cell infiltration of the involved tissue, secretion of inflammatory mediators (e.g., cytokines), and activation of inflammatory pathways (16). Emerging data reveal that IBD pathobiology is also associated with dysregulation of energy sources found within lipids, such as significantly altered lipid levels, secretion of obesity-like mediators (adipokines), and mesenteric fat accumulation (“creeping fat”) (2, 4). As this increase in lipid metabolism might be an adaptive mechanism supporting the high-energy requirements of inflammation, it is possible that their regulation is interdependent. This notion is supported by a recent study demonstrating that blockade of lipogenesis ameliorates intestinal inflammation in mice (33), revealing the immunometabolic feature of the disease. However, the regulation of lipid metabolism in inflammation and the possible mechanisms involving lipids as drivers of intestinal inflammation remain unclear.
TNF-α is markedly enhanced in IBD-affected tissue, and several monoclonal antibodies directed against TNF provide effective treatment options. TNF drives intestinal inflammation, in part, by stimulating cytokine production (23), which relies on receptor signaling pathways leading to activation of the transcription factor NF-κB (27). Elevated TNF is also found in lipid-storing tissues, such as the liver and fat, mediating increases in intracellular lipolysis, leading to elevation of lipid mediators and lipid levels (11). However, in nonlipid-storing tissue such as the intestine, whether TNF has the ability to stimulate lipid metabolism and whether lipid and cytokine regulation is codependent remain unexplored.
Intracellular lipids stored in dynamic organelles known as lipid droplets (LDs) provide metabolic energy for diverse cellular functions (14, 32). LDs are coated with proteins from the perilipin family (PLIN1-5) (8, 31), some of which are involved in the regulation of lipid metabolism (18, 34, 45). LDs are formed at the endoplasmic reticulum (ER) during a complex process involving lipogenesis and multiple maturation steps (3). Although initially LDs were viewed only as lipid-storage organelles in adipocytes and liver cells, emerging data reveal that their elevation in other tissues is associated with disease (17, 48). Among the diverse regulatory roles that LDs play in cellular function (48), they were recently implicated in the storage of enzymes and precursor molecules involved in prostaglandin E2 (PGE2) synthesis (5), supporting an indirect role for these lipid depots in inflammation. However, the regulation and function of LDs in intestinal inflammation is still unclear.
Here, we demonstrate that in inflammatory conditions increased LDs in colonic cells are mediated through TNF receptor signaling, leading to activation of a negative lipogenic regulatory loop involving FOXO3 and PGE2. Increases in TNF-mediated cytokine depend on elevated lipogenesis and are associated with the surface of LDs in the lumina of the ER and Golgi apparatus. These novel findings could provide the opportunity to pursue new therapeutic strategies targeting LDs for the treatment of intestinal inflammatory diseases.
MATERIALS AND METHODS
Cell culture.
Nontransformed human NCM460 colonic epithelial cells (INCELL, San Antonio, TX) were grown in M3Base medium (INCELL) containing 10% fetal bovine serum. Transformed human HT-29 and HCT116 colonic epithelial cells (passages 7–15; ATCC, Manassas, VA) were propagated in complete McCoy's 5A media (Sigma-Aldrich, St. Louis, MO) containing 10% fetal bovine serum (Gibco, Carlsbad, CA). Semiconfluent monolayers were serum starved overnight before the experimental procedures.
Treatments and inhibitors.
Experimental monolayers were stimulated with human recombinant TNF (10 ng/ml; R&D Systems, Minneapolis, MN) for different lengths of time. LD accumulation was stimulated with oleic acid (300 μM, Sigma). The lipid synthase inhibitor, C75 (5 μg/ml, 50 μg/ml; Cayman Chemical, Ann Arbor, MI) was used for LD depletion. Blockade of EGFR was accomplished by AG1517 (1 μM; Calbiochem, San Diego, CA), IKK by PS1145 (9 mM; Sigma), and phosphatidylinositol 3-kinase (PI3K) by wortmannin (200 nM; Calbiochem). For inhibition of PGE2 synthesis, sulindac (2 μM; Sigma) was used. Serum-starved monolayers were preincubated with inhibitors for 30 min before TNF treatments.
Immunofluorescent staining.
Experimental cells grown on coverslips were fixed with 3.7% formaldehyde and immunofluorescently stained for FOXO3 (Cell Signaling Technology, Beverly, MA), IL-8 (Santa Cruz Biotechnology, Santa Cruz, CA), p65 (Cell Signaling Technology) (43, 44), calnexin (30) (Abcam, Cambridge, MA), and syntaxin 6 (19) (Abcam) as described previously (43, 44). For LD staining, fixed monolayers were incubated with 1 μg/ml BODIPY 493/503 (Molecular Probes, Eugene, OR) (38). Slides were washed, mounted with Prolong Gold antifade reagent containing DAPI (Invitrogen, Carlsbad, CA), and observed using fluorescent microscopy (Olympus DP80 with camera software for CellSens Dimensions). For localization studies, z-stack images were acquired with an Olympus Fluoview FV1000 multiphoton laser-scanning microscope (Louisiana Cancer Research Consortium, Imaging Core, New Orleans, LA). Distance and LD size measurements were performed with ImageJ software (NIH).
Protein extraction and immunoblotting.
Protein from experimental cells and colonic mucosa of mice were extracted and immunoblotted as described previously (43, 44). Specific antibodies against the following proteins were used: PLIN2 (Santa Cruz Biotechnology), pFOXO3 (Cell Signaling Technology), cyclooxygenase 2 (COX-2) (Cell Signaling Technology), IκBα (Cell Signaling Technology), p65 (Cell Signaling Technology), IL-8 (Santa Cruz Biotechnology), and β-actin (Cell Signaling Technology) (38, 43, 44). Proteins were visualized with IRDye-conjugated secondary antibodies (LI-COR Biotechnology, Lincoln, NE) using the Odyssey infrared imaging system (LI-COR Biotechnology).
LD isolation.
LDs were isolated by density-gradient centrifugation following a protocol adapted from Ding et al. (13). Semiconfluent HT-29 cells (∼1 × 108 cells) treated with TNF and oleic acid (24 h) were harvested with a rubber policeman in PBS. Cell pellets were resuspended in hypotonic buffer (10 mM Tris·HCl, pH 7.4, 1 mM EDTA) containing protease inhibitors (complete mini; Roche Diagnostics, Mannheim, Germany), incubated on ice for 30 min, and disrupted by Dounce homogenization (20 strokes). Cell homogenates were centrifuged (3,000 g, 4°C, 10 min), and the postnuclear supernatant was adjusted to 0.25 M sucrose concentration. The final volume of 6 ml was transferred to an ultraclear centrifuge tube (Beckman Coulter, Fullerton, CA) and overlaid with 3 ml of 0.125 M sucrose in hypotonic buffer with protease inhibitor and 3 ml buffer only. After centrifugation in a SW 41 rotor (182,000 g, 4°C, 1 h; Beckman Coulter), the tubes were frozen (−80°C) followed by thawing of the top layer (∼3 mm) with warm air to obtain the floating LD fraction.
IL-8 quantification by ELISA and qPCR.
For quantification of intracellular IL-8, cells were suspended in extraction buffer (100 mM Tris·HCl pH 7.4, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate) containing protease inhibitor (10 μl/ml, Sigma) (38), and supernatant was used to determine IL-8 levels using ELISA (R&D Systems) according to the manufacturer's protocol. Expression of IL-8 mRNA was determined using qPCR. Total RNA from experimental cells was isolated using QIAzol Lysis Reagent (Qiagen, Valencia, CA) according to the manufacturer's protocol. DNase-treated mRNA was reverse transcribed with Oligo-dT12-18 primers of the SuperScript First-Strand Synthesis System (Invitrogen) following the standard protocol. For quantification of resulting cDNA, the C1000 Thermal Cycler system (Bio-Rad, Hercules, CA) and iQ SYBR Green DNA double-strand binding dye (iQ SYBR Green Supermix, Bio-Rad) were used. The following primers specifically amplifying IL-8 (CXCL8 forward: 5′-GTGCAGTTTTGCCAAGGAGT-3′, reverse: 5′-CTCTGCACCCAGTTTTCCTT-3′) and hypoxanthine-guanine phosphoribosyltransferase (HPRT-1) (HPRT-1 forward: 5′-GACCAGTCAACAGGGGACAT-3′, reverse: 5′-AACACTTCGTGGGGTCCTTTTC-3′) were utilized (50). To determine the relative levels of IL-8 mRNA, the comparative Ct method was employed.
Chromatin immunoprecipitation assay.
After being cross linked with 2 mM disuccinimidyl glutarate and 1% formaldehyde, cells were lysed, DNA was sheared (200-1,000 bp) by sonication, and fragments were incubated with p65 antibody (Cell Signaling Technology). The specific p65 binding regions were enriched with the chromatin immunoprecipitation (ChIP) assay kit (Millipore, Billerica, MA) according to the manufacturer's protocol. Amplification of p65 bound to IL-8 promotor sequence was accomplished by PCR using the following primers: CXCL8 forward: 5′-TGGCTGGCTTATCTTCACCA-3′, reverse: 5′-CGATTTGCAACTGATGGCCC-3′.
Animals.
C57BL/6J strain, wild-type, and Foxo3-deficient mice (29) were housed in the Biological Resources Laboratory at Tulane University facility. Colonic inflammation was induced by 2.5% dextran sulfate sodium (DSS; MP Biomedicals, Solon, OH) introduced in drinking water for 5 days. All guidelines and experimental procedures were approved by the animal ethics committee, and procedures were applied in accordance with approved animal care protocols.
Histological analysis.
Tissue sections were immunohistostained after antigen retrieval with anti-PLIN2 (1:100, 1 h; LSBio, Seattle, WA). Antibody labeled with horseradish peroxidase was added, followed by incubation with diaminobenzidine chromogen and then counterstained with hematoxylin. Tissues were dehydrated in graded alcohol and xylene and then coverslipped using Permount (Biomeda, Foster City, CA). Images were obtained using the slide scanner Aperio CS2 and prepared by Image Scope software.
Frozen colonic tissue sections fixed with 3.7% formaldehyde and incubated in PBS for 5 min were stained with BODIPY 493/503 (1 μg/ml) for 20 min in the dark (38). After being washed in PBS, coverslips were mounted using ProLong antifade reagent containing DAPI. Images were observed with an Olympus DP80 fluorescent microscope.
Statistical analysis.
Statistical analysis was performed with ANOVA and Student Newman-Keuls posttest or Student's unpaired t-test using GraphPad Instat 3 software (GraphPad Software, La Jolla, CA). All data are represented as means ± SE for a series of experiments, and a P value of <0.05 was considered significant.
RESULTS
Increased LDs associated with colonic inflammation are mediated by TNF-stimulated lipogenesis.
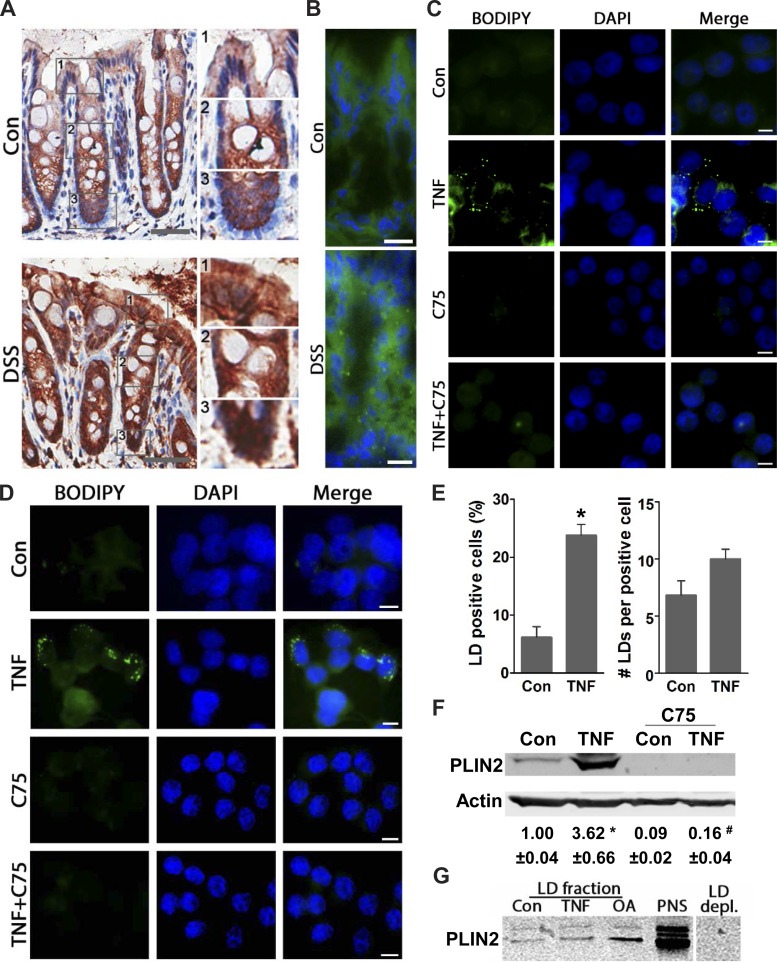
Recently, it has been demonstrated that systemic blockade of lipid synthesis ameliorates colitis in mice (33), revealing dependency of intestinal inflammation on lipogenesis. However, the cellular source of lipids, the mechanisms involved in their regulation, and whether they facilitate intestinal inflammation remain unexplored. Here, we found in the inflamed intestine of mice elevated LDs in colonic and infiltrating immune cells. In control mice, immunohistostaining for the LD coat protein PLIN2, a marker for LDs (34), localizes primarily at the base of colonic crypts, as we described previously (38). In inflamed colons, PLIN2 is found to be increased in epithelial cells along the entirety of the crypts as well as in infiltrating immune cells (Fig. 1A). Similarly, fluorescent BODIPY staining of neutral lipids indicates that elevated LDs are associated with colonic inflammation in mice (Fig. 1B). Although elevated LDs in infiltrating immune cells could also be involved in driving the pathophysiology of intestinal inflammation, in this study, we focused on LD regulation in colonic cells. Because TNF is critical in intestinal inflammation (23), we assessed its ability to elevate LDs in both nontransformed (NCM460) and transformed (HT-29) human colonic cells. In both NCM460 and HT-29 cells, BODIPY staining shows that a few resting cells contain small ∼0.3-μm LDs, whereas TNF (6 h) treatment mediates increases in their size up to 1.0 μm (Fig. 1, C and D). Moreover, TNF (6 h) stimulates increases in the number of LD-positive cells by ∼3.8-fold and the number of LDs per cell by 1.5-fold (Fig. 1E), which progressed more than twofold by 24 h (data not shown), revealing a time-dependent increase. Additionally, immunoblotting demonstrates that TNF significantly elevates PLIN2 by ∼3.6-fold relative to control (Fig. 1F). Similarly, in the LD fraction obtained from HT-29 cells treated with TNF and oleic acid (previously shown to stimulate increases in LDs) (38), we found elevated PLIN2 compared with control cells, further supporting these findings (Fig. 1G). Blockade of lipid synthesis with C75 inhibits TNF-mediated increases in LDs in both NCM460 and HT-29 cells, as shown by BODIPY staining and immunoblotting for PLIN2 (Fig. 1, C–F), suggesting that TNF induces increases in LDs, in part, through lipogenesis. Notably, blockade of lipid synthesis by C75 also diminishes basal levels of PLIN2 (Fig. 1F), revealing that lipogenesis is critical, not only for increases in LDs, but also for maintaining their homeostasis in resting cells. These data demonstrate that colonic inflammation is associated with elevated LDs mediated by TNF-stimulated lipogenesis. Thus we further assessed the regulation and function of TNF-mediated increases in LDs in colonic cells.
Fig. 1.
Increased lipid droplets (LDs) associated with colonic inflammation are mediated via TNF-dependent lipogenesis. A: colonic tissues from control and dextran sulfate sodium (DSS)-treated mice were immunohistostained for PLIN2 (n = 4, scale bar 50). Insets (1–3): enlarged areas of apical, intermediate, and basal portions of the colonic crypts. B: tissues from the same mice were stained with BODIPY 493/503 (n = 4, scale bar = 10 μm). C and D: human colonic NCM460 (nontransformed) and HT-29 (transformed) cells were stimulated with TNF (6 h) with or without C75 and stained for LDs (BODIPY 493/503) (representative images from 3 independent experiments, scale bar = 5 μm). E: graphs present the number of LD-positive cells and LDs per cell in HT-29 cells treated with TNF (6 h) (ImageJ quantification of BODIPY 493/503 stained, n = 300 cells, *P < 0.05 compared with Con). F: total protein from HT-29 cells treated with TNF (6 h) with or without C75 were immunoblotted for the LD coat protein Perilipin 2 (PLIN2) (n = 3, *P < 0.05, compared with Con, #P < 0.05, compared with TNF alone, ANOVA). G: LD fractions obtained from HT-29 control cells, TNF (24 h), and oleic acid (OA, 24 h) were immunoblotted for PLIN2. As a positive control, protein from postnuclear supernatant (PNS) was used, and as negative control LD depleted fraction (LD depl.) from control cells was used.
TNF-stimulated increases in LDs depend on receptor signaling, leading to activation of the PI3K pathway.
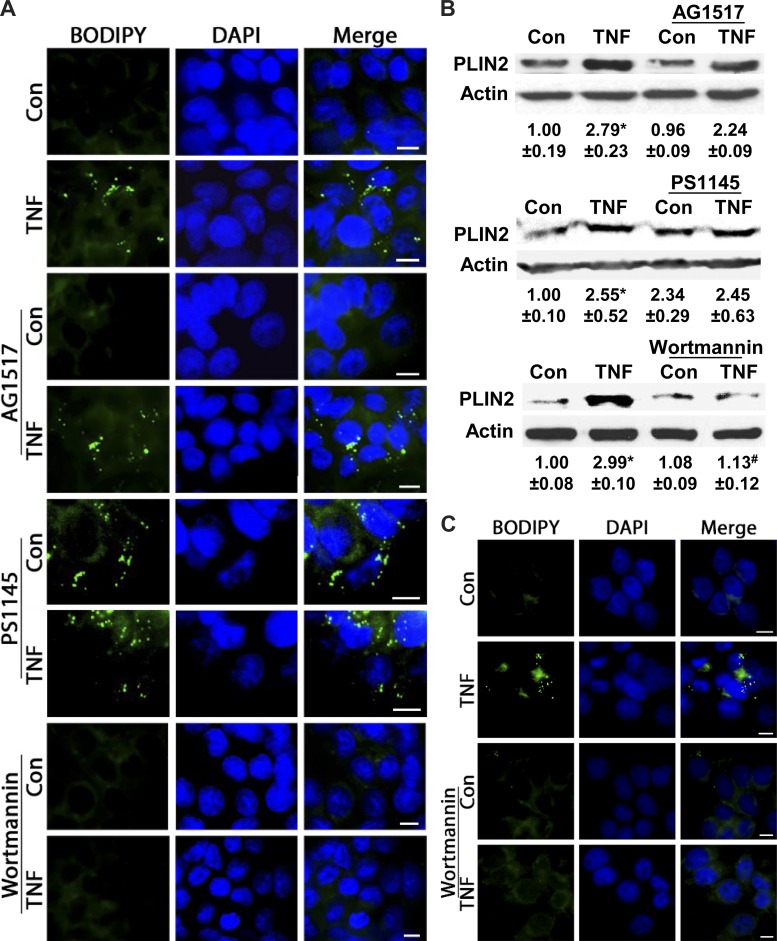
As TNF acts via receptor-mediated activation of downstream pathways (27), we examined whether elevation of LDs is dependent on receptor signaling. Also, because in intestinal cells TNF can transactivate the EGF receptor (EGFR) (49), which also leads to elevated LDs in colonic cells (37), we first assessed whether TNF increases LDs via its own receptor or EGFR. Following pharmacological inhibition of EGFR by AG1517, TNF-mediated increases in LDs are unaffected in HT-29 cells, as shown by BODIPY staining and immunoblotting (Fig. 2, A and B), indicating that EGFR is not involved. Because downstream TNF receptor activation triggers the IKK and PI3K pathways (42), we assessed whether these signal transducers mediate increases in LDs. In HT-29 cells, TNF-mediated increases in LDs remain unaffected following IKK-β blockade with PS1145 (Fig. 2, A and B); however, in resting cells, this blockade alone stimulated LDs. This finding suggests that in colonic cells inducible LDs are independent of IKK-β, yet this pathway might be involved in their homeostasis in a resting state. In contrast, inhibiting PI3K with wortmannin abrogates TNF-mediated increases in LDs, as shown by BODIPY staining and immunoblotting in HT-29 and NCM460 cells (Fig. 2, A–C). While inhibition of lipogenesis by C75 significantly reduces basal levels of PLIN2 (Fig. 1F), wortmannin diminishes only TNF-stimulated increases (Fig. 2B), suggesting that the PI3K pathway might be involved in LD growth. These data reveal that increases in LDs in colonic cells depend in part on TNF receptor-mediated activation of PI3K.
Fig. 2.
TNF-mediated increases in LDs are independent of EGF receptor (EGFR) and IKK but depend on phosphatidylinositol 3-kinase (PI3K). A: HT-29 cells were treated with TNF (6 h) in the presence of pharmacological inhibitors for EGFR (AG1517), IKK-β (PS1145), or PI3K (wortmannin) and stained for LDs (BODIPY 493/503) (representative images from 3 independent experiments, scale bar = 5 μm). B: protein from TNF-stimulated HT-29 cells (6 h) in the presence of AG1517, PS1145, or wortmannin was immunoblotted for the LD coat protein PLIN2. Densitometric analysis of n = 3, *P < 0.05, compared with Con, #P < 0.05, compared with TNF alone, ANOVA. C: TNF-treated NCM460 cells (6 h) with and without wortmannin were stained by BODIPY 493/503 (representative images from 3 independent experiments, scale bar = 5 μm).
TNF-stimulated LDs depend on a lipogenic negative regulatory loop involving FOXO3 and PGE2.
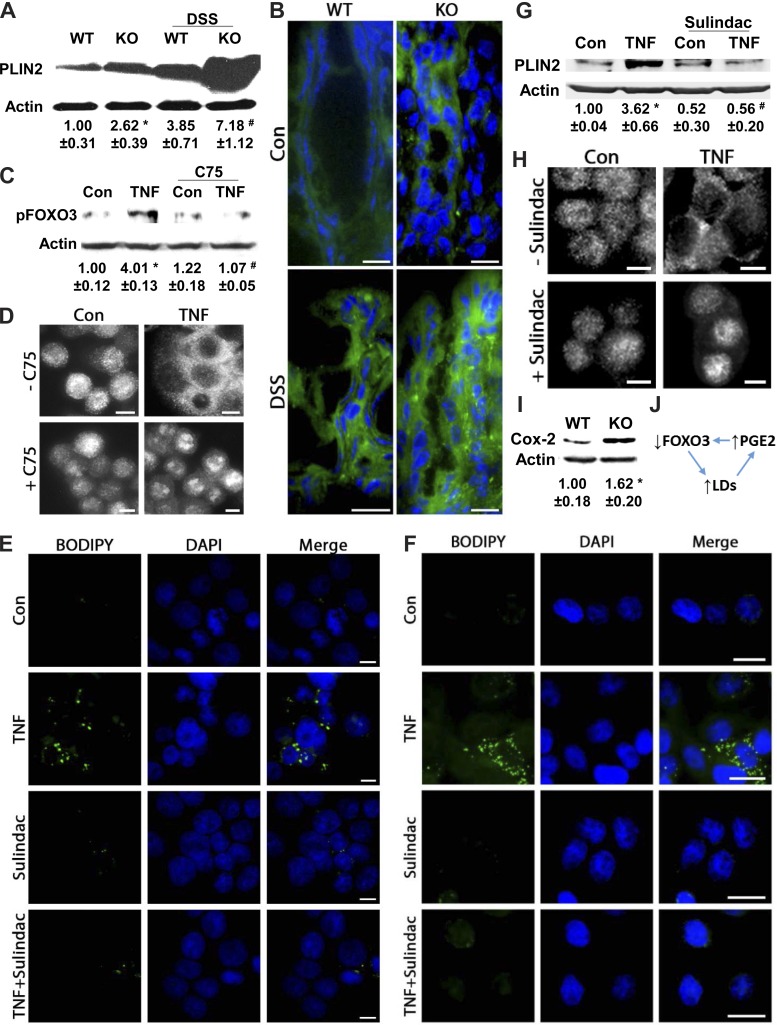
We and others have demonstrated that deficiency in the transcription factor Foxo3 leads to elevated LDs in the liver and colonic cells of mice (38, 47), indicating that increases in LDs depend on loss of FOXO3. Also, loss of FOXO3 is blocked by LD depletion following inhibition of lipogenesis (38). Therefore, we showed that LDs rely on FOXO3 function and that FOXO3 function depends on LDs, supporting existence of an LD-FOXO3 negative regulatory loop (38). Because intestinal inflammation depends on loss of Foxo3 function (44), we investigated whether LD-FOXO3 could be triggered by inflammatory stimuli. In agreement with previous findings (38), PLIN2 expression was significantly increased in colonic mucosa of Foxo3-deficient mice compared with wild-type (Fig. 3A). In an inflammatory setting, PLIN2 levels were increased threefold in wild-type and more than sevenfold in Foxo3-deficient mice. Similarly, BODIPY staining indicates further increases in LDs with inflammation in Foxo3-deficient colon (Fig. 3B). Although it is important to consider involvement of other mechanisms, these data support that, in mouse colon, under both normal and inflammatory conditions, elevated LDs depend, in part, on loss of FOXO3. Because we previously showed that the TNF-mediated loss of FOXO3 occurs, in part, through PI3K (44), we assessed whether this loss is also dependent on lipogenesis. In HT-29 cells, TNF-stimulated FOXO3 phosphorylation, known to be an initial step in inactivation of this transcription factor (10), and translocation from the nucleus to the cytosol are blocked by C75 (Fig. 3, C and D). These data reveal that in colonic cells inflammatory stimuli induce increases in LDs, in part, via loss of FOXO3, which can be blocked by inhibition of lipogenesis, indicating that TNF triggers a LD-FOXO3 negative regulatory loop.
Fig. 3.
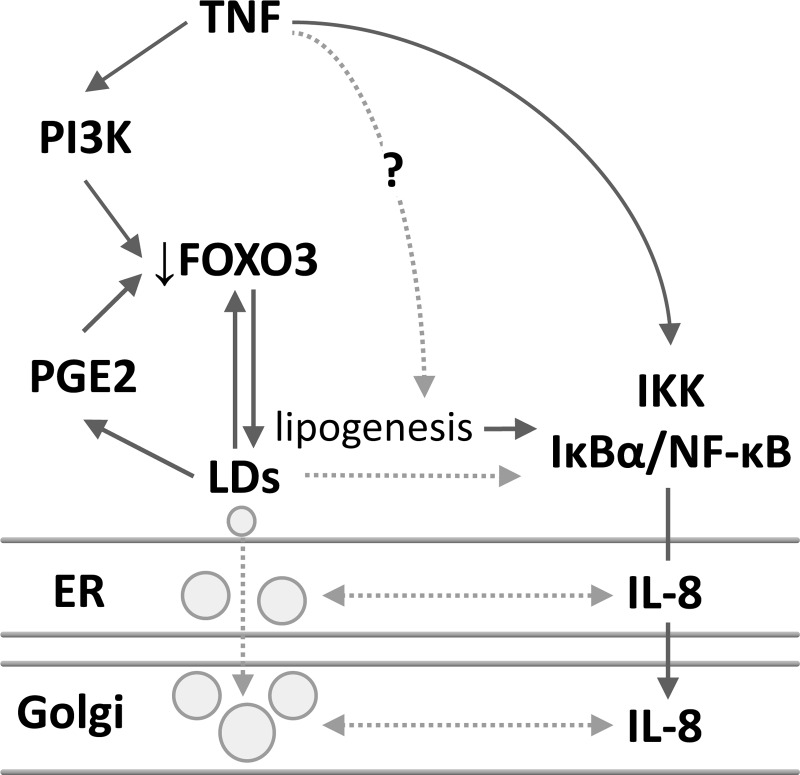
TNF-induced increases in LDs depend on prostaglandin E2 (PGE2) and FOXO3 interplay. A: protein from scraped colonic mucosa of wild-type (WT) and Foxo3-deficient knockout (KO) mice with or without DSS treatment were analyzed for PLIN2 expression by immunoblotting (n = 4, *P < 0.05, compared with Con, # P < 0.05, compared with KO alone, ANOVA). B: BODIPY (493/503) staining of colonic tissues from WT and Foxo3 KO mice treated with DSS (n = 4, scale bar = 10 μm). C: protein from HT-29 cells stimulated with TNF (0.5 h) with or without C75 was immunoblotted for pFOXO3 (corresponding densitometric analysis n = 3, *P < 0.05, compared with Con, #P < 0.05, compared with TNF alone, ANOVA). D: HT-29 cells treated with TNF (1 h) in the presence of C75 were immunofluorescently stained for FOXO3 (Alexa 488); representative images from 3 independent experiments, scale bar = 5 μm. E and F: NCM460 and HT-29 cells treated with TNF (6 h) in the presence of the PGE2 synthesis inhibitor sulindac were stained for LDs (BODIPY 493/503); representative images from 3 independent experiments, scale bar = 5 μm and 10 μm. G: immunoblotting and corresponding densitometric analysis of protein from HT-29 cells treated with TNF (6 h) with and without sulindac (n = 3, *P < 0.05, compared with Con, #P < 0.05, compared with TNF alone, ANOVA). H: HT-29 cells treated with TNF (1 h) with or without sulindac were immunofluorescently stained for FOXO3 (Alexa 488; representative images from 3 independent experiments, scale bar = 5 μm). I: protein from scraped colonic mucosa of WT and Foxo3 KO mice was immunoblotted for cyclooxygenase 2 (COX-2) (n = 3, *P < 0.05 compared with WT, t-test). J: proposed model of LD-FOXO3-PGE2 negative regulatory loop.
Additionally, several studies have shown in resting cells a codependency between LDs and PGE2 synthesis (1, 6, 7); however, it is unclear whether TNF-mediated increases in LDs also rely on PGE2 and whether FOXO3 and PGE2 are codependent. Here, we observe that, in NCM460 and HT-29 cells, inhibition of PGE2 synthesis with sulindac attenuates TNF-mediated increases in LDs, as shown by BODIPY staining (Fig. 3, E and F) and immunoblotting (Fig. 3G). Because sulindac does not affect basal PLIN2 levels (Fig. 3G), as seen following wortmannin treatment (Fig. 2B), we speculate that PGE2 also likely plays a role in LD growth. Moreover, in colonic cells, TNF-mediated FOXO3 translocation from the nucleus to the cytosol (inactivation) is attenuated by sulindac (Fig. 3H), supporting that loss of FOXO3 is dependent in part on PGE2 synthesis. In colonic mucosa of Foxo3-deficient mice, we detect elevated COX-2 levels (Fig. 3I), a mediator of PGE2 synthesis (15). Altogether we show in colonic cells that increased LDs and the loss of FOXO3 depend on PGE2 synthesis, and PGE2 synthesis depends on loss of FOXO3, supporting that the LD-FOXO3 negative regulatory loop involves PGE2 (Fig. 3J).
Increased LDs facilitate TNF-mediated IL-8 transcription by supporting NF-κB activity.
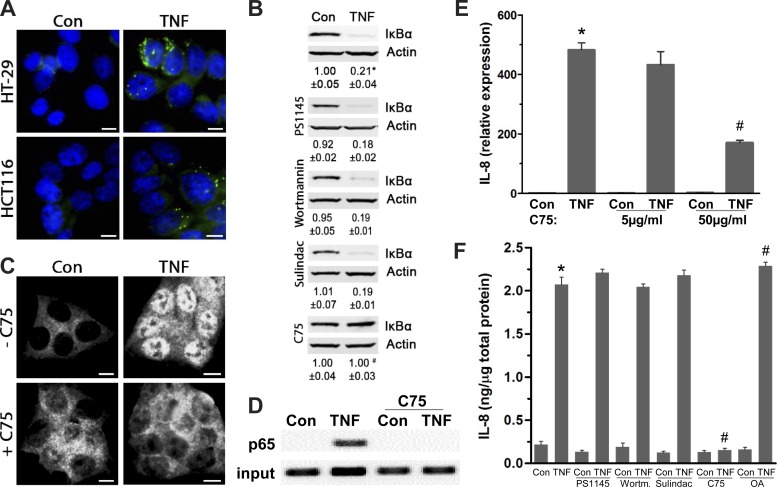
We next assessed whether TNF-stimulated increases in LDs and their regulatory loop are involved in intestinal inflammation. Because intestinal inflammation is dependent on NF-κB-mediated cytokine expression such as IL-8 (23, 27), we examined whether they depend on elevated LDs. As degradation of inhibitory IκBα leading to NF-κB activation is unconventional in HT-29 cells (21), colonic HCT116 cells were employed because they also accumulate LDs in response to TNF treatment, yet to a lesser degree than in HT-29 (Fig. 4A). In HCT116 cells, although TNF-mediated degradation of IκBα was unaffected by PS1145, wortmannin, and sulindac, it was effectively blockaded by C75 (Fig. 4B). This suggests that TNF-mediated IκBα degradation could depend on increases in LDs mediated by lipogenesis, whereas IKK-β, PI3K, and PGE2 mediation of their growth is likely uninvolved. Consequently, C75 prevents TNF-mediated NF-κB activation, as it blocks translocation of p65, a NF-κB subunit (20), from the cytosol to the nucleus and binding to the promoter of IL-8 (ChIP assay) (Fig. 4, C and D). Following treatment with C75 (50 μg/ml), IL-8 mRNA expression is significantly attenuated by 64.5 ± 2.6% (Fig. 4E). At a lower concentration, C75 (5 μg/ml) also attenuates IL-8 mRNA expression but to a lesser extent. Moreover, although TNF-stimulated intracellular IL-8 protein levels are unchanged in the presence of PS1145, wortmannin, and sulindac, they are effectively lowered by C75 (Fig. 4F). As anticipated in HT-29 cells with oleic acid-stimulated LDs (38), TNF-mediated increases in IL-8 are significantly higher relative to TNF alone (Fig. 4F), yet it is important to consider that oleic acid could increase not only lipogenesis but also lipolysis. A similar trend was seen with released IL-8 (data not shown). These data demonstrate that in colonic cells TNF-stimulated NF-κB activation and IL-8 expression are dependent in part on increases in LDs mediated by lipogenesis.
Fig. 4.
Increased LDs facilitate TNF-mediated IL-8 transcription by supporting NF-κB activity. A: HT-29 and HCT116 cells treated with TNF (6 h) were stained for LDs (BODIPY 493/503) (representative images from 3 independent experiments, scale bar = 5 μm). B: protein from HCT116 cells treated with TNF (0.5 h) with or without inhibitors for IKK-β (PS1145), PI3K (wortmannin), PGE2 synthesis (sulindac), or lipogenesis (C75) was immunoblotted for IκBα (n = 4, *P < 0.05, compared with Con, #P < 0.05, compared to TNF alone, ANOVA). C: immunofluorescent staining for p65, a NF-κB subunit (Alexa 594), in HCT116 cells treated with TNF (0.5 h) with and without C75 (representative images from 3 independent experiments, scale bar = 5 μm). D: chromatin immunoprecipitation (ChIP) assay of TNF-mediated p65 binding (0.5 h) to the IL-8 promoter with and without C75 treatment (50 μg/ml); input represents total chromatin levels before immunoprecipitation, indicating that equal amounts of samples were applied to ChIP followed by PCR. E: total RNA was extracted from HT-29 cells treated with TNF (2 h) in the presence of C75 (5 μg/ml and 50 μg/ml), and IL-8 transcription levels were determined with qPCR (n = 3, *P < 0.05, *compared with Con, #P < 0.05, compared with TNF alone). F: in HT-29 cells treated with TNF (4 h) with and without PS1145, wortmannin, sulindac, C75, or OA (24-h pretreatment), intracellular IL-8 protein levels were quantified by ELISA (n = 3, *P < 0.05, compared with Con, #P < 0.05, compared with TNF alone).
Intracellular IL-8 protein is associated with the surface of LDs in the lumen of the ER and Golgi.
Emerging data reveal that, in certain pathophysiological processes, LDs could be physically associated with regulatory protein, as seen in liver cells infected with hepatitis C virus (HCV) (28, 48). Also, because the ER and Golgi apparatus are required for LD maturation and function (48), we assessed in colonic cells whether TNF-mediated increases in LDs and IL-8 protein share any physical association in these secretory pathways. Immunofluorescent costaining of LDs with BODIPY and an IL-8 antibody shows that, in colonic cells treated with TNF, 34.4 ± 4.7% of LD-positive and 14.2 ± 2.6% of LD-negative cells contain IL-8 (Fig. 5, A and B). Furthermore, confocal images of costained IL-8 with PLIN2 demonstrate that the surface of LDs and IL-8 protein are associated (Fig. 5C). Moreover, confocal images demonstrate that TNF-stimulated LDs and IL-8 are found to colocalize with ER membrane protein calnexin, supporting their colocalization inside of the ER (Fig. 5D). Similarly, colocalization of IL-8 and LDs with Golgi membrane protein syntaxin 6 reveals their presence in the Golgi apparatus (Fig. 5E). These data indicate that in colonic cells TNF-stimulated LDs and IL-8 are physically associated within the secretory pathways of the ER and Golgi.
Fig. 5.
In colonic cells, TNF-stimulated intracellular IL-8 protein is associated with the surface of LDs in endoplasmic reticulum (ER) and Golgi. A: HT-29 cells treated with TNF (6 h) with and without C75 were immunofluorescently stained with IL-8 antibody followed by secondary antibody (Alexa 594) and BODIPY 493/503, or IL-8 antibody followed by secondary antibody, or BODIPY 493/503 only, or IgG-only secondary antibody (Alexa 594) (representative images from 3 independent experiments, scale bar = 5 μm). B: graphical representation of IL-8 distribution between LD-positive and LD-negative cells (n = 300 cells). C: orthogonal view of confocal images of PLIN2 (Alexa 488) and IL-8 (Alexa 594). White arrows show colocalization of LDs and IL-8 (blue: nucleus, green: PLIN2, red: IL-8, scale bar = 3 μm). D: orthogonal view of confocal images of BODIPY 493/503, IL-8 (Alexa 594), and calnexin (Alexa 647). White and yellow arrows show colocalization of 2 and 3 colors, respectively (gray: nucleus, green: LDs, red: IL-8, blue: calnexin, scale bar = 3 μm). E: orthogonal view of confocal images of BODIPY 493/503, IL-8 (Alexa 594), and syntaxin 6 (Alexa 647). White and yellow arrows show colocalization of 2 and 3 colors, respectively (gray: nucleus, green: LDs, red: IL-8, blue: syntaxin 6, scale bar = 3 μm).
DISCUSSION
Intestinal inflammation has been recently characterized by elevated lipid mediators found within the circulation, as well as within the affected tissue (2, 4); however, the role of intracellular lipids in the pathophysiology of the disease is unclear. Because increased lipids could provide the metabolic support necessary for the inflammatory process, it is plausible that their regulation and function might be coregulated with inflammatory mediators. Here, we demonstrate in the inflamed colon that increased intracellular LDs are facilitated by TNF receptor signaling and are dependent, in part, on a lipogenic regulatory network involving FOXO3 and PGE2. We propose a model in which increased lipogenesis-mediating elevation in LDs could support NF-κB activity, thus facilitating production of cytokines that are associated with the surface of LDs found in the ER and Golgi (Fig. 6). These novel findings demonstrate that intestinal inflammation is dependent on intracellular lipid metabolism, providing an opportunity to develop new therapeutic strategies for treatment.
Fig. 6.
Proposed regulatory model of intestinal inflammation dependent on elevated LDs mediated by a negative FOXO3 and PGE2 regulatory loop. TNF receptor signaling leading to PI3K-mediated loss of FOXO3 appears to be the initial step that stimulates lipogenesis, initiates LD growth, and PGE2 synthesis. Increased lipogenesis and PGE2 synthesis further maintain the loss of FOXO3 function. When there are low cellular needs for lipids from LDs, lipogenesis and PGE2 synthesis would be attenuated, and FOXO3 activity recovered, supporting the existence of a negative LD-FOXO3-PGE2 regulatory loop. TNF-stimulated lipogenesis leads to elevated LDs and facilitates NF-κB-mediated IL-8, which is associated with the surface of LDs found in the ER and Golgi.
In the last decade, numerous studies have demonstrated that augmented lipid metabolism promotes inflammation associated with obesity, yet the regulatory and functional interdependency between lipids and inflammatory mediators in human diseases is not well understood. Although findings among animal models and human studies differ as to whether TNF stimulates lipolysis or lipogenesis or both (11), TNF-dependent lipid metabolism is most likely tissue specific. Distinct to what is known about lipid-storing adipose and liver tissues (11, 41), in colonic epithelial cells, we demonstrated that TNF stimulates lipogenesis, leading to increases in LDs. Although similar findings are demonstrated in skin cells associated with inflammation (12), it is important to take into account that, in epithelial cells, TNF might also promote lipolysis. Also, the role of elevated LDs in immune cells in intestinal inflammation needs further investigation, as one study demonstrates that mouse neutrophils containing high levels of LDs could have an enhanced immune response (40). Moreover, downstream of the TNF receptor, select signaling is utilized to increase LDs. We and others have shown in different cell types that activation of PI3K is important for increasing LDs following various stimuli (36–38), which recently has been supported with findings demonstrating its critical role in initiation of LD growth (39). In contrast, the IKK pathway appears uninvolved in the TNF induction of LDs, yet IKK-β might be critical in maintaining their homeostasis in resting colonic cells. In liver cells, IKK-α is essential for HCV-induced LDs (28), suggesting a tissue-specific role for different subunits of this kinase in LD regulation. Therefore, in colonic cells, multiple pathways could be involved in the regulation of LD homeostasis and their TNF-mediated increases.
Downstream of TNF receptor signaling, LD elevation is dependent on a negative regulatory loop consisting of FOXO3 and PGE2 synthesis. We and others have previously demonstrated in resting cells the existence of a LD-FOXO3 negative regulatory loop that includes Sirtuin6 (22, 38). Here we showed that this regulatory network is utilized by TNF receptor signaling and also includes PGE2. Because LD formation is a multistep process (3) and because PGE2 synthesis requires lipid precursors, it is likely that PGE2 plays a role in LD growth following lipogenesis. Limited studies have also described the PGE2-FOXO3 regulatory network in muscle tissue (9), suggesting that a similar mechanism might be utilized in intestinal inflammation and energy-producing muscle tissue. Moreover, because FOXO3 levels are considerably low in IBD-affected tissue (35), it is plausible that this molecule is at the intersection between lipid metabolism and inflammation. Because increases in LDs and their growth require multiple pathways and genes, it is important to take into account that a FOXO3-PGE2 regulatory network could be one of the mechanisms triggered by TNF to synchronize the metabolic energy found within lipids with production of inflammatory mediators.
Functionally, with colonic inflammation, elevated LDs could promote NF-κB activity, consequently facilitating cytokine expression. Emerging studies in lipid-storing tissues have shown that NF-κB activity depends on increased LDs. The loss of function of Sirtuin6, leading to lipogenesis-mediated increases in LDs (22, 38), stimulates NF-κB-dependent cytokine expression (25, 46). Moreover, adipose triglyceride lipase, an LD-associated protein that conducts lipolysis, when attenuated leads to elevated LDs and NF-κB-mediated cytokine production in adipocytes (26). Thus, because LDs are dynamic organelles dependent on both lipogenesis and lipolysis, and because regulators of these processes could stimulate cytokine expression, their role in intestinal inflammation could be critical. Also, as blockade of upstream pathways involved in LD growth did not attenuate cytokines, it is plausible that signaling from unstored lipids could contribute to inflammation. Together, we speculate in colonic cells that TNF-stimulated lipogenesis leading to elevated LDs could be critical in driving cytokine expression. Additionally, in colonic cells, we found that intracellular cytokines were associated with the surface of LDs within the ER and Golgi apparatus. It is unclear at this point whether the association between LDs and IL-8 has a function, but it is plausible that this could play a role in cytokine secretion. This notion could be supported by recent findings in polarized colonic monolayers in which stimulated LDs utilize the ER to be released to the basolateral side (3), supporting a need to further investigate codependency of their release.
IBD is associated with changes in lipids (2, 4), which is a novel immunometabolic characteristic of the disease requiring further understanding. Although the abnormal dynamics of LD regulation are not well understood, evolving studies reveal their significance in diverse cellular functions (24, 48) and also their association with many metabolic diseases, including obesity, diabetes, atherosclerosis, fatty liver, cancer, and inflammation (17). Our study provides new insight into mechanisms of regulation of intestinal epithelial LDs and underlines the significance of their function in cytokine production that could be utilized as a new therapeutic target in the treatment of intestinal inflammation.
GRANTS
This work is supported by a Senior Investigator Award from the Crohn's and Colitis Foundation of America (CCFA no. 1953) and NIH RO1 award (CA160809).
DISCLOSURES
Suzana D. Savkovic wishes to disclose ownership in Pegasus Biosolution, LLC. No other conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.H., M.C., and S.D.S. conception and design of research; S.H., C.C., H.P., R.M., D.B., and M.C. performed experiments; S.H., C.C., H.P., R.M., D.B., M.C., and S.D.S. analyzed data; S.H., H.P., R.M., and S.D.S. interpreted results of experiments; S.H. prepared figures; S.H., C.C., H.P., R.M., D.B., M.C., S.E.C., and S.D.S. approved final version of manuscript; C.C., H.P., and S.D.S. edited and revised manuscript; S.D.S. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. Wentao Qi for providing technical assistance for the project.
REFERENCES
- 1.Accioly MT, Pacheco P, Maya-Monteiro CM, Carrossini N, Robbs BK, Oliveira SS, Kaufmann C, Morgado-Diaz JA, Bozza PT, Viola JP. Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon cancer cells. Cancer Res 68: 1732–1740, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Baur P, Martin FP, Gruber L, Bosco N, Brahmbhatt V, Collino S, Guy P, Montoliu I, Rozman J, Klingenspor M, Tavazzi I, Thorimbert A, Rezzi S, Kochhar S, Benyacoub J, Kollias G, Haller D. Metabolic phenotyping of the Crohn's disease-like IBD etiopathology in the TNF(DeltaARE/WT) mouse model. J Proteome Res 10: 5523–5535, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Beilstein F, Carriere V, Leturque A, Demignot S. Characteristics and functions of lipid droplets and associated proteins in enterocytes. Exp Cell Res 340: 172–179, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Bertin B, Desreumaux P, Dubuquoy L. Obesity, visceral fat and Crohn's disease. Curr Opin Clin Nutr Metab Care 13: 574–580, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Bozza PT, Magalhaes KG, Weller PF. Leukocyte lipid bodies—Biogenesis and functions in inflammation. Biochim Biophys Acta 1791: 540–551, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bozza PT, Pacheco P, Yu W, Weller PF. NS-398: cyclooxygenase-2 independent inhibition of leukocyte priming for lipid body formation and enhanced leukotriene generation. Prostaglandins Leukot Essent Fatty Acids 67: 237–244, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Bozza PT, Payne JL, Morham SG, Langenbach R, Smithies O, Weller PF. Leukocyte lipid body formation and eicosanoid generation: cyclooxygenase-independent inhibition by aspirin. Proc Natl Acad Sci USA 93: 11091–11096, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res 48: 2547–2559, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Breuel W, Krause M, Schneider M, Harzer W. Genetic stretching factors in masseter muscle after orthognathic surgery. Br J Oral Maxillofac Surg 51: 530–535, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Burgering BM, Kops GJ. Cell cycle and death control: long live Forkheads. Trends Biochem Sci 27: 352–360, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Xun K, Chen L, Wang Y. TNF-alpha, a potent lipid metabolism regulator. Cell Biochem Funct 27: 407–416, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Choi JJ, Park MY, Lee HJ, Yoon DY, Lim Y, Hyun JW, Zouboulis CC, Jin M. TNF-alpha increases lipogenesis via JNK and PI3K/Akt pathways in SZ95 human sebocytes. J Dermatol Sci 65: 179–188, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Ding Y, Zhang S, Yang L, Na H, Zhang P, Zhang H, Wang Y, Chen Y, Yu J, Huo C, Xu S, Garaiova M, Cong Y, Liu P. Isolating lipid droplets from multiple species. Nat Protoc 8: 43–51, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Dugail I, Hajduch E. A new look at adipocyte lipid droplets: towards a role in the sensing of triacylglycerol stores? Cell Mol Life Sci 64: 2452–2458, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng L, Xia Y, Garcia GE, Hwang D, Wilson CB. Involvement of reactive oxygen intermediates in cyclooxygenase-2 expression induced by interleukin-1, tumor necrosis factor-alpha, and lipopolysaccharide. J Clin Invest 95: 1669–1675, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev 13: 3–10, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg AS, Obin MS. Many roads lead to the lipid droplet. Cell Metab 7: 472–473, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Heid HW, Moll R, Schwetlick I, Rackwitz HR, Keenan TW. Adipophilin is a specific marker of lipid accumulation in diverse cell types and diseases. Cell Tissue Res 294: 309–321, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Heinonen MT, Kanduri K, Lahdesmaki HJ, Lahesmaa R, Henttinen TA. Tubulin- and actin-associating GIMAP4 is required for IFN-gamma secretion during Th cell differentiation. Immunol Cell Biol 93: 158–166, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huxford T, Huang DB, Malek S, Ghosh G. The crystal structure of the IkappaBalpha/NF-kappaB complex reveals mechanisms of NF-kappaB inactivation. Cell 95: 759–770, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Jobin C, Haskill S, Mayer L, Panja A, Sartor RB. Evidence for altered regulation of I kappa B alpha degradation in human colonic epithelial cells. J Immunol 158: 226–234, 1997. [PubMed] [Google Scholar]
- 22.Kim HS, Xiao C, Wang RH, Lahusen T, Xu X, Vassilopoulos A, Vazquez-Ortiz G, Jeong WI, Park O, Ki SH, Gao B, Deng CX. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab 12: 224–236, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kmiec Z. Cytokines in inflammatory bowel disease. Arch Immunol Ther Exp (Warsz) 46: 143–155, 1998. [PubMed] [Google Scholar]
- 24.Kuhnlein RP. Thematic review series: Lipid droplet synthesis and metabolism: from yeast to man. Lipid droplet-based storage fat metabolism in Drosophila. J Lipid Res 53: 1430–1436, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lappas M. Anti-inflammatory properties of sirtuin 6 in human umbilical vein endothelial cells. Mediators Inflamm 2012: 597514, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lettieri Barbato D, Tatulli G, Aquilano K, Ciriolo MR. Inhibition of age-related cytokines production by ATGL: a mechanism linked to the anti-inflammatory effect of resveratrol. Mediators Inflamm 2014: 917698, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Yin Q, Wu H. Structural basis of signal transduction in the TNF receptor superfamily. Adv Immunol 119: 135–153, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q, Pene V, Krishnamurthy S, Cha H, Liang TJ. Hepatitis C virus infection activates an innate pathway involving IKK-alpha in lipogenesis and viral assembly. Nat Med 19: 722–729, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin L, Hron JD, Peng SL. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity 21: 203–213, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Logan S, Agbaga MP, Chan MD, Brush RS, Anderson RE. Endoplasmic reticulum microenvironment and conserved histidines govern ELOVL4 fatty acid elongase activity. J Lipid Res 55: 698–708, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Londos C, Sztalryd C, Tansey JT, Kimmel AR. Role of PAT proteins in lipid metabolism. Biochimie 87: 45–49, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol 7: 373–378, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Matsuo S, Yang WL, Aziz M, Kameoka S, Wang P. Fatty acid synthase inhibitor C75 ameliorates experimental colitis. Mol Med 20: 1–9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McIntosh AL, Senthivinayagam S, Moon KC, Gupta S, Lwande JS, Murphy CC, Storey SM, Atshaves BP. Direct interaction of Plin2 with lipids on the surface of lipid droplets: a live cell FRET analysis. Am J Physiol Cell Physiol 303: C728–C742, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Min M, Peng L, Yang Y, Guo M, Wang W, Sun G. MicroRNA-155 is involved in the pathogenesis of ulcerative colitis by targeting FOXO3a. Inflamm Bowel Dis 20: 652–659, 2014. [DOI] [PubMed] [Google Scholar]
- 36.Pauloin A, Chanat E. Prolactin and epidermal growth factor stimulate adipophilin synthesis in HC11 mouse mammary epithelial cells via the PI3-kinase/Akt/mTOR pathway. Biochim Biophys Acta 1823: 987–996, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Penrose H, Heller S, Cable C, Makboul R, Chadalawada G, Chen Y, Crawford SE, Savkovic SD. Epidermal growth factor receptor mediated proliferation depends on increased lipid droplet density regulated via a negative regulatory loop with FOXO3/Sirtuin6. Biochem Biophys Res Commun 469: 370–376, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qi W, Fitchev PS, Cornwell ML, Greenberg J, Cabe M, Weber CR, Roy HK, Crawford SE, Savkovic SD. FOXO3 growth inhibition of colonic cells is dependent on intraepithelial lipid droplet density. J Biol Chem 288: 16274–16281, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rohwedder A, Zhang Q, Rudge SA, Wakelam MJ. Lipid droplet formation in response to oleic acid in Huh-7 cells is mediated by the fatty acid receptor FFAR4. J Cell Sci 127: 3104–3115, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Schlager S, Goeritzer M, Jandl K, Frei R, Vujic N, Kolb D, Strohmaier H, Dorow J, Eichmann TO, Rosenberger A, Wolfler A, Lass A, Kershaw EE, Ceglarek U, Dichlberger A, Heinemann A, Kratky D. Adipose triglyceride lipase acts on neutrophil lipid droplets to regulate substrate availability for lipid mediator synthesis. J Leukoc Biol 98: 837–850, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sethi JK, Xu H, Uysal KT, Wiesbrock SM, Scheja L, Hotamisligil GS. Characterisation of receptor-specific TNFalpha functions in adipocyte cell lines lacking type 1 and 2 TNF receptors. FEBS Lett 469: 77–82, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Sizemore N, Lerner N, Dombrowski N, Sakurai H, Stark GR. Distinct roles of the Ikappa B kinase alpha and beta subunits in liberating nuclear factor kappa B (NF-kappa B) from Ikappa B and in phosphorylating the p65 subunit of NF-kappa B. J Biol Chem 277: 3863–3869, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Snoeks L, Weber CR, Turner JR, Bhattacharyya M, Wasland K, Savkovic SD. Tumor suppressor Foxo3a is involved in the regulation of lipopolysaccharide-induced interleukin-8 in intestinal HT-29 cells. Infect Immun 76: 4677–4685, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snoeks L, Weber CR, Wasland K, Turner JR, Vainder C, Qi W, Savkovic SD. Tumor suppressor FOXO3 participates in the regulation of intestinal inflammation. Lab Invest 89: 1053–1062, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Straub BK, Stoeffel P, Heid H, Zimbelmann R, Schirmacher P. Differential pattern of lipid droplet-associated proteins and de novo perilipin expression in hepatocyte steatogenesis. Hepatology 47: 1936–1946, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Sun H, Wu Y, Fu D, Liu Y, Huang C. SIRT6 regulates osteogenic differentiation of rat bone marrow mesenchymal stem cells partially via suppressing the nuclear factor-kappaB signaling pathway. Stem Cells 32: 1943–1955, 2014. [DOI] [PubMed] [Google Scholar]
- 47.Tao R, Wei D, Gao H, Liu Y, Depinho RA, Dong XC. Hepatic FoxOs regulate lipid metabolism via modulation of expression of the nicotinamide phosphoribosyltransferase gene. J Biol Chem 286: 14681–14690, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Welte MA. Expanding roles for lipid droplets. Curr Biol 25: R470–R481, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaoka T, Yan F, Cao H, Hobbs SS, Dise RS, Tong W, Polk DB. Transactivation of EGF receptor and ErbB2 protects intestinal epithelial cells from TNF-induced apoptosis. Proc Natl Acad Sci USA 105: 11772–11777, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X, Ding L, Sandford AJ. Selection of reference genes for gene expression studies in human neutrophils by real-time PCR. BMC Mol Biol 6: 4, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]