Abstract
Exercise stimulates hepatic mitochondrial adaptations; however, the mechanisms remain largely unknown. Here we tested whether FGF21 plays an obligatory role in exercise induced hepatic mitochondrial adaptations by testing exercise responses in FGF21 knockout mice. FGF21 knockout (FGF21-KO) and wild-type (WT) mice (11–12 wk of age) had access to voluntary running wheels for exercise (EX) or remained sedentary for 8 wk. FGF21 deficiency resulted in greater body weight, adiposity, serum cholesterol, insulin, and glucose concentrations compared with WT mice (P < 0.05). In addition, hepatic mitochondrial complete palmitate oxidation, β-hydroxyacyl-CoA dehydrogenase (β-HAD) activity, and nuclear content of PGC-1α were 30–50% lower in FGF21-KO mice compared with WT mice (P < 0.01). EX effectively lowered body weight, adiposity, serum triglycerides, free fatty acids, and insulin and normalized mitochondrial complete palmitate oxidation in the FGF21-KO mice, whereas the reduced hepatic β-HAD activity and lowered nuclear content of PGC-1α in FGF21-KO mice were not restored by EX. In addition, EX increased hepatic CPT-1α mRNA expression and ACC phosphorylation (a marker of increased AMPK activity) and reduced hepatic triacylglycerol content in both genotypes. However, FGF21-KO mice displayed a lower EX-induced increase in the mRNA expression of the hepatic gluconeogenic gene, PEPCK, compared with WT. In conclusion, FGF21 does not appear necessary for exercise-induced systemic and hepatic mitochondrial adaptations, but the increased adiposity, hyperinsulinemia, and impairments in hepatic mitochondrial function induced by FGF21 deficiency can be partially rescued by daily wheel running exercise.
Keywords: mitochondria, mitochondrial function, exercise, metabolism
the liver plays a vital role in maintaining euglycemia, in part through gluconeogenic processes during times of fasting or exercise. Hepatic mitochondria are instrumental in providing the ATP necessary to fuel this energy-costly process through the oxidation of fatty acids that are lipolyzed from hepatic and adipose stores (6). Exercise is a stimulus that challenges the liver to maintain euglycemia and hepatic mitochondria to produce the necessary ATP to maintain this endogenous glucose production. Evidence suggests that this exercise-induced challenge ultimately leads to greater hepatic mitochondrial content and function. Eight weeks of treadmill training has been shown to increase the activity of complexes I, IV, and V of the electron transport chain (41). In addition, daily voluntary wheel running led to greater complete palmitate oxidation, enzymatic activities of β-hydroxyacyl-CoA dehydrogenase (β-HAD) and citrate synthase (markers of mitochondrial function and content, respectively), and the protein content of COX IV and cytochrome c in hyperphagic obese Otsuka Long-Evans Tokushima rats (34, 36). Recently, our laboratory has demonstrated that 4 wk of voluntary wheel running or treadmill training in healthy, lean Sprague-Dawley rats increases various indexes of hepatic mitochondrial function and content, including mitochondrial respiration, TCA cycle flux, pyruvate dehydrogenase activity, and citrate synthase activity (13). Despite the accumulating evidence that exercise training results in greater hepatic mitochondrial content and function, mechanisms mediating these changes have yet to be elucidated.
Fibroblast growth factor 21 (FGF21) is a novel endocrine factor that plays a key role in the regulation of carbohydrate and lipid metabolism (16). Exogenous administration of FGF21 or FGF21 analogs, as well as transgenic overexpression of FGF21, results in an elevation of hepatic metabolic processes such as β-oxidation, ketogenesis, TCA cycle flux, and oxygen consumption, which is accompanied by increased expression of gluconeogenic genes (G6Pase, PEPCK), as well as those of cytochrome c, AMPKα1, CPT1α and CPT1β, and PGC-1α (5, 11, 32). While these changes are suggestive of improvements in mitochondrial function, the pharmacological levels of FGF21 achieved in these instances far exceed physiological fluctuations in FGF21, leaving many questions unanswered about the physiology of FGF21. In mice, the role of hepatic FGF21 in the physiology of fasting has been most extensively characterized and has been shown to be involved in the regulation of mitochondrial fatty acid oxidation and ketone body production (2, 27, 32). More recently, elevations in FGF21 have been reported with exercise and it has been proposed that FGF21 may mediate some of the metabolic benefits of exercise (8, 21, 22). However, a positive correlation between exercise and FGF21 is not always reported and it appears that the duration and intensity of the exercise along with the time point at which blood samples are taken may all influence FGF21 levels, making an interpretation of these data more difficult (25, 31, 39).
Therefore, the purpose of the present investigation was to directly determine the role of FGF21 in exercise-induced hepatic mitochondrial adaptations in FGF21 knockout mice. It was hypothesized that FGF21 knockout mice would not display the same exercise-induced adaptations in hepatic metabolic processes and gene transcription that would occur in wild-type (WT) mice with normal FGF21 signaling. Findings from this research may aid in identifying specific mechanisms responsible for hepatic mitochondrial adaptations induced by exercise.
METHODS
Animal protocol.
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri-Columbia and Harry S Truman Memorial Veterans Affairs Hospital. FGF21 knockout mice on a C57BL/6 background (FGF21-KO) and age-matched, but not littermate C57BL/6 WT mice controls were bred by Taconic Biosciences (Hudson, NY) and kindly provided by Eli Lilly (Indianapolis, IN). Male FGF21-KO and WT mice (11–12 wk of age) were provided with a running wheel to promote voluntary wheel running, the chosen mode of exercise, for 8 wk and were designated as FGF21-KO-EX (n = 14) and WT-EX (n = 15). Separate groups of FGF21-KO (n = 9) and WT (n = 10) mice remained sedentary without access to running wheels and were designated as FGF21-KO-SED and WT-SED. All mice were individually housed during the intervention in temperature-controlled animal quarters (21°C) with a 0600–1800 light and 1800-0600 dark cycle. All groups were provided standard rodent chow (Formulab 5008; Purina Mills, Brentwood, MO) for ad libitum feeding in new cages at the beginning of each week. Running wheel revolutions were monitored and counted continuously through the 8-wk intervention by use of a Sigma BC 509 bike computer (St. Charles, IL). Running distance was obtained every day between 0800 and 1000. Body mass and food consumption were measured on the same day each week throughout the study. To calculate feeding efficiency, a measurement that provides an index of energy balance, the change in body weight was divided by the amount of food consumed [Δ body weight (g)/g intake] during defined periods.
After the 8-wk intervention, running wheels were locked for 24 h and all mice were then fasted for 5 h, anesthetized with pentobarbital sodium (100 mg/kg), and then exsanguinated by removal of the heart. Retroperitoneal, epididymal, and inguinal adipose tissue fat pads were excised and weights were recorded.
Body composition.
Fat mass, lean mass, and percentages of body fat and lean mass were measured with an EchoMRI 4in1-1100 analyzer (EchoMRI; Houston, TX).
Tissue homogenization and mitochondrial isolation procedures.
Livers were quickly excised from anesthetized mice and either flash frozen in liquid nitrogen or placed in ice-cold isolation buffer (in mM: 220 mannitol, 70 sucrose, 10 Tris-base, 1 EDTA; pH 7.4). Hepatic mitochondria were prepared as previously described (28, 38).
Palmitate oxidation.
Oxidation of [1-14C]palmitate (American Radiochemicals; St. Louis, MO) was measured in whole liver homogenate and fresh isolated hepatic mitochondria preparations. The collection and measurement of 14C-CO2 palmitate oxidation allowed for the estimation of complete fatty acid oxidation and was conducted as previously described (34).
Citrate synthase and β-HAD activity.
Hepatic citrate synthase and β-HAD activity were measured as previously described by Srere (40) and Bass et al. (3), respectively, as well as previously described by our laboratory (34).
Intrahepatic lipid content and liver morphology.
Liver tissue was formalin fixed and paraffin embedded. Hematoxylin and eosin (H&E) stains were done to examine liver morphology. Intrahepatic triacylglycerol content was determined by a biochemical assay as previously described (34).
Hepatic glycogen content.
Glycogen content in liver tissue was measured as previously described by Aschenbach et al. (1) and by our laboratory (34).
Gene expression.
Phosphoenolpyruvate carboxykinase (PEPCK), glucose 6-phosphatase (G6Pase), peroxisome proliferator-activated receptor-α (PPARα), peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC-1α), carnitine palmitoyltransferase 1 (CPT1α), fibroblast growth factor receptor substrate 2 (FRS2), β-Klotho, and mitochondrial transcription factor A (TFAM) mRNA expression were quantified as previously described (35). Briefly, to examine PEPCK and G6Pase, the primer dilution mix [nuclease-free water, and both forward and reverse primers (Sigma), Fast SYBR Green Master Mix kit (ABI)] and cDNA sample (50 ng) were loaded to a 96-well microplate and placed into the ABI 7500 Fast Sequence Detection System (Applied Biosystems, Carlsbad, CA) for polymerization. FGF21 gene expression was assessed by loading TaqMan Master Mix (ABI), FGF21 primers and probe (Applied Biosystems), and cDNA samples (50 ng) into a 96-well microplate, which was placed into the ABI 7500 Fast Sequence Detection System (Applied Biosystems) for polymerization. Once polymerization was completed, results were quantified by the DDCT technique relative to the cyclophilin-B (cycB) housekeeping gene.
Serum measurements.
Serum glucose, aspartate aminotransferases, alanine aminotransferases, and lipids were all determined on the Cobas c311 clinical chemistry analyzer (Roche Diagnostics, Indianapolis, IN). Serum ketones were measured with a commercially available β-hydroxybutyrate Liquicolor kit (Stanbio, Boerne, TX). Serum total FGF21 (mouse/rat FGF21 kit Quantikine ELISA; R&D Systems, Minneapolis, MN), insulin (ultrasensitive mouse insulin ELISA kit; Crystal Chem, Downers Grove, IL), and adiponectin (mouse adiponectin ELISA kit; BioVendor, Asheville, NC) were measured by using commercially available assays and following manufacturer-provided protocols.
Nuclear extraction.
Nuclear and cytoplasmic protein fractions from liver were isolated by using a commercially available NE-PER nuclear and cytoplasmic extraction reagents kit (Thermo Scientific, Rockford, IL).
Western blotting.
Western blot analyses were performed to determine protein content of the following: oxidative phosphorylation (OXPHOS) electron transport chain complexes I through V (MitoProfile Total OXPHOS Rodent WB Antibody Cocktail; Abcam, Cambridge, MA), PGC-1α (EMD Millipore, Billerica, MA), mitochondrial transcription factor A (TFAM; Santa Cruz Biotechnology, Dallas, TX), PEPCK (Santa Cruz Biotechnology), G6Pase (Santa Cruz Biotechnology), PPARα (Santa Cruz Biotechnology), fibroblast growth factor receptor 2 (FGFR2; Abcam), FRS2 (Abcam) and phospho-FRS2 (Cell Signaling, Danvers, MA), β-Klotho (Santa Cruz Biotechnology), pyruvate carboxylase (PC; Abcam), cAMP-responsive element-binding protein (CREB) and phospho-CREB(Ser133) (Cell Signaling), AMP-activated protein kinase (AMPK) and phospho-AMPK(Thr 172) (Cell Signaling), and acetyl-CoA carboxylase (ACC) and phospho-ACC (Cell Signaling).
Phosphorylation status (with phosphospecific antibodies) was calculated from the density of the phosphoprotein band divided by density of the total protein with use of the appropriate antibody (33, 34). Membranes stained with 0.1% amido black (Sigma) were quantified to control for differences in protein loading or transfer of band densities as previously described (34). Hepatic FGF21 protein content was assessed with a commercially available mouse/rat FGF21 kit Quantikine ELISA (R&D Systems).
Statistics.
Each outcome measure was examined in 9 to 15 animals per group. Main effects of exercise (SED vs. EX) and genotype (WT vs. FGF21-KO) were examined through a two-way analysis of variance (IBM SPSS, version 20.0; SPSS, Chicago, IL), and significant main effects (0.05) were followed with Fisher least significant difference post hoc comparisons. Values are reported as means ± standard errors (SE), and a P value of ≤0.05 denotes a statistically significant difference.
RESULTS
Animal characteristics.
WT-EX and FGF21-KO-EX groups ran, on average, 7.8 and 7 km (respectively) each night with no significant difference in average weekly running distance between the two groups (P ≥ 0.05; Table 1). Heart mass to-body mass ratio was significantly higher in the EX groups (WT-EX and FGF21-KO-EX) compared with their SED counterparts (WT-SED and FGF21-KO-SED) (P ≤ 0.05; Table 1), indicating an adequate stimulus for training adaptations.
Table 1.
Animal characteristics
| Variable | WT-SED | WT-EX | FGF21KO-SED | FGF21KO-EX |
|---|---|---|---|---|
| Body weight, g | 29.3 ± 1.2 | 26.9 ± 0.5†‡ | 37.4 ± 0.8*‡ | 31.6 ± 0.8†* |
| Running distance, km/day | NA | 7.75 ± 0.60 | NA | 6.96 ± 0.73 |
| Heart weight/liver weight, mg/g | 5.24 ± 0.16 | 5.83 ± 0.19† | 4.58 ± 0.14* | 5.74 ± 0.23†* |
| Liver weight, mg | 1,251.5 ± 56.5 | 1,262.6 ± 28.6 | 1,693.2 ± 69.3* | 1,565.1 ± 38.7* |
| Liver weight/body weight, mg/g | 42.68 ± 0.76 | 47.04 ± 1.02† | 45.29 ± 1.49* | 49.77 ± 1.16†* |
| Fat mass, g | 5.3 ± 0.9 | 2.7 ± 0.4†‡ | 10.8 ± 0.4*‡ | 5.6 ± 0.6†* |
| Lean mass, g | 22.9 ± 0.6 | 22.9 ± 0.3 | 25.9 ± 0.4* | 25.1 ± 0.4* |
| Percent body fat, % | 17 ± 2 | 10 ± 1† | 29 ± 1* | 17 ± 1†* |
| Percent lean mass, % | 79 ± 2 | 86 ± 1† | 69 ± 1* | 80 ± 1†* |
| Food consumption, g/wk | 25.8 ± 0.2 | 32.3 ± 0.7† | 28.6 ± 0.2* | 33.7 ± 0.8†* |
| Feeding efficiency | 0.024 ± 0.004 | 0.006 ± 0.002† | 0.033 ± 0.003 | 0.009 ± 0.003† |
Values are means ± SE (n = 9–15).
Significant genotype main effect (P ≤ 0.05);
significant exercise main effect (P ≤ 0.05);
significantly different than WT-SED and FGF21KO-EX (P ≤ 0.05).
NA, not applicable. WT, wild-type; FGF21-KO, FGF21 knockout; SED, sedentary; EX, exercise.
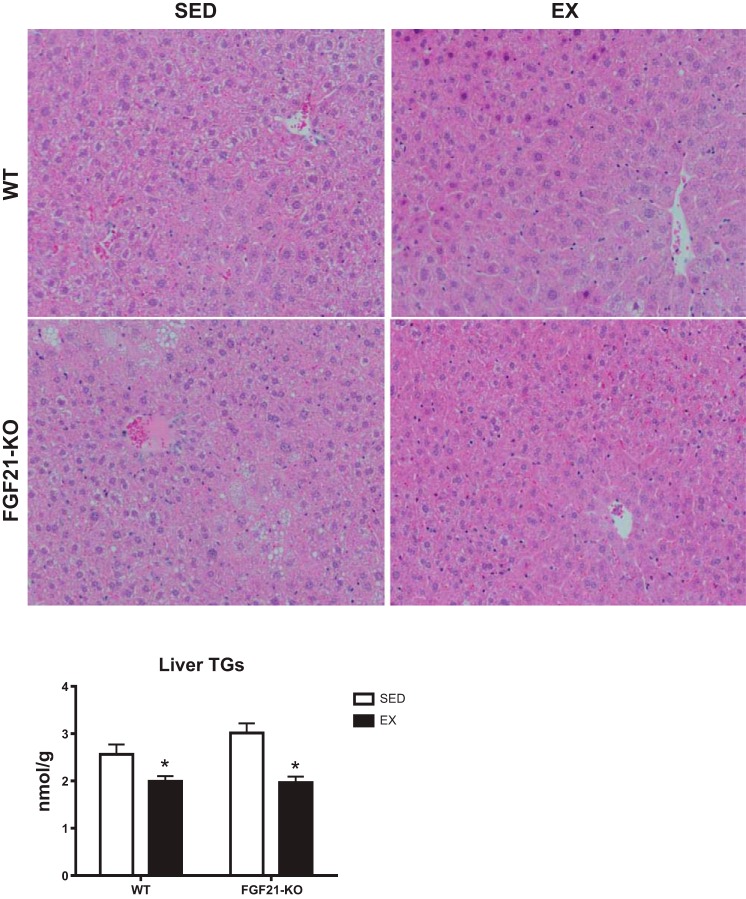
FGF21-KO mice had greater body weight and adiposity than WT (P < 0.001), while EX lowered these two variables in both genotypes (P < 0.001; Table 1; Fig. 1). Furthermore, lean mass was elevated in the FGF21-KO mice (P < 0.05; Table 1). In examining percentages of body fat and lean mass (P < 0.05), an absence of FGF21 resulted in a greater percentage of body fat and a reduction in percentage of lean mass compared with WT mice. Additionally, EX lowered the percentage of body fat while increasing the percentage of lean mass compared with SED animals in each genotype (P < 0.05; Table 1). These observed increases in body weight and adiposity are, in part, due to the greater food consumption in the FGF21-KO mice (P < 0.05). EX also led to greater food intake (P < 0.05; Table 1). Despite this greater food consumption, 8 wk of EX still lowered body weight as is reflected in significant reductions in feed efficiency (P < 0.05; Table 1). Furthermore, a lack of FGF21 and/or exercise resulted in a significantly greater liver mass to body mass ratio (P < 0.05; Table 1). In addition to lowering body weight and adiposity, EX also reduced hepatic triglycerides in both genotypes (P < 0.05; Fig. 2). Representative H&E images of the liver indicate greater lipid vacuolization in the FGF21-KO-SED mice compared with WT mice, which was normalized with 8 wk of EX (Fig. 2).
Fig. 1.
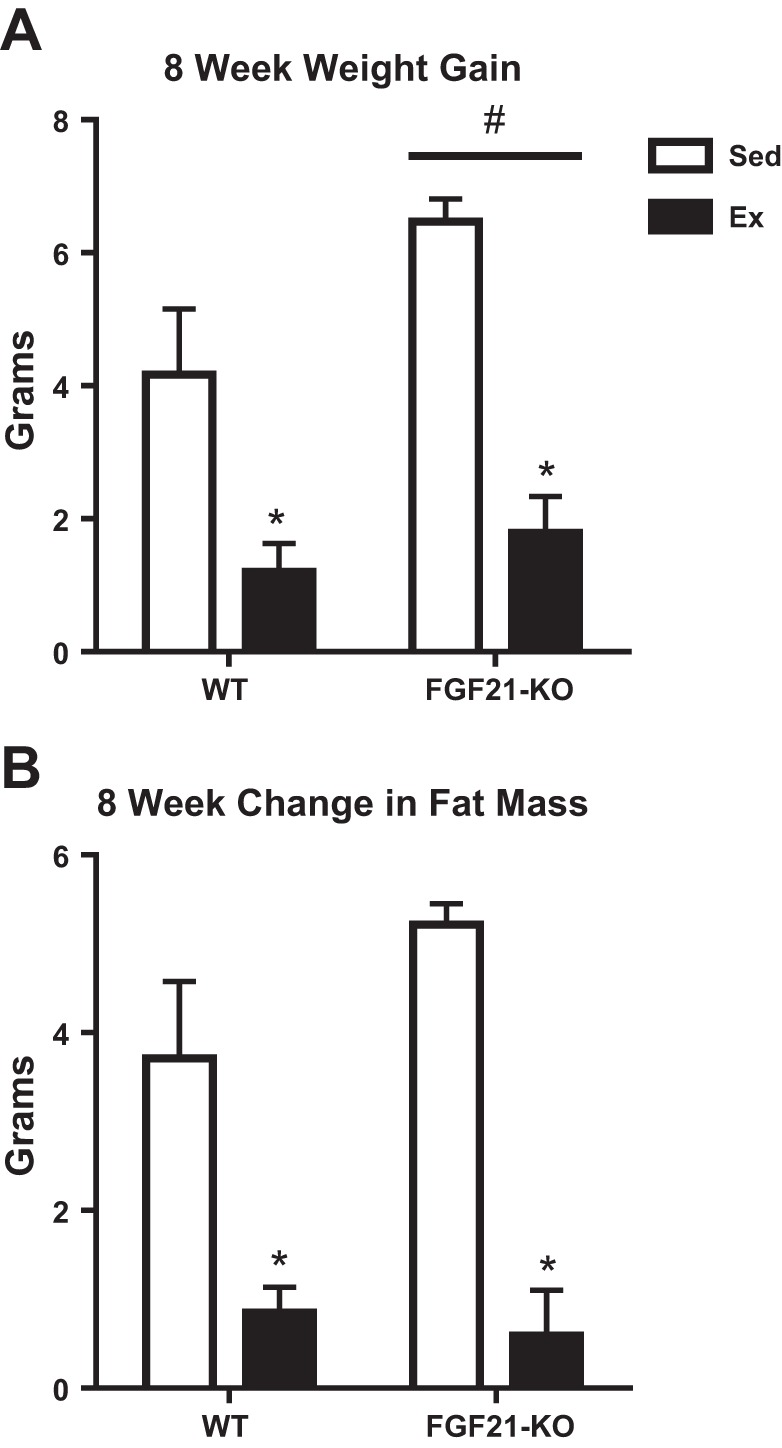
Effects of exercise and/or a lack of FGF21 on changes in body weight (A) and fat mass over the course of 8 wk (B). WT, wild-type; FGF21-KO, FGF21 knockout; SED, sedentary; EX, exercise. Values are means ± SE (n = 9–15). #Significant genotype main effect, P ≤ 0.05; *significant exercise main effect, P ≤ 0.05.
Fig. 2.
Effects of exercise and/or absence of FGF21 on hepatic triglycerides. Representative images of liver H&E staining and measured liver triglyceride (TAGS) content for each group. WT, wild-type; FGF21-KO, FGF21 knockout; SED, sedentary; EX, exercise. Values are means ± SE (n = 9–15). *Significant exercise main effect, P ≤ 0.05.
Eight weeks of EX did not alter circulating FGF21 concentrations in the WT mice. However, FGF21 deficiency caused elevations in 5-h fasted serum cholesterol, insulin, and glucose concentrations compared with WT mice (P < 0.05); EX effectively lowered serum triglycerides, free fatty acids, insulin, and adiponectin in WT and FGF21-KO mice (P < 0.05, Table 2). Furthermore, FGF21-KO mice displayed evidence of suppressed ketogenesis, with ∼40% lower serum ketones (β-hydroxybutyrate) compared with WT mice (P < 0.05; Table 2).
Table 2.
Serum measures
| Variable | WT-SED | WT-EX | FGF21KO-SED | FGF21KO-EX |
|---|---|---|---|---|
| FGF21, pg/ml | 892.50 ± 199.63 | 858.60 ± 130.97 | BDL | BDL |
| Insulin, ng/ml | 1.23 ± 0.10 | 0.82 ± 0.08† | 2.61 ± 0.29* | 1.95 ± 0.23†* |
| Glucose, mg/dl | 280.20 ± 10.75 | 280.53 ± 15.77 | 332.22 ± 21.89* | 350.84 ± 13.21* |
| β-Hydroxybutyrate, μmol/l | 41.64 ± 4.29‡ | 28.04 ± 2.69† | 27.12 ± 2.38* | 24.04 ± 2.13†* |
| Adiponectin, μg/ml | 21.38 ± 1.05 | 18.10 ± 0.64† | 20.17 ± 0.92 | 17.85 ± 0.49† |
| TG, mg/dl | 61.06 ± 4.69 | 47.33 ± 2.10† | 64.44 ± 3.91 | 57.89 ± 2.53† |
| Cholesterol, mg/dl | 95.37 ± 4.96 | 91.42 ± 1.90 | 111.51 ± 4.28* | 99.10 ± 4.28* |
| FFA, μM | 769.44 ± 53.55 | 598.89 ± 32.73† | 813.11 ± 26.14 | 680.22 ± 35.05† |
Values are means ± SE (n = 9–15).
Significant genotype main effect (P ≤ 0.05);
significant exercise main effect (P ≤ 0.05);
significant differences vs.
WT-EX and FGF21KO-SED (P ≤ 0.05). BDL, below detectable limits; TG, triglyceride; FFA, free fatty acid.
Markers of hepatic mitochondrial function.
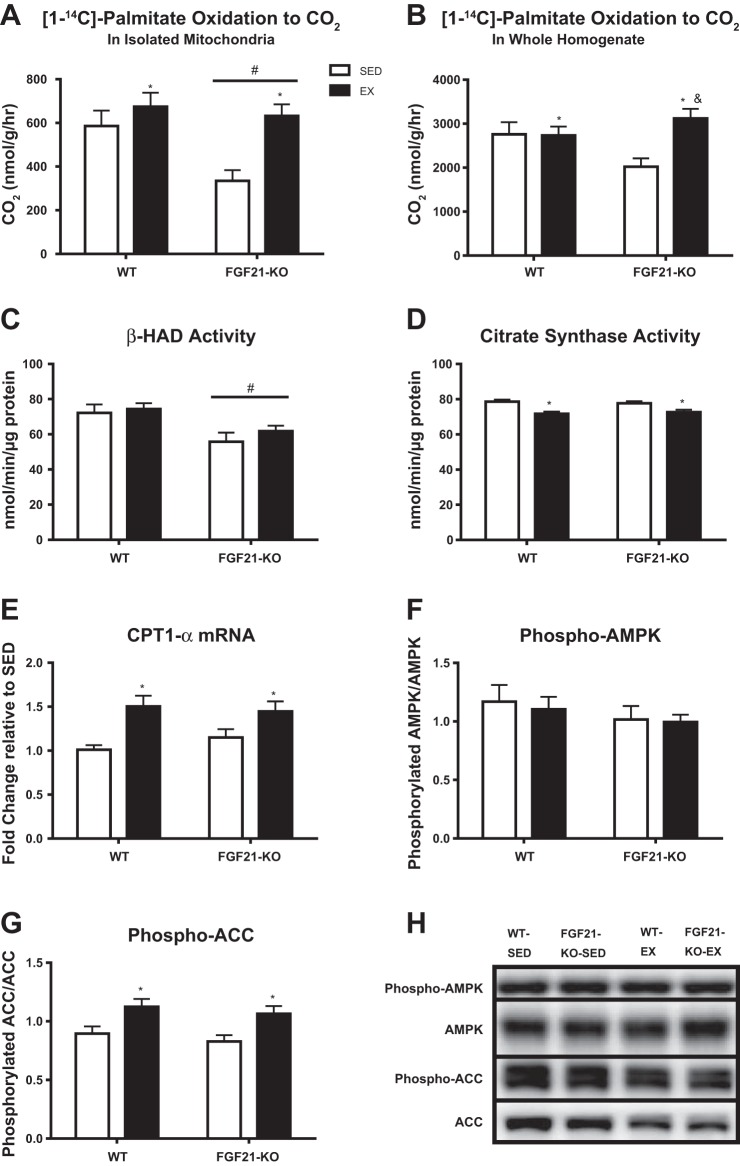
A major finding in the present investigation is that FGF21 deficiency reduced complete disposal of palmitate oxidation to CO2 in isolated liver mitochondria, yet this effect was completely rescued with daily EX (P < 0.05; Fig. 3A). Examining complete [1-14C]palmitate oxidation in whole homogenate shows again that EX increases fatty acid oxidation and this effect is likely driven by the significant increase in fatty acid oxidation in the FGF21-KO-EX mice compared with the FGF21-KO-SED group (P < 0.05; Fig. 3B). Moreover, lack of FGF21 lowered the activity of β-HAD (P = 0.001; Fig. 3C), the rate-limiting enzyme in β-oxidation, a reduction that was not reversed with EX. Interestingly, EX suppressed hepatic citrate synthase activity in both genotypes (P < 0.05; Fig. 3D).
Fig. 3.
Effects of exercise and/or a lack of FGF21 on complete [1-14C]-palmitate oxidation to CO2 (isolated mitochondria) (A), complete [1-14C]-palmitate oxidation to CO2 (whole homogenate) (B), β-HAD enzymatic activity (in whole homogenate) (C), citrate synthase activity (whole homogenate) (D), CPT1α mRNA expression to WT-SED (E), phosphorylated AMPK to AMPK protein content (F), phosphorylated ACC to ACC protein content (G). Representative Western blots shown in H. Representative images do not reflect the order in which they appear in the graphs. WT, wild-type; FGF21-KO, FGF21 knockout; SED, sedentary; EX, exercise. Values are means ± SE (n = 9–15). #Significant genotype main effect, P ≤ 0.05; *significant exercise main effect, P ≤ 0.05; &significant differences vs. FGF21KO-SED, P ≤ 0.05.
Additional analyses were performed on liver tissue to examine several key regulators of fat oxidation in an attempt to help explain the observed reduction of complete palmitate oxidation in the FGF21-KO-SED group and how EX rescued the deficit. CPT-1α was examined because of its role in fatty acid entry into the mitochondria, and it was observed that EX upregulated (P < 0.05; Fig. 3E) this important gene involved in fatty acid oxidation in both WT and FGF21-KO mice. The ratio of phosphorylated AMPK to total AMPK hepatic protein content was then measured because of the ability of AMPK to act as an energy sensor and ability to upregulate fatty acid oxidation in the liver during times of energy deprivation. Although AMPK and phosphorylation status of AMPK were not different among groups (Fig. 3F), ACC phosphorylation status (phosphorylated ACC/total ACC) at Ser-79 [inhibitory site (17) that is known to be phosphorylated by AMPK] was increased with EX in both WT and FGF21-KO mice (P < 0.05; Fig. 3G), albeit both phosphorylated ACC and total ACC protein content was lowered by EX (P < 0.05; representative images shown in Fig. 3H).
Proteins involved in mitochondrial biogenesis.
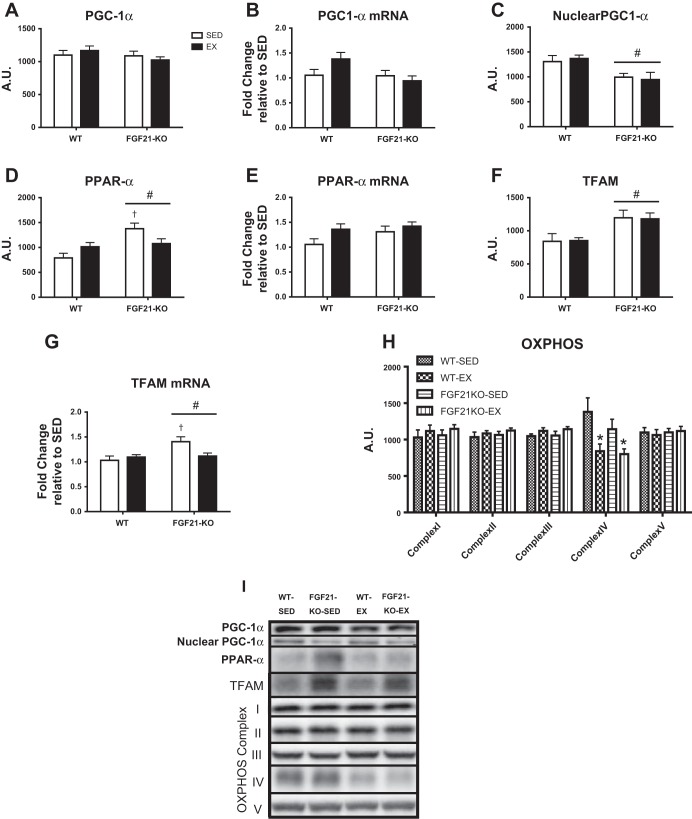
We assessed PGC-1α, a potent coactivator of transcriptional processes that plays a vital role in multiple metabolic processes, including fatty acid oxidation and mitochondrial biogenesis. Neither the ablation of FGF21 nor 8 wk of EX altered PGC-1α protein content or mRNA expression (Fig. 4, A and B, respectively). Transcriptional (active) PGC-1α resides in the nucleus where it can actively coactivate transcription factors that bind to DNA. It was found that FGF21-KO mice had significant reductions in the nuclear content of PGC-1α (P < 0.05; Fig. 4C), which was not rescued by exercise. The protein content of PPARα, a transcription factor involved in the transcription of fatty acid oxidation related genes and FGF21, was elevated in the FGF21-KO mice compared with WT animals (P < 0.05, Fig. 4D). However, PPARα mRNA expression was not affected by EX or genotype (Fig. 4E).
Fig. 4.
Effects of exercise and/or absence of FGF21 on PGC-1α protein content (A), PGC-1α mRNA expression to WT-SED (B), PGC-1α protein content found in the nucleus (C), PPARα protein content (D), PPARα mRNA expression to WT-SED (E), TFAM protein content (F), TFAM mRNA expression to WT-SED (G), and oxidative phosphorylation (H). Representative Western blots shown in I. Representative images do not reflect the order in which they appear in the graphs. WT, wild-type; FGF21-KO, FGF21 knockout; SED, sedentary; EX, exercise. Values are means ± SE (n = 9–15). #Significant genotype main effect, P ≤ 0.05; †significant differences vs. WT-SED and FGF21KO-EX, P ≤ 0.05. A.U., arbitrary units.
To examine the effects of the observed reduction in the nuclear content of PGC-1α on mitochondrial biogenesis, we measured hepatic TFAM, an important transcription factor involved in mitochondrial biogenesis, and OXPHOS protein content. Despite reduction in the nuclear content of PGC-1α, FGF21-KO mice had greater protein content and gene expression of TFAM (P < 0.05; Fig. 4, F and G, respectively). Interestingly, TFAM mRNA expression was lowered with EX in FGF21-KO mice. When examining the complexes in OXPHOS, we found that EX reduced complex IV protein content in both genotypes (P < 0.05; Fig. 4H).
Gluconeogenesis.
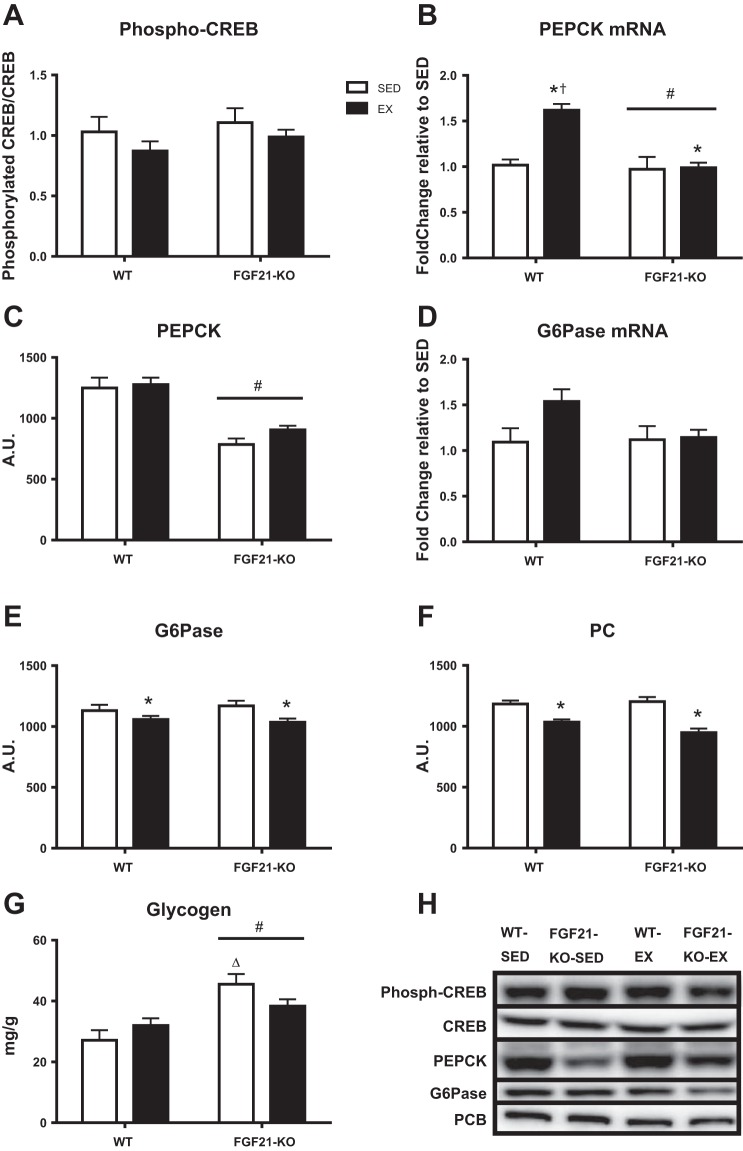
Mechanisms involved in gluconeogenesis were analyzed to determine how they were influenced by EX and/or a lack of FGF21. The hepatic protein content of activated CREB (phosphorylated CREB/total CREB), a transcription factor responsible for transcribing gluconeogenic genes, was found to not be different among groups (Fig. 5A). However, mRNA expression of PEPCK, another important enzyme involved in gluconeogenesis, was significantly increased in the WT-EX mice (P ≤ 0.001), but not in FGF21-KO-EX mice (Fig. 5B). FGF21-KO mice also had lower PEPCK protein content compared with WT mice (P ≤ 0.001), which was not affected by EX (Fig. 5C). Unlike PEPCK, G6Pase mRNA expression was not affected by EX or ablating FGF21 (Fig. 5D); however, EX did lower the protein content of both G6Pase (P < 0.01; Fig. 5E) and PC (P ≤ 0.001; Fig. 5F). Hepatic glycogen is another contributor to endogenous glucose production and may affect the need for gluconeogenesis, as well as the protein content and gene expression of important enzymes involved in gluconeogenesis and/or vice versa. In the present report, hepatic glycogen content was elevated in FGF21-KO mice (P < 0.05; Fig. 5G).
Fig. 5.
Effects of exercise and/or lack of FGF21 on hepatic phosphorylated cAMP-responsive element-binding protein (CREB) protein content (A), PEPCK mRNA expression to WT-SED (B), PEPCK protein content (C), G6Pase mRNA expression to WT-SED (D), G6Pase protein content (E), pyruvate carboxylase (PC) protein content (F), and hepatic glycogen content (G). Representative Western blots shown in H. Representative images do not reflect the order in which they appear in the graphs. WT, wild-type; FGF21-KO, FGF21 knockout; SED, sedentary; EX, exercise. Values are means ± SE (n = 9–15). #Significant genotype main effect, P ≤ 0.05; *significant exercise main effect, P ≤ 0.05; †significant differences vs. WT-SED and FGF21KO-EX, P ≤ 0.05; Δsignificant differences vs. WT-SED, P ≤ 0.05. A.U., arbitrary units.
FGF21 signaling mediators.
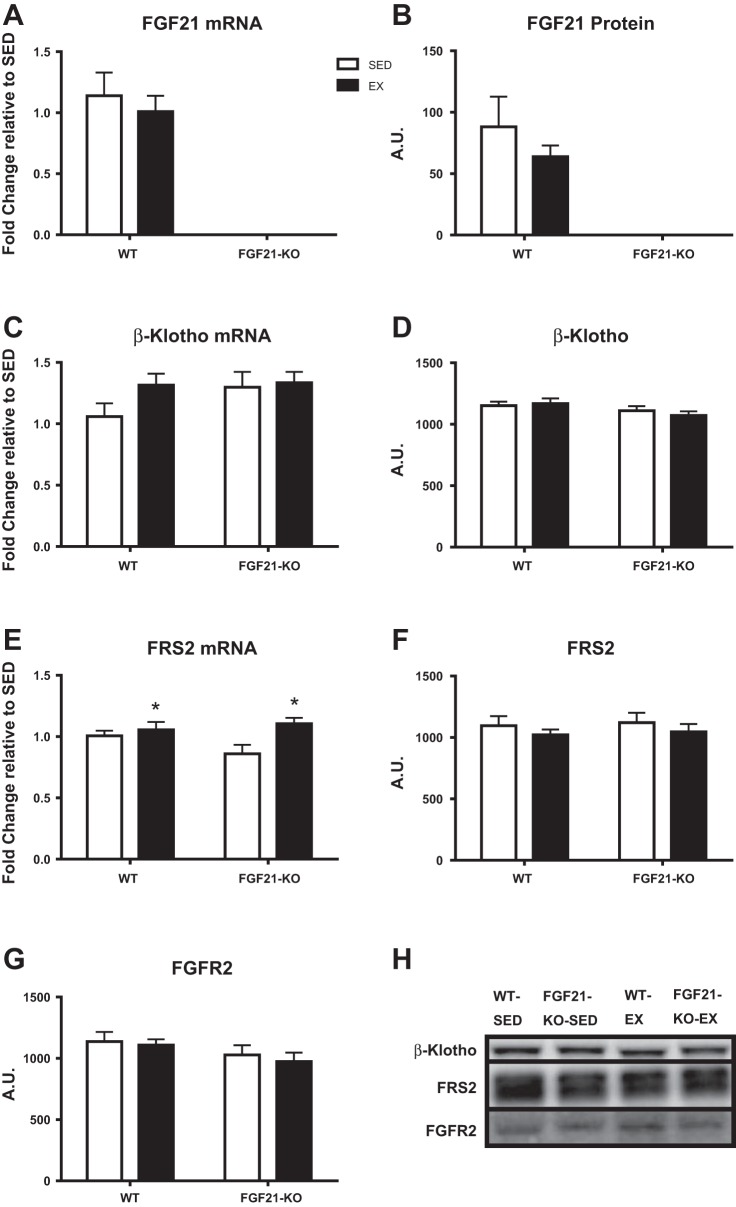
We have previously reported that exercise increased and/or rescued FGF21 signaling mediators in OLETF rats when exercise was used to prevent obesity (12). In the present investigation, the effects of exercise and/or a lack of FGF21 on FGF21 signaling mediators was assessed. To confirm the deletion of FGF21, hepatic FGF21 mRNA expression and protein content were assessed. FGF21 was not detected in FGF21-KO mice, and neither gene expression nor protein content differed between the WT-SED and WT-EX mice (Fig. 6, A and B, respectively). Furthermore, neither protein content nor gene expression of β-Klotho or FGFR2 was affected by EX or a lack of FGF21 (Fig. 6). FRS2 protein content was also not affected by either treatment; however, FRS2 gene expression was increased by 8 wk of EX in both genotypes (P < 0.05; Fig. 6).
Fig. 6.
Effects of exercise and/or lack of FGF21 on hepatic FGF21 mRNA expression (A), FGF21 protein content (B), Beta-Klotho mRNA expression to WT-SED (C), Beta-Klotho protein content (D), FRS2 mRNA expression to WT-SED (E), FRS2 protein content (F), and FGFR2 protein content (G). Representative Western blots shown in H. WT, wild-type; FGF21-KO, FGF21 knockout; SED, sedentary; EX, exercise. Values are means ± SE (n = 9–15). *Significant exercise main effect, P ≤ 0.05. Representative images do not reflect the order in which they appear in the graphs. A.U., arbitrary units.
DISCUSSION
Exercise is a known stimulus for increasing hepatic mitochondrial function (13), yet the mechanisms responsible for these adaptations remain to be elucidated. FGF21, a powerful metabolic regulator known to be induced by fasting (4, 15), is one potential mediator of exercise-induced adaptations. The present investigation examined, for the first time, the role of FGF21 in improvements in mitochondrial function that occur in response to exercise. Here we report novel findings suggesting that FGF21 does not appear necessary for exercise-induced hepatic mitochondrial adaptations and that impairments in hepatic fatty acid oxidation induced by FGF21 deficiency can be partially rescued by daily wheel running exercise.
In the present study, lack of FGF21 resulted in low-fat chow-fed mice developing greater body weight, adiposity, hyperinsulinemia, hyperglycemia, and dyslipidemia in sedentary mice. We also demonstrate that 8 wk of voluntary wheel exercise can largely normalize most of these abnormalities. The oxidation of fatty acids is the primary means of energy production in the liver to support other processes such as endogenous glucose production (gluconeogenesis). In the present investigation, as expected, a lack of FGF21 resulted in diminished palmitate oxidation in sedentary mice. Importantly, 8 wk of EX normalized the effects of FGF21 deficiency on hepatic fatty acid oxidation. We have previously found that 6, 16, and 36 wk of daily voluntary wheel running prevents obesity and enhances (24, 34, 36) fatty acid oxidation in hyperphagic OLETF rats and that 4 wk of daily voluntary wheel running induced a 40% increase in complete palmitate oxidation in healthy lean Sprague-Dawley rats compared with sedentary rats (13). Here, 8 wk of daily voluntary wheel running did not increase complete palmitate oxidation in the healthy lean wild-type mice. The reason for the discrepancy is unknown, but it is possible that adaptations in hepatic fat oxidation with exercise take longer in mice compared with rats (13, 24). Our findings that a deficit of hepatic fatty acid oxidation occurs in the absence of FGF21 agrees with previous reports (10, 32), and it is well documented that FGF21 also regulates whole body fat oxidation and energy expenditure (7, 29, 45). These changes in fat oxidation and energy expenditure due to a lack of FGF21 most likely played a major role in the elevated body weight found in FGF21 KO mice.
FGF21 increases hepatic fatty acid oxidation (32), as well as transcription of genes important to this process (26). Acute FGF21 administration leads to increases in hepatic PGC-1α gene expression (11, 32), whereas administration of FGF21 for 8 days results in the increased gene expression of CPT1α, CPT1β, and AMPKα1. Moreover, knocking down FGF21 gene expression leads to a failure to upregulate genes involved in hepatic β-oxidation (medium, long, and very long chain acyl-CoA dehydrogenase), and CPT1α in response to a ketogenic diet (2). Here we report that a lack of FGF21 resulted in reduced hepatic palmitate oxidation in sedentary mice, which was normalized with daily wheel running exercise in the absence of changes in PGC-1α or PPARα protein content and gene expression. Although PGC-1α transcriptional activity was not directly assessed, nuclear protein content of PGC-1α was found to be lower in the FGF21-KO groups, and 8 wk of EX failed to change the nuclear content of this important cotranscription factor. It is entirely possible that exercise upregulated other transcription factors, and/or changes in PPARα or PGC-1α content or location were not necessary to accommodate the necessary increases in genes associated with fatty acid oxidation. PGC-1α cotranscribes CPT-1α (37), and an increase in CTP-1 mRNA expression was observed in the FGF21-KO group with EX despite a reduction in nuclear PGC-1α.
The upregulation in CPT-1α may also explain the EX-induced increase in fatty acid oxidation, since entry into the mitochondria through CPT-1α is the rate-limiting step of β-oxidation (9, 20, 30). This step in the process can be inhibited by ACC activity, the enzyme responsible for the rate-limiting step of fatty acid synthesis and the production of malonyl-CoA. Malonyl-CoA has an inhibitory effect on CPT-1α and can therefore limit fat oxidation (9, 30). ACC is deactivated upon phosphorylation on ser79 by AMPK (9, 17). In the present study, EX increased hepatic ACC ser79 phosphorylation status (increased ratio of phosphorylated ACC to total ACC) in both FGF21-KO and WT mice, which is indicative of increased AMPK activity. Taken together, these data suggest that exercise, at least in part, rescued fatty acid oxidation in FGF21-KO mice by increasing AMPK activity and promoting increased fatty acid entry into the mitochondria through CPT-1α.
Not only is PGC-1α important for hepatic fatty acid oxidation, it is also vital to mitochondrial biogenesis. PGC-1α aids in the transcription of nuclear respiratory factor-1 (NRF-1), which in turn is responsible for the transcription of TFAM, an important transcription factor for mitochondrial biogenesis (44). TFAM, transcribed from nuclear DNA, is then able to translocate to mitochondrial DNA and transcribe proteins from the mitochondrial genome including subunits of electron transport chain complexes (43, 44). Despite a reduction in hepatic nuclear PGC-1α protein content, FGF21-KO mice had greater TFAM protein content and gene expression. However, this elevation in TFAM in the FGF21-KO mice did not appear to affect the protein content of electron transport chain complexes. Surprisingly, hepatic complex IV protein content was actually reduced in EX groups; the reason for this reduction is unknown. The protein content of these electron transport chain complexes are considered markers of mitochondrial content (23). Citrate synthase activity, another marker of mitochondrial content (23), was also reduced in response to voluntary wheel running. These findings contrast those of a previous wheel running study from our group using rats (36). Discrepancies in these findings may be due to a larger liver weight-to-body weight ratio in voluntary wheel running mice compared with sedentary mice. Perhaps a larger liver-to-body weight ratio reduces the gluconeogenic burden in these mice and increased mitochondrial density is not needed, but rather the quality of the mitochondria is increased. This is strictly speculation and these findings warrant future, more in-depth studies.
The transcription factor CREB and cotranscription factor PGC-1α are responsible for upregulating hepatic gluconeogenic genes. Previous studies have shown that this upregulation of gene expression is stimulated both by FGF21 signaling as well as exercise (13, 14, 32). Although exercise and the deletion of FGF21 did not influence the protein content of PGC-1α or phosphorylated CREB in the present report, the absence of FGF21 resulted in a blunting of PEPCK content in both the sedentary and voluntary wheel running groups. Furthermore, 8 wk of voluntary wheel running upregulated the hepatic gene expression of PEPCK in WT mice yet was unable to do so in the absence of FGF21. Large decreases in PEPCK protein content have been shown to lead to small reductions in gluconeogenesis (6), and it is not known whether the reductions in PEPCK protein content in the FGF21-KO groups were sufficient to lower gluconeogenesis in these mice. However, if a decrement in gluconeogenesis did occur in the FGF21-KO mice, then it is possible that the greater glycogen content in these animals was a compensatory mechanism to help maintain endogenous glucose production. Using Sprague-Dawley rats, our laboratory previously found that PEPCK mRNA expression and the protein content of phosphorylated CREB increased in response to voluntary wheel running in a fasted condition, while hepatic G6Pase expression remained unchanged (13). Similarly, in the present report, whereas 8 wk of EX increased PEPCK expression, the protein content of the gluconeogenic enzymes G6Pase and PC were decreased. Taken together, these data suggest that not all gluconeogenic enzymes respond to an exercise stimuli in the same manner. Similar findings have been previously reported (18). Importantly, these data also suggest that wheel running exercise does not have the same ability to upregulate hepatic PEPCK in the absence of FGF21.
We have previously demonstrated that voluntary wheel running for 36 wk not only prevents obesity in hyperphagic OLETF rats but also increases or rescues FGF21 signaling mediators (β-Klotho, FGFR2, and FRS2) while at the same time lowering circulating FGF-21 levels (12). In the present study, 8 wk of voluntary wheel running exercise increased hepatic FRS2 mRNA expression in both genotypes but did not affect hepatic gene expression or protein content of FGF21 or any of the other FGF21 signaling mediators assessed. Additionally, exercise did not alter circulating FGF21 levels in the WT mice. The literature regarding exercise-induced changes in FGF21 is inconsistent. Although some studies have found increases in FGF21 with a single exercise bout (4, 21) and that the liver contributes to the release of FGF21 during exercise (19), others have reported changes after weeks of training but no changes following acute exercise (8). Moreover, consistent with our observations in obese rats (12), circulating FGF-21 levels are reduced following exercise training in overweight and obese individuals (39, 46) and in the elderly following 5 wk of cycle ergometer (42). The discrepancies in the literature may be due to the exercise conditions studied, the timing of blood collection in relation to the last exercise bout, and/or a number of other variables known to influence FGF21 levels both acutely and chronically including diet composition, fasting duration, and the health/age/sex/species of the subject/animal tested.
The use of FGF21-KO mice provided a means to directly determine the extent to which FGF21 contributes to exercise-associated improvements in hepatic metabolism. Collectively, our data do not support a strong role for exercise-induced enhancement of FGF21 signaling in mediating exercise-induced increases in hepatic fatty acid oxidation. However, given the pleotropic nature of FGF21, it is possible that it is important for exercise-associated benefits in other tissues, and additional investigations are warranted to examine this more thoroughly.
In conclusion, data from the present investigation indicate that FGF21 deficiency results in greater body weight, adiposity, dyslipidemia, and insulin resistance in mice fed a low-fat chow diet. In addition, FGF21 deficiency led to reductions in markers of hepatic mitochondrial function and biogenesis. However, our findings suggest that FGF21 may not be necessary for exercise-induced systemic and hepatic mitochondrial adaptations, since 8 wk of daily wheel running exercise effectively corrected many, but not all, of these abnormalities. Importantly, daily exercise normalized impairments in hepatic fatty acid oxidation induced by FGF21 deficiency. Collectively, these data further support the metabolic importance of both intact FGF21 signaling and daily exercise on liver health and also highlight some of the independent effects of each on these metabolic processes.
GRANTS
This research was supported by an ACSM Foundation Research Grant from the American College of Sports Medicine Foundation (J. A. Fletcher), as well as National Institutes of Health Grants T32 AR 048523-07 (J. A. Fletcher and E. M. Morris) and DK-088940 (J. P. Thyfault) and Veterans Affairs Grants Merit (1I01BX002567-01, J. P. Thyfault) and VHA-CDA2 IK2BX001299 (R. S. Rector).
DISCLOSURES
A. Butterfield and J. W. Perfield II are paid employees of Eli Lilly and Company and may own company stock or possess stock options. The remaining authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
J.A.F., J.W.P., J.P.T., and R.S.R. conception and design of research; J.A.F., G.M.M., A.B., J.P.T., and R.S.R. performed experiments; J.A.F., G.M.M., J.P.T., and R.S.R. analyzed data; J.A.F., M.A.L., R.D.S., E.M.M., J.W.P., J.P.T., and R.S.R. interpreted results of experiments; J.A.F. and R.S.R. prepared figures; J.A.F., M.A.L., J.W.P., J.P.T., and R.S.R. drafted manuscript; J.A.F., M.A.L., R.D.S., G.M.M., E.M.M., A.B., J.W.P., J.P.T., and R.S.R. edited and revised manuscript; J.A.F., M.A.L., R.D.S., G.M.M., E.M.M., A.B., J.W.P., J.P.T., and R.S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the excellent technical assistance of Radheya Naik, Tasnim Farha Haq, and Kayla Kanosky. This work was supported with resources and the use of facilities at the Harry S Truman Memorial Veterans Hospital in Columbia, MO.
REFERENCES
- 1.Aschenbach WG, Hirshman MF, Fujii N, Sakamoto K, Howlett KF, Goodyear LJ. Effect of AICAR treatment on glycogen metabolism in skeletal muscle. Diabetes 51: 567–573, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5: 426–437, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem 10: 198–206, 1969. [DOI] [PubMed] [Google Scholar]
- 4.Berglund ED, Kang L, Lee-Young RS, Hasenour CM, Lustig DG, Lynes SE, Donahue EP, Swift LL, Charron MJ, Wasserman DH. Glucagon and lipid interactions in the regulation of hepatic AMPK signaling and expression of PPARα and FGF21 transcripts in vivo. Am J Physiol Endocrinol Metab 299: E607–E614, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Berglund ED, Li CY, Bina HA, Lynes SE, Michael MD, Shanafelt A Butterfield, Kharitonenkov A, Wasserman DH. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology 150: 4084–4093, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgess SC, He T, Yan Z, Lindner J, Sherry AD, Malloy CR, Browning JD, Magnuson MA. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab 5: 313–320, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149: 6018–6027, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Cuevas-Ramos D, Almeda-Valdes P, Meza-Arana CE, Brito-Cordova G, Gomez-Perez FJ, Mehta R, Oseguera-Moguel J, Aguilar-Salinas CA. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS One 7: e38022, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobrzyn P, Dobrzyn A, Miyazaki M, Cohen P, Asilmaz E, Hardie DG, Friedman JM, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc Natl Acad Sci USA 101: 6409–6414, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher FM, Chui PC, Nasser IA, Popov Y, Cunniff JC, Lundasen T, Kharitonenkov A, Schuppan D, Flier JS, Maratos-Flier E. Fibroblast growth factor 21 limits lipotoxicity by promoting hepatic fatty acid activation in mice on methionine and choline-deficient diets. Gastroenterology 147: 1073–1083.e6, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher FM, Estall JL, Adams AC, Antonellis PJ, Bina HA, Flier JS, Kharitonenkov A, Spiegelman BM, Maratos-Flier E. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology 152: 2996–3004, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fletcher JA, Meers GM, Laughlin MH, Ibdah JA, Thyfault JP, Rector RS. Modulating fibroblast growth factor 21 in hyperphagic OLETF rats with daily exercise and caloric restriction. Appl Physiol Nutr Metab 37: 1054–1062, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fletcher JA, Meers GM, Linden MA, Kearney ML, Morris EM, Thyfault JP, Rector RS. Impact of various exercise modalities on hepatic mitochondrial function. Med Sci Sports Exerc 46: 1089–1097, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman JE. Role of glucocorticoids in activation of hepatic PEPCK gene transcription during exercise. Am J Physiol Endocrinol Metab 266: E560–E566, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Galman C, Lundasen T, Kharitonenkov A, Bina HA, Eriksson M, Hafstrom I, Dahlin M, Amark P, Angelin B, Rudling M. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab 8: 169–174, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Gimeno RE, Moller DE. FGF21-based pharmacotherapy—potential utility for metabolic disorders. Trends Endocrinol Metab 25: 303–311, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Ha J, Daniel S, Broyles SS, Kim KH. Critical phosphorylation sites for acetyl-CoA carboxylase activity. J Biol Chem 269: 22162–22168, 1994. [PubMed] [Google Scholar]
- 18.Haase TN, Ringholm S, Leick L, Bienso RS, Kiilerich K, Johansen S, Nielsen MM, Wojtaszewski JF, Hidalgo J, Pedersen PA, Pilegaard H. Role of PGC-1α in exercise and fasting-induced adaptations in mouse liver. Am J Physiol Regul Integr Comp Physiol 301: R1501–R1509, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Hansen JS, Clemmesen JO, Secher NH, Hoene M, Drescher A, Weigert C, Pedersen BK, Plomgaard P. Glucagon-to-insulin ratio is pivotal for splanchnic regulation of FGF-21 in humans. Mol Metab 4: 551–560, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jambor de Sousa UL, Koss MD, Fillies M, Gahl A, Scheeder MR, Cardoso MC, Leonhardt H, Geary N, Langhans W, Leonhardt M. CPT1alpha over-expression increases long-chain fatty acid oxidation and reduces cell viability with incremental palmitic acid concentration in 293T cells. Biochem Biophys Res Commun 338: 757–761, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Kim KH, Kim SH, Min YK, Yang HM, Lee JB, Lee MS. Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS One 8: e63517, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KH, Lee MS. FGF21 as a stress hormone: the roles of FGF21 in stress adaptation and the treatment of metabolic diseases. Diabetes Metab J 38: 245–251, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N, Schroder HD, Boushel R, Helge JW, Dela F, Hey-Mogensen M. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 590: 3349–3360, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laye MJ, Rector RS, Borengasser SJ, Naples SP, Uptergrove GM, Ibdah JA, Booth FW, Thyfault JP. Cessation of daily wheel running differentially alters fat oxidation capacity in liver, muscle, and adipose tissue. J Appl Physiol 106: 161–168, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, Kebebew E, Pacak K, Chen KY, Celi FS. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab 19: 302–309, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Wong K, Walsh K, Gao B, Zang M. Retinoic acid receptor beta stimulates hepatic induction of fibroblast growth factor 21 to promote fatty acid oxidation and control whole-body energy homeostasis in mice. J Biol Chem 288: 10490–10504, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Mohammadi M, Potthoff MJ. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 63: 4057–4063, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris EM, Meers GM, Booth FW, Fritsche KL, Hardin CD, Thyfault JP, Ibdah JA. PGC-1α overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. Am J Physiol Gastrointest Liver Physiol 303: G979–G992, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy M, Samms R, Warner A, Bolborea M, Barrett P, Fowler MJ, Brameld JM, Tsintzas K, Kharitonenkov A, Adams AC, Coskun T, Ebling FJ. Increased responses to the actions of fibroblast growth factor 21 on energy balance and body weight in a seasonal model of adiposity. J Neuroendocrinol 25: 180–189, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Park EA, Cook GA. Differential regulation in the heart of mitochondrial carnitine palmitoyltransferase-I muscle and liver isoforms. Mol Cell Biochem 180: 27–32, 1998. [PubMed] [Google Scholar]
- 31.Pedersen LR, Olsen RH, Jurs A, Astrup A, Chabanova E, Simonsen L, Wisloff U, Haugaard SB, Prescott E. A randomised trial comparing weight loss with aerobic exercise in overweight individuals with coronary artery disease: the CUT-IT trial. Eur J Prev Cardiol 22: 1009–1017, 2015. [DOI] [PubMed] [Google Scholar]
- 32.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci USA 106: 10853–10858, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rector RS, Thyfault JP, Laye MJ, Morris RT, Borengasser SJ, Uptergrove GM, Chakravarthy MV, Booth FW, Ibdah JA. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. J Physiol 586: 4241–4249, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol 294: G619–G626, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Rector RS, Uptergrove GM, Borengasser SJ, Mikus CR, Morris EM, Naples SP, Laye MJ, Laughlin MH, Booth FW, Ibdah JA, Thyfault JP. Changes in skeletal muscle mitochondria in response to the development of type 2 diabetes or prevention by daily wheel running in hyperphagic OLETF rats. Am J Physiol Endocrinol Metab 298: E1179–E1187, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rector RS, Uptergrove GM, Morris EM, Borengasser SJ, Laughlin MH, Booth FW, Thyfault JP, Ibdah JA. Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. Am J Physiol Gastrointest Liver Physiol 300: G874–G883, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhee J, Inoue Y, Yoon JC, Puigserver P, Fan M, Gonzalez FJ, Spiegelman BM. Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1): requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. Proc Natl Acad Sci USA 100: 4012–4017, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruiz-Ramirez A, Chavez-Salgado M, Peneda-Flores JA, Zapata E, Masso F, El-Hafidi M. High-sucrose diet increases ROS generation, FFA accumulation, UCP2 level, and proton leak in liver mitochondria. Am J Physiol Endocrinol Metab 301: E1198–E1207, 2011. [DOI] [PubMed] [Google Scholar]
- 39.Scalzo RL, Peltonen GL, Giordano GR, Binns SE, Klochak AL, Paris HL, Schweder MM, Szallar SE, Wood LM, Larson DG, Luckasen GJ, Hickey MS, Bell C. Regulators of human white adipose browning: evidence for sympathetic control and sexual dimorphic responses to sprint interval training. PLoS One 9: e90696, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srere PA. Citrate synthase. Methods Enzymol 13: 3–5, 1969. [Google Scholar]
- 41.Sun L, Shen W, Liu Z, Guan S, Liu J, Ding S. Endurance exercise causes mitochondrial and oxidative stress in rat liver: effects of a combination of mitochondrial targeting nutrients. Life Sci 86: 39–44, 2010. [DOI] [PubMed] [Google Scholar]
- 42.Taniguchi H, Tanisawa K, Sun X, Kubo T, Higuchi M. Endurance exercise reduces hepatic fat content and serum fibroblast growth factor 21 levels in elderly men. J Clin Endocrinol Metab 101: 191–198, 2016. [DOI] [PubMed] [Google Scholar]
- 43.Wredenberg A, Wibom R, Wilhelmsson H, Graff C, Wiener HH, Burden SJ, Oldfors A, Westerblad H, Larsson NG. Increased mitochondrial mass in mitochondrial myopathy mice. Proc Natl Acad Sci USA 99: 15066–15071, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Veniant MM. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58: 250–259, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang SJ, Hong HC, Choi HY, Yoo HJ, Cho GJ, Hwang TG, Baik SH, Choi DS, Kim SM, Choi KM. Effects of a three-month combined exercise programme on fibroblast growth factor 21 and fetuin-A levels and arterial stiffness in obese women. Clin Endocrinol (Oxf) 75: 464–469, 2011. [DOI] [PubMed] [Google Scholar]