Abstract
Objective
Microvascular insufficiency represents a major cause of end-organ failure among diabetics. The current studies were undertaken to determine whether dysregulation of the angiopoietins/Tie-2 system would result in an impairment of smooth muscle cell (SMC) recruitment and vascular maturation, which contributes to impaired angiogenesis in diabetes.
Methods and Results
Tie-2 expression was significantly attenuated, whereas angiopoietin-2 (Ang-2) was increased in db/db mice subjected to myocardial ischemia. Our morphological analysis showed that the number of SMC coverage area per neovessel was significantly reduced in db/db mice. This was accompanied by a significant reduction of myocardial capillary density and arteriole formation. Interestingly, Angiopoietin-1(Ang-1)–induced SMC recruitment and vessel outgrowth were severely impaired in db/db mice. Our in vitro studies further demonstrated that exposure of mouse heart endothelial cells to high glucose resulted in a significant upregulation of Ang-2 and a downregulation of Tie-2 expression. These alterations led to a significant impairment of Ang-1–induced Akt and eNOS phosphorylation, along with a remarkable impairment of Ang-1–induced endothelial cell migration and endothelial cell spheroid sprouting. Ang-1 gene transfer restored Tie-2 expression and rescued these abnormalities in diabetes.
Conclusions
Our findings underscore the important role of Ang-1–Tie-2 signaling in the diabetes-induced impairment of vascular maturation and angiogenesis.
Keywords: hyperglycemia, angiopoietins/Tie-2, myocardial ischemia, angiogenesis, type II diabetes
This study was to determine whether dysregulation of the angiopoietins/Tie-2 system would result in an impairment of smooth muscle cell recruitment, vascular maturation, and angiogenesis in diabetes. Our studies demonstrated that disruption of Angiopoietin-1/Tie-2 signaling and vascular maturation is an important contributor to the impaired angiogenesis in diabetes.
Angiogenesis requires coordinated signaling events among a variety of angiogenic factors and their receptors. Tie-2 is endothelial-specific receptor tyrosine kinase. Angiopoietin-1 (Ang-1) and Angiopoietin-2 (Ang-2) are the two key ligands for the Tie-2 receptor. Ang-1 binds to the Tie-2 receptor and induces Tie-2 phosphorylation.1–3 Ang-2 has been identified as a natural antagonist of Ang-1, inhibiting Ang-1–mediated Tie-2 phosphorylation and angiogenesis.4 Angiogenesis is mainly regulated by the interplay between vascular endothelial growth factor (VEGF) and angiopoietins.5,6 VEGF is required to initiate neovessel formation, whereas Ang-1 is required for further maturation of the neovessel by recruitment of smooth muscle cell (SMC).5,6 Disruption of Ang-1–Tie-2 signaling has been shown to impede vascular maturation and disrupt vessel formation in the developing embryo.4 Ang-2 has been identified as a vessel-destabilizing agent that plays a predominant role in controlling vessel regression. In the absence of VEGF, increased Ang-2 leads to endothelial cell apoptosis, immature neovessel destabilization and neovessel regression.7
In patients with diabetes mellitus, coronary collateral vessel formation is significantly impaired during myocardial ischemia.8 The molecular mechanisms underlying the impairment of angiogenesis in diabetes have generated much interest but, so far, have remained largely unidentified. Recent studies have revealed that diabetic abnormal angiogenesis is closely associated with an abnormality of the angiopoietins/Tie-2 system.9 Our previous studies have demonstrated that disturbed angiopoietins/Tie-2 balance contributes to the hyperglycemic exacerbation of myocardial infarction and impairment of myocardial ischemia-induced neovessel formation in type 1 diabetes.10 However, what might be wrong with myocardial ischemia-induced neovessel formation and what is the molecular basis that leads to these abnormalities remain unknown. Ang-1 is required for SMC recruitment and neovessel maturation during myocardial ischemia.11 After an early phase of plasticity associated with robust angiogenic response, SMCs were recruited into the infarct neovessels to form muscular arteries and veins, which improve myocardial blood supply and facilitate myocardial repair.11–15 To date, little is known about the role of the angiopoietins/Tie-2 system on diabetic impaired myocardial angiogenesis and vascular maturation. Whether disruption of Ang-1–Tie-2 signaling occurs in the diabetic heart, which may contribute to the formation of immature vasculature, and thus leading to neovessel regression and reduction of angiogenesis in response to myocardial ischemia, has not yet been determined.
In this study, we hypothesized that diabetes impairs myocardial angiogenesis by a mechanism involving disruption of Ang-1–Tie-2 signaling and vascular maturation. To test our hypotheses, we characterized the expression of the angiopoietins/Tie-2 in vivo using a db/db mouse model subjected to myocardial ischemia, and, in vitro, using mouse heart myocardial endothelial cells (MHMECs) under hyperglycemic conditions. We examined SMC recruitment, neovessel maturation, and the angiogenic response to ischemia or Ang-1 stimulation. Our data indicated that SMC recruitment and myocardial ischemia-induced neovessel maturation were severely impaired in db/db mice. Furthermore, hyperglycemia disrupted Ang-1–Tie-2 signaling and attenuated Ang-1–induced SMC recruitment and angiogenesis.
Materials and Methods
Experimental Diabetic Mouse Myocardial Ischemia Model10,16
C57BLKS/J and db/db mice (12 to 14 weeks of age) were purchased from Jackson Laboratories (Bar Harbor, Me). Myocardial ischemia was achieved by ligation of the left anterior descending coronary artery (LAD). The sham control underwent the surgery without the LAD ligation. All procedures were in conformance with the Institute for Laboratory Animal Research Guide for the Care and Use of Laboratory Animals and were approved by the Vanderbilt University Institutional Animal Care and Use Committee.
Western Blot Analysis
For angiopoietins/Tie-2 and VEGF expression, the membranes were immunoblotted with Ang-1, Ang-2, Tie-2, and VEGF antibodies (1:1000, Santa Cruz). For eNOS and Akt phosphorylation, the membranes were immunoblotted with rabbit antiphospho-Akt and antiphospho-eNOS (1:1000, Cell Signaling). Total eNOS, Akt, and loading control β-Actin were detected using anti-Akt, Anti-eNOS, and anti–β-Actin (1:1000, Cell Signaling Technology) on the same nitrocellulose blots after stripping.
Analysis of Myocardial Capillary and Arteriole Densities10
Sections were incubated with fluorescerin-labeled Isolectin B4 (1:200, Molecular Probe, Invitrogen) and Cy3-conjugated anti–α smooth muscle actin (SMC, 1:100; Sigma). Myocardial capillary and arteriole (SMA-positive coated neovessels) densities were measured using image acquisition and analysis software (Image J, NIH). To assess the acquisition of a muscular coat by infarct neovessels, the density of coated vessels in the infarcted border zone area was stained for SMA. SMC coverage per neovessel in the infarcted border zone area was measured using image analysis software (Image J, NIH).
Mouse Aortic Ring Sprouting Ex Vivo Model16,17
Mouse aortas were isolated from C57BLKS/J and db/db mice. Vessel outgrowth at day 5 was examined using a Nikon TE-300 microscope. The area of vessel outgrowth was quantified using image acquisition and analysis software (Image J, NIH).
Endothelial Cell and Smooth Muscle Cell Immunostaining18
To characterize Ang-1–induced SMC recruitment, specific EC and SMC markers were directly applied to ex vivo aortic culture explants. Briefly, the cultured explants were incubated with specific cell markers: fluorescein isothiocyanate (FITC)-labeled mouse CD31 antibody (1:100, BD Biosciences) for ECs; and Cy3-conjugated anti–α smooth muscle actin (1:100) for SMCs. SMC recruitment was quantified by measuring the relative area of SMC/EC coverage using image acquisition and analysis software (Image J, NIH).
Endothelial Cell Migration and Spheroid Angiogenesis Assays
Endothelial cell migration and endothelial cell spheroid sprouting assays were performed as previously described.16,17
Statistical Analysis
The results are expressed as the mean±SD. Statistical analysis was performed using ANOVA followed by a t test corrected for multiple comparisons (Student–Newman–Keuls). Significance was set at P<0.05.
Results
Dysregulation of the Myocardial Angiopoietins/Tie-2 System in db/db Mice Subjected to Myocardial Ischemia
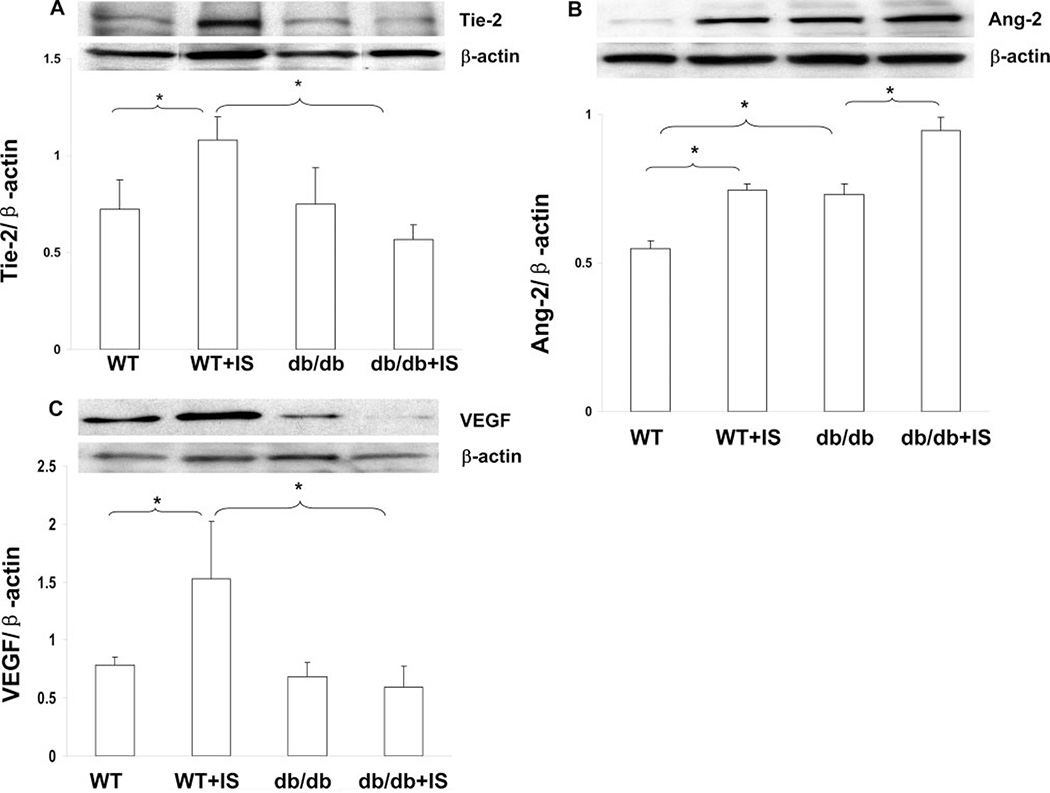
Wild-type (WT) mouse hearts exposed to ischemia for 24 hours showed a significant increase in Tie-2 expression, whereas myocardial ischemia (IS)-induced Tie-2 expression was significantly blunted in db/db mouse hearts (Figure 1A). Relative to WT mice, db/db mice had enhanced Ang-2 expression which was further increased by myocardial ischemia (Figure 1B). Ang-1 expression did not change significantly in either WT or db/db mouse hearts (data not shown). To examine the correlation between angiopoietins/Tie-2 and VEGF in diabetes-induced impairment of angiogenesis, we also examined myocardial VEGF level. Myocardial ischemia –induced VEGF expression was diminished in db/db mouse hearts subjected to ischemia for 24 hours (Figure 1C).
Figure 1.
Representative Western blot and densitometry analysis of myocardial angiopoietins/Tie-2 and VEGF expression after 24 hours myocardial ischemia: Tie-2 was downregulated (A), whereas Ang-2 was upregulated in db/db mice (B). VEGF expression was diminished in db/db mice (C). n = 5 to 10, *P<0.05.
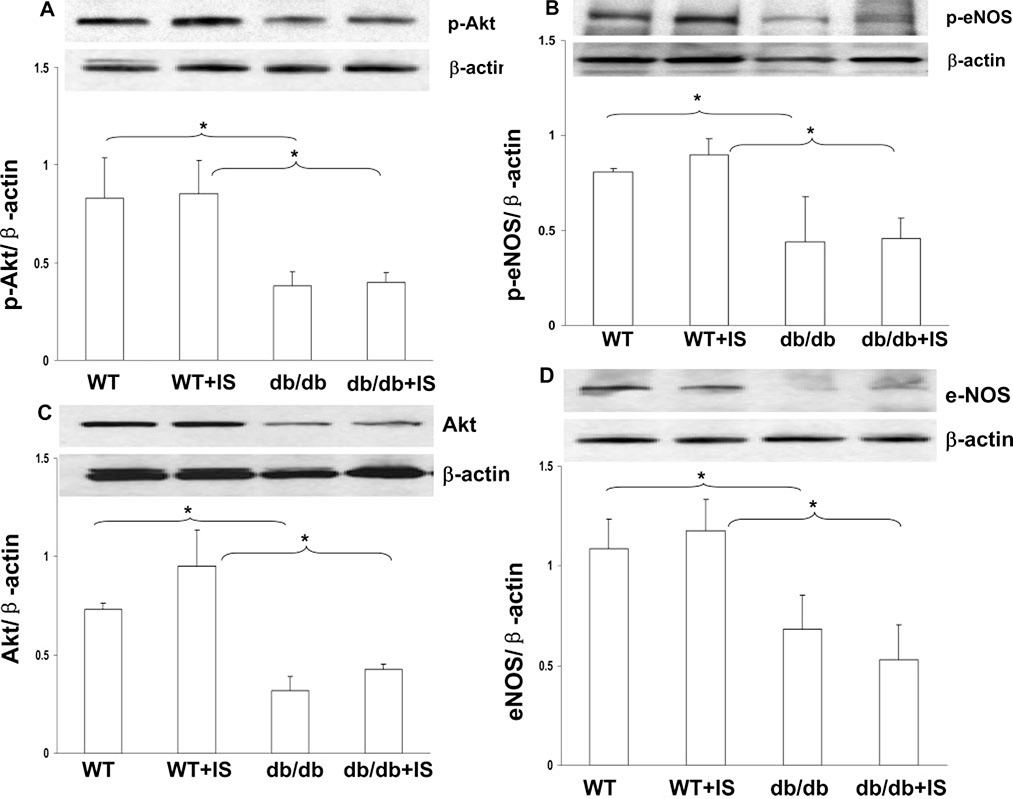
Impaired Akt and eNOS Phosphorylation in db/db Mice
Next, we examined Akt and eNOS phosphorylation in db/db mice. Our Western blot analysis data showed that Akt and eNOS phosphorylation was significantly reduced in the db/db mouse hearts (Figure 2A and 2B). Additionally, total Akt and eNOS expression was also significantly reduced in db/db mice both at basal level and subjected to myocardial ischemia (Figure 2C and 2D).
Figure 2.
Diabetic db/db mice demonstrated decreased Akt (A) and eNOS (B) phosphorylation compared with WT mice. Total Akt (C) and eNOS (D) protein expression was reduced in db/db mouse hearts compared with WT mice at the baseline level and after myocardial ischemia. n = 3 to 4, *P<0.05.
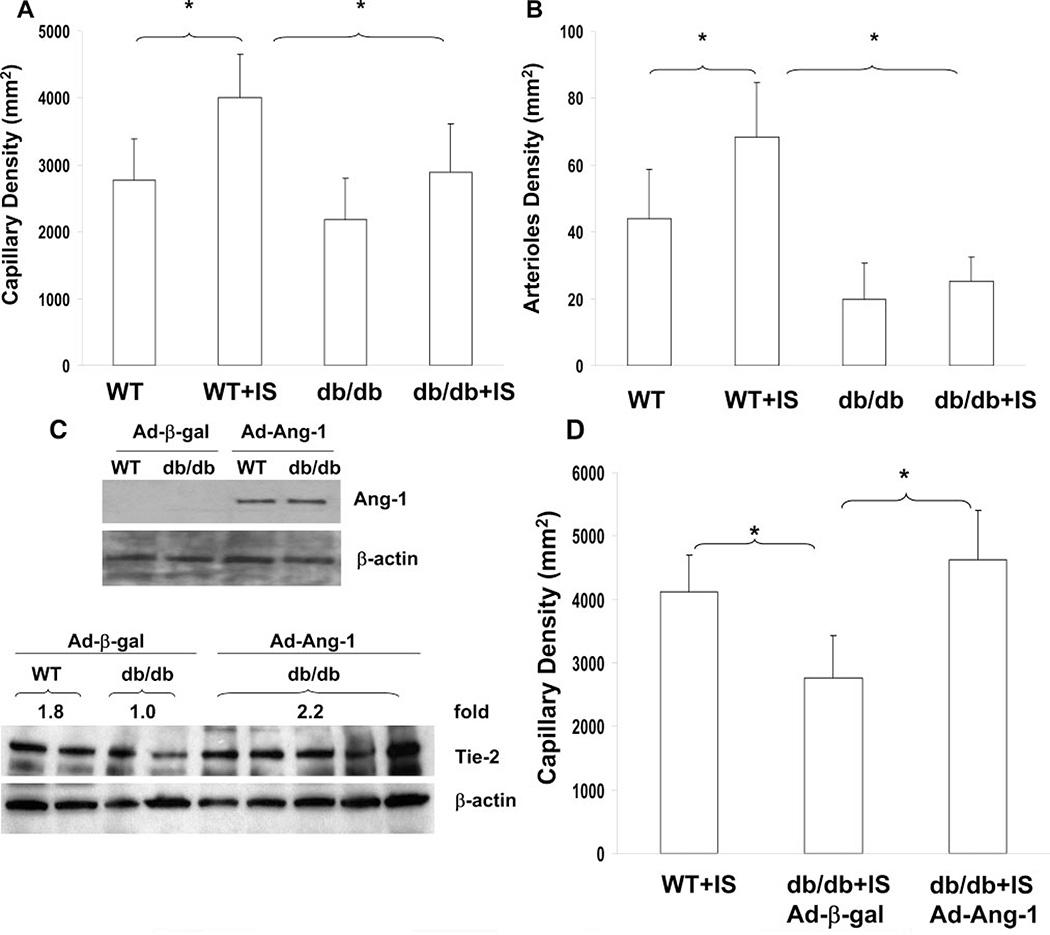
Myocardial Ischemia–Induced Capillary and Arteriole Formation Are Impaired in db/db Mice
WT mouse hearts subjected to ischemia showed a significant increase in myocardial capillary density in the border zone of the infarcted myocardium (Figure 3A). Furthermore, the number of arterioles in the border zone of the infarcted myocardium was also significantly increased (Figure 3B). Myocardial ischemia–induced capillary and arteriole formation in the border and remote area of the infarcted myocardium were significantly less in the hearts of db/db mice compared to WT mice (Figure 3A and 3B; supplemental Figure I and II, available online at http://atvb.ahajournals.org). To further determine whether rescuing impaired Ang-1–Tie-2 signaling can reverse impaired angiogenesis in the diabetic heart, diabetic db/db mice were given an intravenous injection of 1 × 109 PFU Ad-Ang-1. Ang-1 protein expression was significantly increased in the hearts of Ad-Ang-1–treated db/db mice. Ang-1 gene transfer resulted in the restoration of Tie-2 expression in db/db mice (Figure 3C). Furthermore, Ang-1 gene transfer led to a significant improvement in myocardial capillary formation in the db/db mice (Figure 3D).
Figure 3.
A and B, Myocardial ischemia-induced capillary and arteriole formation was significantly reduced in db/db mice. C, Myocardial Ang-1 and Tie-2 expression was increased in Ad-Ang-1–treated db/db mice. D, Systemic administration of Ad-Ang-1 significantly increased capillary density in db/db mice. n = 6 to 8, *P<0.05.
Impairment of Neovessel Maturation in db/db Mice Subjected to Myocardial Ischemia
As shown in Figure 4A, the border zone of the infarcted myocardial area contained a significant number of mature, coated neovessels in WT mice, whereas few coated neovessels were found in db/db mouse hearts after 14 days of ischemia (Figure 4A). Quantitative analysis of SMC coverage further revealed that 62.6% of the neovessels in the border zone of the infarcted myocardial area were stained positive for SMA after 14 days of myocardial ischemia in WT mice. The relative ratio of SMC/neovessel coverage was considerably reduced; only 27.9% of nevessels were covered with SMA in db/db mouse hearts (Figure 4A).
Figure 4.
A, Immunostaining and quantitative analysis of SMC coverage of neovessels in the infarcted myocardial border zone (n = 6 mice). B, CD31/SMC coverage of Ang-1–induced SMC recruitment in aortic ring explants (n = 10 mice), *P<0.05.
Ang-1–Induced SMC Recruitment Is Impaired in db/db Mice
Stimulation of aortic rings isolated from WT mice with Ang-1 (250 ng/mL) led to a significant increase in SMC recruitment (Figure 4B). Ang-1–induced SMC/EC coverage was significantly less in aortic rings isolated from db/db mice than in those from WT mice (Figure 4B). The area of vessel outgrowth in response to Ang-1 stimulation was significantly reduced in the aortic rings isolated from db/db diabetic mice than in those from WT mice (supplemental Figure III).
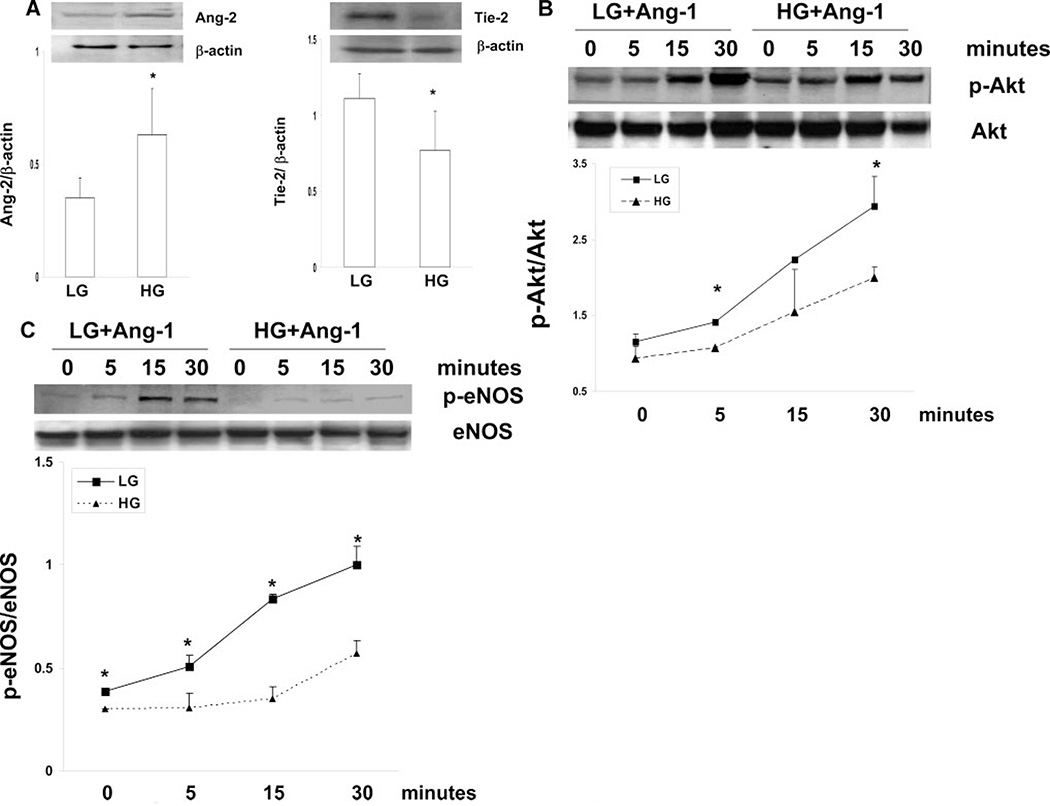
High Glucose Dysregulates Angiopoietins/Tie-2 Expression and Impairs Ang-1–Induced Angiogenesis
Exposure of MHMECs to high glucose (HG; 25 mmol/L) resulted in a significant increase in Ang-2 expression; this was accompanied by a dramatic decrease in Tie-2 expression as compared to low glucose conditions (LG; 5 mmol/L; Figure 5A). Ang-1 expression was undetectable in MHMECs both under LG and HG conditions (data not shown). Stimulation of MHMECs with Ang-1 (250 ng/mL) led to a gradual increase in Akt and eNOS phosphorylation under LG conditions. Ang-1–induced Akt and eNOS phosphorylation were significantly blunted under HG conditions (Figure 5B and 5C). This was accompanied by a significant reduction of MHMEC migration in response to Ang-1 (supplemental Figure IV). Furthermore, Ang-1–induced endothelial cell spheroid sprouts were dramatically suppressed under HG conditions (supplemental Figure IV).
Figure 5.
A, Ang-2 and Tie-2 protein expression under LG or HG conditions in MHMECs (n = 5 to 6). B and C, Ang-1–induced Akt and eNOS phosphorylation under LG or HG conditions in MHMEC (n = 3 to 4), *P<0.05.
To examine whether overexpression of Ang-1 to rescue Ang-1–Tie-2 signaling can restore angiogenesis under HG conditions, MHMECs were first infected with Ad–Ang-1 for 24 hours. The infected cells were then exposed to HG conditions for 72 hours. Overexpression of Ang-1 significantly improved the HG-induced impairment of MHMEC spheroid sprouting (supplemental Figure V).
Discussion
In this study, we have demonstrated that Tie-2 expression was significantly attenuated, whereas Ang-2 expression was increased under hyperglycemia and in db/db mice subjected to myocardial ischemia. Ang-1 plays a critical role in regulating endothelial sprouting and SMC recruitment, whereas Ang-1–induced SMC recruitment and ischemia-induced neovessel maturation were impaired in db/db mice. These were accompanied by a significant reduction of angiogenesis. Restoration of Tie-2 expression by Ang-1 gene transfer reversed the impaired angiogenesis in db/db mouse hearts. Our present study has provided additional mechanistic evidence that hyperglycemia may impair angiogenesis by a mechanism involving disruption of Ang-1–Tie-2 signaling in diabetes.
Diabetic reduced angiogenesis is associated with defective angiogenic growth factor expression as well as the impairment of angiogenic signaling transduction, such as the loss Akt/eNOS signaling.19–21 Tie-2 expression is specifically localized to the vascular endothelium and is upregulated by hypoxia and ischemia.22–26 Reduction of Tie-2 expression has been found in the experimental pulmonary hypertension model. Furthermore, restoration of Tie-2 expression by Ang-1 gene transfer protects the pulmonary microvasculature and attenuates pulmonary hypertension progression.27 Our data for the first time have demonstrated that myocardial ischemia–induced Tie-2 expression was significantly blunted in db/db mice. Exposure of MHMECs to high glucose resulted in a significant reduction of Tie-2 expression. This was accompanied by a significant impairment of Ang-1–induced Akt/eNOS phosphorylation and angiogenesis. These results led us to speculate that reduction of Tie-2 expression and disruption of Ang-1–Tie-2 signaling under hyperglycemic conditions may be the underlying cause of impaired angiogenesis in diabetics. This notion was further substantiated by our data which revealed that overexpression of Ang-1 restored Tie-2 expression and significantly improved angiogenesis both in vitro and in vivo myocardial angiogenesis models.
Sustained elevation of Ang-2 expression has been shown to disrupt the formation of capillary-like structures and impaired angiogenesis.28,21 Hindlimb ischemia–induced collateral artery growth and angiogenesis were significantly impaired in the transgenic overexpression of Ang-2 mice.29 Furthermore, chronic systemic delivery of Ang-2 resulted in a dramatic reduction of myocardial vasculature.30 Recent clinical studies revealed that the plasma Ang-2 level was increased in patients with diabetes.31–34 Intriguingly, administration of Ang-2 into the eyes of normal rats led to a dose-dependent pericyte loss whereas heterozygous Ang-2 deficiency completely prevented diabetes-induced pericyte loss.35 Exposure of mesangial cells to high glucose dramatically increased Ang-2 expression and significantly inhibited capillary tubule formation.9 Consistent with these findings, our data showed that Ang-2 expression was upregulated in MHMECs under hyperglycemic conditions and in diabetic db/db mice. Furthermore, Ang-1–induced endothelial cell spheroid sprouting was significantly decreased in MHMECs in the presence of exogenous Ang-2 (unpublished data). As such, we reasoned that elevation of Ang-2 might be competition with Ang-1 and negatively interfere with Ang-1–Tie-2 signaling and impair angiogenesis in diabetes. Our data showed that Ang-1 gene transfer rescued the hyperglycemia-induced impairment of endothelial sprouting, further suggesting the possibility that the overexpression of Ang-1 may overcome endogenous blocking by Ang-2, thereby restoring angiogenesis in diabetes.
Neovessel maturation is critical for the stabilization and repairing of the myocardial infarcted area. After an early phase of plasticity associated with robust angiogenic response, SMCs were recruited into the neovessels to form muscular arteries and veins, which improve myocardial blood supply and facilitate myocardial repair.12–15,36 Recruitment of SMCs in the myocardial infarcted area is also important for the prevention of neovessel from regression.12–15,36 VEGF is required to initiate neovessel formation, whereas Ang-1–Tie-2 signaling is required for SMC recruitment and neovessel maturation.5,6 Importantly, Ang-1–Tie-2 signaling is crucial in their development as stable and leak-resistant neovessels during myocardial ischemia. Data from our laboratory and other investigators revealed that Ang-1 gene transfer improved neovessel density in acute myocardial infarction rat11 and STZ hyperglycemic mice.10 Furthermore, Ang-1 gene transfer stabilized the angiogenic response for longer duration, and led to the formation of larger number of matured vascular structures.11 In light of these findings, we have examined SMC recruitment and neovessel maturation in diabetic d/db mouse using ex vivo and in vivo models. Our data clearly demonstrated that Ang-1–induced SMC recruitment and myocardial ischemia-induced neovessels maturation in the border zone of infarcted area were severely impaired in db/db mice, suggesting that reduction of SMC coverage and formation of immature neovessels in the border zone of infarcted area might be another possible mechanistic explanation for the impairment of myocardial angiogenesis in diabetes.
There is a notable dearth of knowledge about the impairment of VEGF expression, which has been linked to insufficient angiogenesis in diabetic wound healing.37–39 Our present studies have extended these findings, demonstrating that myocardial ischemia-induced VEGF expression is also impaired in db/db mice. In contrast to these results, recent studies also revealed that the expression of VEGF remained unchanged in the myocardium of diabetic patients. However, VEGFR phosphorylation and its downstream Akt and eNOS phosphorylation were severely impaired.40 Furthermore, in diet-induced diabetic mice, plasma VEGF level was increased whereas VEGFR, Akt, and eNOS phosphorylation were decreased.41 Taken together, these findings suggest that impaired angiogenesis in diabetes is not only attributable to the reduction of the VEGF level, but is also involved in the impairment of VEGF/VEGFR signaling transduction. Our present data demonstrated that VEGF expression and Ang-1–Tie-2 signaling were significantly blunted after coronary occlusion in db/db mouse hearts, further supporting the notion that deficits in postischemic angiogenesis in diabetes were associated with the dysregulation of a complex angiogenesis-regulatory factors and its signaling.41,42
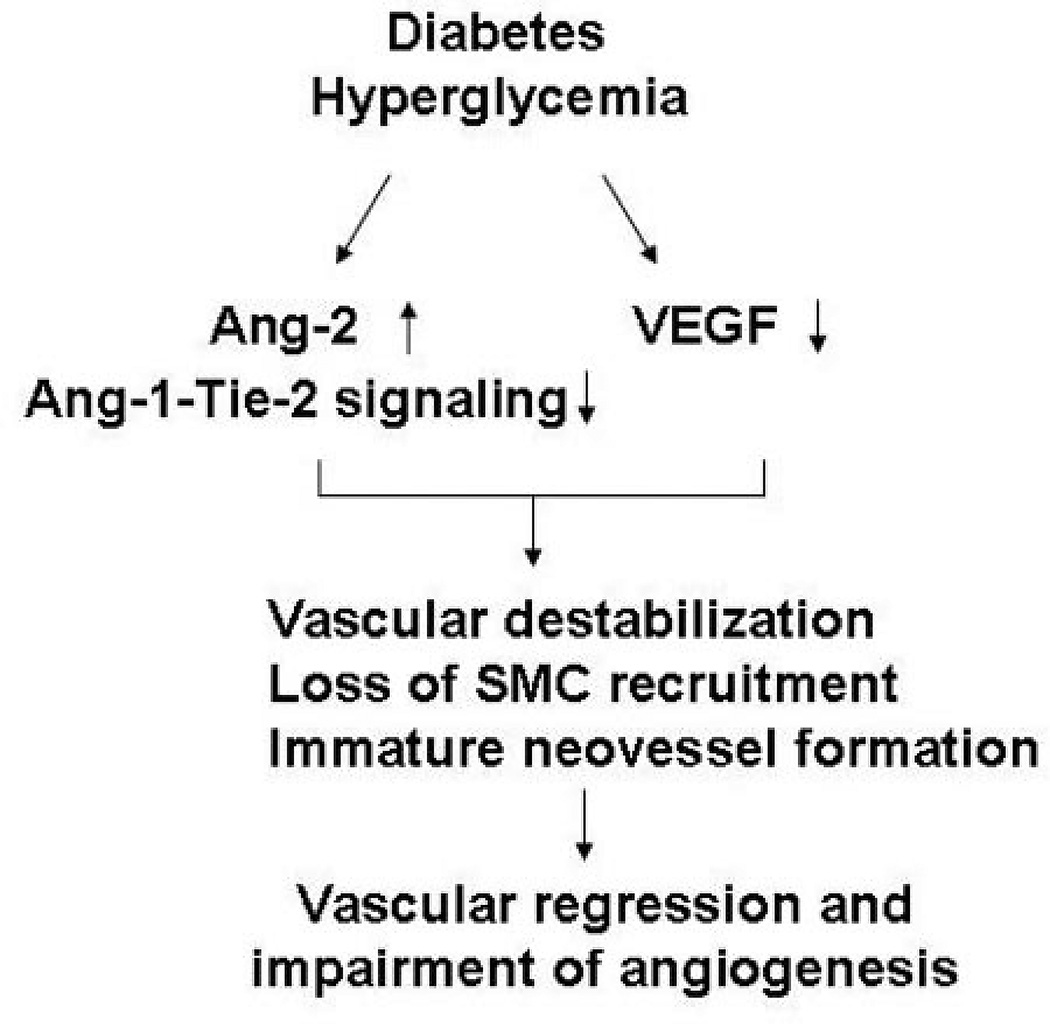
In summary, our present data demonstrate that, in the absence of VEGF, hyperglycemia disrupts the angiopoietins/Tie-2 balance in favor of Ang-2, and blunts Ang-1–Tie-2 angiogenic signaling, thus leading to the impairment of SMC recruitment, immature neovessel formation, and reduced angiogenesis (Figure 6). Given that disruption of Ang-1–Tie-2 signaling and vascular maturation is an important contributor to the impairment of angiogenesis in diabetes, therefore, rescue of immature vasculature should be considered as a novel therapeutic strategy in treatment of diabetic impaired myocardial collateral and angiogenesis complications.
Figure 6.
Current working hypothesis of intraceullar mechanisms by which hyperglycemia causes impairement of angiogenesis in diabetes. Hyperglycemia disrupts the angiopoietins/Tie-2 balance in favor of Ang-2 and attenuates Ang-1–Tie-2 signaling, thus leading to loss of SMC recruitment, immature neovessel formation, and neovessel regression in the deficiency of VEGF.
Supplementary Material
Acknowledgments
Sources of Funding This work was supported by grants from the American Heart Association grant 0565196B and NIH grant DK074995 to J.X. Chen.
Footnotes
Disclosures
None.
References
- 1.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 2.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 3.Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282:468–471. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- 4.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 5.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 6.Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18:5356–5362. doi: 10.1038/sj.onc.1203035. [DOI] [PubMed] [Google Scholar]
- 7.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abaci A, Oguzhan A, Kahraman S, Eryol NK, Unal S, Arinc H, Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–2242. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 9.Singh AK, Gudehithlu KP, Pegoraro AA, Singh GK, Basheerudin K, Robey RB, Arruda JA, Dunea G. Vascular factors altered in glucose-treated mesangial cells and diabetic glomeruli. Changes in vascular factors impair endothelial cell growth and matrix. Lab Invest. 2004;84:597–606. doi: 10.1038/labinvest.3700082. [DOI] [PubMed] [Google Scholar]
- 10.Tuo QH, Zeng H, Stinnett A, Yu H, Aschner JL, Liao DF, Chen JX. Critical role of angiopoietins/Tie-2 in hyperglycemic exacerbation of myocardial infarction and impaired angiogenesis. Am J Physiol Heart Circ Physiol. doi: 10.1152/ajpheart.01250.2007. In press. [DOI] [PubMed] [Google Scholar]
- 11.Shujia J, Haider HK, Idris NM, Lu G, Ashraf M. Stable therapeutic effects of mesenchymal stem cell-based multiple gene delivery for cardiac repair. Cardiovasc Res. 2008;77:525–533. doi: 10.1093/cvr/cvm077. [DOI] [PubMed] [Google Scholar]
- 12.Dobaczewski M, Akrivakis S, Nasser K, Michael LH, Entman ML, Frangogiannis NG. Vascular mural cells in healing canine myocardial infarcts. J Histochem Cytochem. 2004;52:1019–1029. doi: 10.1369/jhc.3A6210.2004. [DOI] [PubMed] [Google Scholar]
- 13.Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid Redox Signal. 2006;8:1907–1939. doi: 10.1089/ars.2006.8.1907. [DOI] [PubMed] [Google Scholar]
- 14.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164:665–677. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zymek P, Bujak M, Chatila K, Cieslak A, Thakker G, Entman ML, Frangogiannis NG. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J Am Coll Cardiol. 2006;48:2315–2323. doi: 10.1016/j.jacc.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 16.Chen JX, Zeng H, Tuo QH, Yu H, Meyrick B, Aschner JL. NADPH oxidase modulates myocardial Akt, ERK1/2 activation and angiogenesis after hypoxia/reoxygenation. Am J Physiol Heart Circ Physiol. 2007;292:H1664–H1674. doi: 10.1152/ajpheart.01138.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen JX, Zeng H, Lawrence ML, Blackwell TS, Meyrick B. Angiopoietin-1-induced angiogenesis is modulated by endothelial NADPH oxidase. Am J Physiol Heart Circ Physiol. 2006;291:H1563–H1572. doi: 10.1152/ajpheart.01081.2005. [DOI] [PubMed] [Google Scholar]
- 18.Zhu WH, Han J, Nicosia RF. Requisite role of p38 MAPK in mural cell recruitment during angiogenesis in the rat aorta model. J Vasc Res. 2003;40:140–148. doi: 10.1159/000070711. [DOI] [PubMed] [Google Scholar]
- 19.Waltenberger J. Impaired collateral vessel development in diabetes: potential cellular mechanisms and therapeutic implications. Cardiovasc Res. 2001;49:554–560. doi: 10.1016/s0008-6363(00)00228-5. [DOI] [PubMed] [Google Scholar]
- 20.Marfella R, Esposito K, Nappo F, Siniscalchi M, Sasso FC, Portoghese M, Di Marino MP, Baldi A, Cuzzocrea S, Di FC, Barboso G, Baldi F, Rossi F, D’Amico M, Giugliano D. Expression of angiogenic factors during acute coronary syndromes in human type 2 diabetes. Diabetes. 2004;53:2383–2391. doi: 10.2337/diabetes.53.9.2383. [DOI] [PubMed] [Google Scholar]
- 21.Larger E, Marre M, Corvol P, Gasc JM. Hyperglycemia-induced defects in angiogenesis in the chicken chorioallantoic membrane model. Diabetes. 2004;53:752–761. doi: 10.2337/diabetes.53.3.752. [DOI] [PubMed] [Google Scholar]
- 22.Oh H, Takagi H, Suzuma K, Otani A, Matsumura M, Honda Y. Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J Biol Chem. 1999;274:15732–15739. doi: 10.1074/jbc.274.22.15732. [DOI] [PubMed] [Google Scholar]
- 23.Lin TN, Wang CK, Cheung WM, Hsu CY. Induction of angiopoietin and Tie receptor mRNA expression after cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2000;20:387–395. doi: 10.1097/00004647-200002000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Ray PS, Estrada-Hernandez T, Sasaki H, Zhu L, Maulik N. Early effects of hypoxia/reoxygenation on VEGF, ang-1, ang-2 and their receptors in the rat myocardium: implications for myocardial angiogenesis. Mol Cell Biochem. 2000;213:145–153. doi: 10.1023/a:1007180518474. [DOI] [PubMed] [Google Scholar]
- 25.Shyu KG, Chang CC, Wang BW, Kuan P, Chang H. Increased expression of angiopoietin-2 and Tie2 receptor in a rat model of myocardial ischaemia/ reperfusion. Clin Sci (Lond) 2003;105:287–294. doi: 10.1042/CS20030025. [DOI] [PubMed] [Google Scholar]
- 26.Ray PS, Sasaki H, Estrada-Hernandez T, Zu L, Maulik N. Effects of hypoxia/reoxygenation on angiogenic factors and their tyrosine kinase receptors in the rat myocardium. Antioxid Redox Signal. 2001;3:89–102. doi: 10.1089/152308601750100560. [DOI] [PubMed] [Google Scholar]
- 27.Zhao YD, Campbell AI, Robb M, Ng D, Stewart DJ. Protective role of angiopoietin-1 in experimental pulmonary hypertension. Circ Res. 2003;92:984–991. doi: 10.1161/01.RES.0000070587.79937.F0. [DOI] [PubMed] [Google Scholar]
- 28.Lee OH, Fueyo J, Xu J, Yung WK, Lemoine MG, Lang FF, Bekele BN, Zhou X, Alonso MA, Aldape KD, Fuller GN, Gomez-Manzano C. Sustained angiopoietin-2 expression disrupts vessel formation and inhibits glioma growth. Neoplasia. 2006;8:419–428. doi: 10.1593/neo.06109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reiss Y, Droste J, Heil M, Tribulova S, Schmidt MH, Schaper W, Dumont DJ, Plate KH. Angiopoietin-2 impairs revascularization after limb ischemia. Circ Res. 2007;101:88–96. doi: 10.1161/CIRCRESAHA.106.143594. [DOI] [PubMed] [Google Scholar]
- 30.Bureau W, Van SP, Jones J, Han RN, Ward NL, Stewart DJ, Dumont DJ. Chronic systemic delivery of angiopoietin-2 reveals a possible independent angiogenic effect. Am J Physiol Heart Circ Physiol. 2006;291:H948–H956. doi: 10.1152/ajpheart.00734.2005. [DOI] [PubMed] [Google Scholar]
- 31.Lim HS, Lip GY, Blann AD. Angiopoietin-1 and angiopoietin-2 in diabetes mellitus: relationship to VEGF, glycaemic control, endothelial damage/dysfunction and atherosclerosis. Atherosclerosis. 2005;180:113–118. doi: 10.1016/j.atherosclerosis.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Lim HS, Blann AD, Chong AY, Freestone B, Lip GY. Plasma vascular endothelial growth factor, angiopoietin-1, and angiopoietin-2 in diabetes: implications for cardiovascular risk and effects of multifactorial intervention. Diabetes Care. 2004;27:2918–2924. doi: 10.2337/diacare.27.12.2918. [DOI] [PubMed] [Google Scholar]
- 33.Chung NA, Makin AJ, Lip GY. Measurement of the soluble angiopoietin receptor tie-2 in patients with coronary artery disease: development and application of an immunoassay. Eur J Clin Invest. 2003;33:529–535. doi: 10.1046/j.1365-2362.2003.01173.x. [DOI] [PubMed] [Google Scholar]
- 34.Lee KW, Lip GY, Blann AD. Plasma angiopoietin-1, angiopoietin-2, angiopoietin receptor tie-2, and vascular endothelial growth factor levels in acute coronary syndromes. Circulation. 2004;110:2355–2360. doi: 10.1161/01.CIR.0000138112.90641.7F. [DOI] [PubMed] [Google Scholar]
- 35.Hammes HP, Lin J, Wagner P, Feng Y, Vom HF, Krzizok T, Renner O, Breier G, Brownlee M, Deutsch U. Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes. 2004;53:1104–1110. doi: 10.2337/diabetes.53.4.1104. [DOI] [PubMed] [Google Scholar]
- 36.Ren G, Michael LH, Entman ML, Frangogiannis NG. Morphological characteristics of the microvasculature in healing myocardial infarcts. J Histochem Cytochem. 2002;50:71–79. doi: 10.1177/002215540205000108. [DOI] [PubMed] [Google Scholar]
- 37.Chou E, Suzuma I, Way KJ, Opland D, Clermont AC, Naruse K, Suzuma K, Bowling NL, Vlahos CJ, Aiello LP, King GL. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic States: a possible explanation for impaired collateral formation in cardiac tissue. Circulation. 2002;105:373–379. doi: 10.1161/hc0302.102143. [DOI] [PubMed] [Google Scholar]
- 38.Frank S, Hubner G, Breier G, Longaker MT, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem. 1995;270:12607–12613. doi: 10.1074/jbc.270.21.12607. [DOI] [PubMed] [Google Scholar]
- 39.Altavilla D, Saitta A, Cucinotta D, Galeano M, Deodato B, Colonna M, Torre V, Russo G, Sardella A, Urna G, Campo GM, Cavallari V, Squadrito G, Squadrito F. Inhibition of lipid peroxidation restores impaired vascular endothelial growth factor expression and stimulates wound healing and angiogenesis in the genetically diabetic mouse. Diabetes. 2001;50:667–674. doi: 10.2337/diabetes.50.3.667. [DOI] [PubMed] [Google Scholar]
- 40.Sasso FC, Torella D, Carbonara O, Ellison GM, Torella M, Scardone M, Marra C, Nasti R, Marfella R, Cozzolino D, Indolfi C, Cotrufo M, Torella R, Salvatore T. Increased vascular endothelial growth factor expression but impaired vascular endothelial growth factor receptor signaling in the myocardium of type 2 diabetic patients with chronic coronary heart disease. J Am Coll Cardiol. 2005;46:827–834. doi: 10.1016/j.jacc.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Hazarika S, Dokun AO, Li Y, Popel AS, Kontos CD, Annex BH. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res. 2007;101:948–956. doi: 10.1161/CIRCRESAHA.107.160630. [DOI] [PubMed] [Google Scholar]
- 42.Schiekofer S, Galasso G, Sato K, Kraus BJ, Walsh K. Impaired revascularization in a mouse model of type 2 diabetes is associated with dysregulation of a complex angiogenic-regulatory network. Arterioscler Thromb Vasc Biol. 2005;25:1603–1609. doi: 10.1161/01.ATV.0000171994.89106.ca. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.