Abstract
Interpretation of the pathologic margin of a specimen from a resected tumor is important because local recurrence can be predicted by the presence of tumor cells in the resection margin. Although a sufficient resection margin is recommended in the resection of gastrointestinal adenocarcinoma, it is not usually regarded strictly in cases of mesenchymal tumor, especially gastrointestinal stromal tumor (GIST), because the tumor is usually encapsulated or well demarcated, and not infiltrative. Therefore, margin positivity is not rare in the pathological evaluation of surgically or endoscopically resected GIST, and does not always indicate incomplete resection. Although a GIST may have a tumor-positive pathologic margin, complete resection may be achieved if no residual tumor is visible, and long-term survival can be predicted as in the cases with a negative pathologic margin.
Keywords: Gastrointestinal stromal tumors, Margin, Endoscopic resection
INTRODUCTION
A sufficient resection margin is recommended for complete resection of malignant tumors and reduction of the risk of residual tumor or recurrence. Especially in patients with adenocarcinoma in the gastrointestinal (GI) tract, survival can be affected by tumor recurrence from a microscopic residual tumor in the resection margin. Therefore, surgical resection of adenocarcinomas in the GI tract is recommended to close the abdomen after confirmation of the tumor-negative pathologic margin in frozen sections.
On the contrary, the interpretation of the pathologic margin in GI sarcomas such as gastrointestinal stromal tumor (GIST) is controversial. Unlike adenocarcinomas, GI sarcomas could show no significant difference in tumor recurrence or survival between the group with a negative pathologic margin and with a positive pathologic margin even though no residual tumor is visible. Little is known about R1 resection of GISTs from a retrospective institutional analysis with a small sample. Herein, the treatment strategy for GIST will be discussed with the interpretation of pathologic margins.
TREATMENT STRATEGY FOR GIST ACCORDING TO PATHOLOGIC MARGIN
Although a tumor-negative pathologic margin is commonly assumed to mean curative endoscopic or surgical resection of the malignant tumor, well-designed prospective randomized data to support this assumption are lacking. However, patients with residual sarcoma due to incomplete resection (R2) show poor clinical outcomes when compared with patients with R0 resection or even a microscopically positive resection margin (R1 resection) [1].
A microscopic positive margin has been reported to possibly have no influence on the disease-free survival of patients or even tumor recurrence in GIST [2]. In general, limited resection is recommended for achieving results comparable with those of extended resection. Surgical resection should include a margin of at least 1 cm of normal tissue and an intraoperative evaluation of frozen section [3]. In endoscopic resection, a sufficient vertical resection margin cannot be achieved for GISTs, which are located in the muscularis propria layer. As endoscopic submucosal dissection can only dissect the layer beneath the GIST, the resection margin may still involve by tumor cells irrespective of the completeness of the resection. If endoscopic full-thickness resection is performed, R0 resection can be achieved as well as surgical resection. A positive resection margin has been reported as ineffective for predicting the recurrence of small GISTs [4].
In a previous study that evaluated the risk of recurrence in R0 and R1 resection cases, recurrence-free survival was not significantly different between the two groups [5]. R1 surgical resection was associated with large tumor size and tumor rupture, and the risk of recurrence was associated with tumor rupture.
As the interpretation of pathologic margin is influenced by tumor contraction and fixation after resection, it can be affected by various factors during the process of tissue preparation. Therefore, R1 resection does not always mean incomplete resection because a false-positive margin may be assessed based on tissue contraction, encapsulation, or tumor rupture.
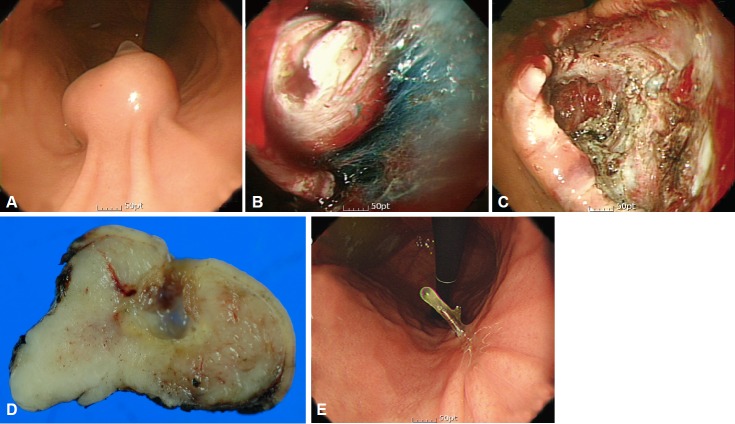
R1 resection is more frequent in endoscopic resection than in surgical resection because a sufficient resection margin is difficult to attain in endoscopic resection. In a recent study that compared endoscopic and surgical resections of GIST, R1 resection was more frequent in the endoscopic resection group than in the surgical resection group [6]. However, the recurrence rate with R1 resection in the endoscopic group was low (2.9%, 2/69), but not significantly different from that in surgical resection. Although the sample size of endoscopic resection cases was not sufficient to demonstrate the safety of R1 resection, it may be proposed that close serial follow-up can be useful if endoscopic resection is performed without evidence of residual tumor in spite of a microscopically tumor-positive resection margin (Fig. 1). These findings are consistent with those of a previous study that concluded that tumor size and not a microscopically tumor-positive margin was associated with disease-specific survival [7]. Therefore, serial endoscopic follow-up is recommended for GIST cases with R1 endoscopic resection if no residual tumor is evident immediately after endoscopic resection. As residual or recurrent tumor is possible, close follow-up is mandatory for patients who undergo R1 resection.
Fig. 1.
Endoscopic resection of a gastrointestinal stromal tumor (GIST). (A) Endoscopic image of a GIST. (B) Submucosal dissection of a GIST. (C) Iatrogenic ulcer immediately after endoscopic resection. (D) Fixation of resected specimen. (E) Follow-up after endoscopic resection.
CONCLUSIONS
Endoscopic complete resection may be achieved for GISTs, unlike adenocarcinoma, even in cases with tumor-positive pathologic margins. However, close follow-up should be maintained for the detection of local recurrence.
Footnotes
Conflicts of Interest: The author has no financial conflicts of interest.
REFERENCES
- 1.Gouveia AM, Pimenta AP, Capelinha AF, de la Cruz D, Silva P, Lopes JM. Surgical margin status and prognosis of gastrointestinal stromal tumor. World J Surg. 2008;32:2375–2382. doi: 10.1007/s00268-008-9704-8. [DOI] [PubMed] [Google Scholar]
- 2.Ng EH, Pollock RE, Munsell MF, Atkinson EN, Romsdahl MM. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg. 1992;215:68–77. doi: 10.1097/00000658-199201000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol. 2002;33:466–477. doi: 10.1053/hupa.2002.124122. [DOI] [PubMed] [Google Scholar]
- 4.Kim MY, Park YS, Choi KD, et al. Predictors of recurrence after resection of small gastric gastrointestinal stromal tumors of 5 cm or less. J Clin Gastroenterol. 2012;46:130–137. doi: 10.1097/MCG.0b013e31821f8bf6. [DOI] [PubMed] [Google Scholar]
- 5.McCarter MD, Antonescu CR, Ballman KV, et al. Microscopically positive margins for primary gastrointestinal stromal tumors: analysis of risk factors and tumor recurrence. J Am Coll Surg. 2012;215:53–59. doi: 10.1016/j.jamcollsurg.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joo MK, Park JJ, Kim H, et al. Endoscopic versus surgical resection of GI stromal tumors in the upper GI tract. Gastrointest Endosc. 2016;83:318–326. doi: 10.1016/j.gie.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 7.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]