Abstract
The prevalence of cardiovascular implantable electronic devices as life-prolonging and life-saving devices has evolved from a treatment of last resort to a first-line therapy for an increasing number of patients. As these devices become more and more popular in the general population, dental providers utilizing instruments and medications should be aware of dental equipment and medications that may affect these devices and understand the management of patients with these devices. This review article will discuss the various types and indications for pacemakers and implantable cardioverter-defibrillators, common drugs and instruments affecting these devices, and management of patients with these devices implanted for cardiac dysrhythmias.
Key Words: CIED, Pacemakers, ICD, Tachyarrhythmias, Bradycardia, Sensing, Ultrasonic, Radiographs, Electrosurgery, Cauterization, Apex locator, Electromagnetic interference, Defibrillation, Electrocautery
Recent statistics point to an increasing number of patients in North America with cardiovascular implantable electronic devices (CIEDs), which include implantable cardiac pacemakers, implanted cardioverter-defibrillators (ICDs), cardiac resynchronization devices, and implantable cardiac monitors. In 2012, it was estimated that at least 3 million patients have these devices implanted and more than 250,000 new devices are implanted each year.1
Of major concern to the dentist practitioner is the possibility of electromagnetic interference (EMI) and electromagnetic disturbance from electrosurgery/electrocautery devices, apex locators, lasers, electric handpieces, radiation, and other electronic sources. Additionally, vasoactive drugs, such as epinephrine-containing local anesthetics and other sympathomimetics that may be administered during anesthetic management, may have significant effects upon patients who suffer from tachyarrhythmias. Several guidelines have already been promulgated for surgeons and anesthesia providers in the medical field, and parallel treatment decisions can also be considered for dental/oral surgeons after substantive review of the literature.
With the increasing prevalence of such patients seeking dental, oral, and maxillofacial procedures, an in-depth review of the available evidence and current guidelines may assist the dental practitioner in delivering optimal and safe care to patients presenting with various CIEDs.
PACEMAKERS
Indications and Function of Pacemakers
Pacemakers are generally indicated for patients suffering from symptomatic bradycardias to help initiate cardiac depolarization when native pacemakers are not providing for an adequate number of cardiac contractions. Pacers are implanted in patients with sick sinus syndrome, tachycardia-bradycardia syndrome, atrial fibrillation with sinus node dysfunction, third-degree atrioventricular block, chronotropic incompetence, prolonged QT syndrome, and cardiac resynchronization therapy with biventricular pacing (Figure 1).
Figure 1. .
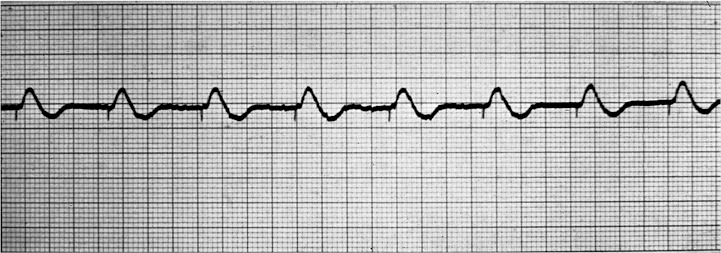
Paced rhythm with pacer “spike.”
A universally adopted classification system developed by the North American Society of Pacing and Electrophysiology (NASPE) Group and the British Pacing and Electrophysiology (BPE) Group use a 5-position letter arrangement to describe pacemaker function. This NASPE/BPE Generic (NBG) code is described in the Table. The first 3 positions describe (a) the chamber location paced, (b) the sensing location of the leads, and (c) the response to those lead inputs. The fourth position describes the programmability of the unit to respond to the sensed rate, and the last position describes the pacer's response to controlling tachycardias. It is important to note that the fifth position describes not only pacing, but also the delivery of shocks for life-threatening tachyarrhythmias.2
Pacemaker Terminology: North American Society of Pacing and the British Pacing and Electrophysiology Group*

Pacemaker Terminology
As depicted in the Table, a patient with complete third-degree block with normal SA function may have a “VAT” pacemaker implanted to restore normal, synchronous atrioventricular function such that a ventricular depolarization follows an atrial depolarization. A pacer programmed in VAT mode will sense an atrial (A) beat and thus trigger (T) a ventricular (V) depolarization. DDD programming is described as the most sophisticated, yet it is the most commonly employed mode in implantable pacers. Both atria and ventricles are sensed, triggered, or inhibited and the pacer becomes the primary method of regulating cardiac electrical function. The rather recent simplification of the fourth and fifth positions now gives the CIED team information only on the presence of rate modulation and location, if any, of multisite (more than 1 lead in a single chamber) pacing.
Pacemaker devices are comprised of 2 main parts: the main pulse generator and the insulated wire cardiac leads. Various configurations exist from single lead to dual leads, and combinations of paced cardiac chambers vary. Generally, in a single-lead pacemaker, either the ventricle or atrium can be paced. Dual-chamber pacers stimulate depolarization in the atrium and a single ventricle. When signals to 2 ventricles (biventricular pacers) are sent, these devices are termed multiple-chamber pacers. Biventricular pacing is also referred to as cardiac resynchronization therapy. In many cases, the right atrium and/or ventricle is paced and leads can both inhibit and initiate chamber depolarization to establish atrioventricular synchrony.
To externally control the function of pacemakers, most units employ a magnetic reed valve that is controlled by an externally placed magnet. Generally, these devices are implanted in the region of the left pectoralis muscle, either subpectorally or subcutaneously. Once the pacemaker is “closed” by placing a magnet in close proximity to the pulse generator, it begins to pace asynchronously despite the patient's underlying cardiac rhythm. To evaluate the function of a pacemaker, evaluation of a continuous electrocardiogram (ECG) is generally advised in consultation with a cardiologist and electrophysiologist. Additionally, many pacemakers have the capability to be “interrogated” as to their function and frequency of events with proprietary software specific to the manufacturer of the device.
Generally, the lithium-ion batteries in contemporary pacemakers have a working life of approximately 10 years. Function and sensing abilities may begin to degrade over a gradual period of time rather than there being an abrupt cessation of pacing. However, EMI coupled with low battery states can lead to problems in both sensing and pacing modes.3 Classically, to circumvent the rhythm disturbances that electrocautery or electrosurgery may induce in these devices, practitioners will often place a magnet near the pacemaker to enable asynchronous pacing (pacing without sensing the intrinsic rhythm of the patient). The resultant asynchronous rate varies from 85 to 100 bpm, and more importantly, the antitachycardia sensing mode of the unit can also be disabled, leaving the patient susceptible to harmful tachyarrhythmias.3 In one study, problems were encountered in restoring the magnet-activated asynchronous pacer back to original programming.4
Oversensing, or the inhibition of implanted pacemaker activity by signals not normally detected, is the most common problem associated with pacemaker malfunction. The inhibition of the pacer signal generally leads to a “failure to pace” condition that often results in symptomatic bradycardias. Vigorous movement in the pectoralis and rectus abdominis muscle groups often lead to oversensing problems and usual treatment consists of reverting back to an asynchronous mode with magnet activation.5
Conversely, pacemaker-induced tachycardia, or endless-loop and pacemaker-reentrant tachycardia, can be triggered by a premature atrial or ventricular contraction in dual-chamber (atrium and ventricle) sensing pacemakers. Retrograde conduction into the atria allows for continued ventricular contraction. Tachycardia can ensue in a manner much like circus reentry rhythms seen in conditions such as Wolff-Parkinson-White syndrome. The use of cardiostimulatory drugs—eg, epinephrine, atropine, or isoproterenol—can also trigger pacemaker-induced tachycardia. Safeguards against runaway rate are built into many pacers with upper-limit rate programming. The traditional antitachyarrhythmic agent, adenosine, can also be considered to terminate endless-loop conditions, as well as returning to an asynchronous pacing condition with a magnet placed over the pacemaker.6
Myocardial infarction occurring in patients with a pacemaker can be difficult to detect because of the abnormal morphology already present on ECG tracings. The typical pacemaker-implanted patient presenting with acute myocardial infarction would be an older male with diabetes mellitus, previous myocardial infarction, and/or congestive heart failure. Similar to patients presenting with preexisting left bundle branch block, pacemaker-influenced rhythms may obscure ST-segment elevation myocardial infarction (Figure 2).7
Figure 2. .

Medtronic MRI-compitable pacemaker.
IMPLANTABLE CARDIOVERTER-DEFIBRILLATORS
ICDs are generally indicated for patients with unstable or potentially fatal tachyarrhythmias. Similar to external defibrillators, ICDs have the ability to continuously monitor and analyze heart rhythms for ventricular fibrillation (VF) or ventricular tachycardia (VT) and deliver a shock intrathoracically. Contemporary ICDs not only provide defibrillation (up to 30–39 J) but may also provide cardioversion and antitachycardia pacing.8
ICD placement is indicated for patients who may have suffered previous cardiac arrest due to VF or hemodynamically unstable VTs. Additionally, prophylactic implantation of an ICD is indicated based on a variety of factors such as structural malformations leading to sustained VT, medication- or substance abuse–induced VT or VF, low left ventricular ejection fraction due to prior infarction, long QT syndrome, impending cardiac transplantation, catecholaminergic tachyarrhythmias while on beta-blocker therapy, Brugada syndrome, cardiac sarcoidosis, giant cell myocarditis, or Chagas disease.9
ICD components are similar to those of pacers, with a pulse generator and wire leads that continuously monitor and analyze cardiac rhythm, but they deliver defibrillation/cardioversion shocks. Most of these devices are implanted under local anesthesia in combination with sedation in the subpectoral or subcutaneous region of the chest. ICD units are programmable with a high degree of specificity in distinguishing fatal arrhythmias from potentially stable tachyarrhythmias.4 Additionally, ICDs respond not only to rhythm analysis, but also to prolonged or sustained high cardiac rates that fulfill defibrillation criteria (Figure 3).10
Figure 3. .

Typical ICD and right atrial and right ventricular lead placement.
When an ICD detects sustained periods of VT or VF, the device charges capacitors and discharges via the right ventricular lead to the device itself (“hot can”—where the generation unit is a conductor) or to other leads. Analysis recurs and persistent arrhythmias are treated repeatedly with shocks. In perfusing tachycardias, low-energy cardioversion shocks (0.1–5 J) synchronized to the R waves of the ECG are provided. Events are recorded within the device and can be recalled through device interrogation. Interrogation in newer models of ICDs often involve a wireless connection device placed in very close proximity to the generator to determine device manufacturer, model, settings, recorded cardiac events, battery condition, and lead status. The use of a magnet, unlike that in pacemakers, will suspend monitoring of VT or VF so that no shocks will be delivered.11
Of the problems reported with ICDs, lead failure and dislodgement can occur in 7% of patients, followed by infection and total lead displacement, both at 3%.12 Patient anxiety from multiple shocks, a form of posttraumatic stress, can also lead to increased circulating catecholamines, which may further precipitate cardioversion or defibrillation. Repeated shocks (3 or more over a 24-hour period) due to polymorphic or monomorphic VTs may be termed an “electrical storm” that is associated with high mortality rates.13
PREOPERATIVE EVALUATION
As with any proposed procedure/surgery and anesthetic plan, a detailed and focused physical examination and medical history must be performed. For those patients with CIEDs, particular attention should be placed on obtaining a thorough cardiovascular history with complete details on historical events and/or surgical interventions. Activity tolerance should be documented. Patients can be questioned regarding perceived shock history when ICDs have been placed. Current recommendations include device interrogation within 30 days prior to the procedure by the CIED team consisting of physicians, nurses, and technicians caring for the patient. Specific consultation and recommendation from cardiology and CIED specialists for dental procedures should be documented thoroughly and revised when changes to medical history and presentation occur.
Moderate sedation providers utilizing ECG monitoring and dentists employing general anesthesia are urged to obtain a 12-lead ECG along with close consultation of the CIED team for specific recommendations regarding the patient's tolerance for procedures, especially when prolonged, and administration of potentially vasoactive medications. Preoperative evaluation includes a baseline ECG rhythm strip, which can be compared with an earlier 12-lead ECG to verify proper CIED function. Of particular note, a 12-lead ECG on a patient with only 1 paced ventricle will display a left bundle branch block.11
A high priority should be placed upon ensuring optimal function and proper programming of the device by relating specifics about the surgical course, specific instrumentation, and anesthetic plan to the CIED team.14 The American Society of Anesthesiologists (ASA) and other groups have provided specific recommendations on the use of surgical instruments, imaging devices, lasers, and dental instruments that are important considerations in perioperative care. A thorough discussion is warranted with the CIED team in regards to specific dental instrumentation and imaging that will be utilized. This will be discussed in the perioperative section to follow.
It is important to note that the majority of patients with pacemakers or ICDs generally fall into a higher risk stratification relative to physical status. It is not uncommon to assign such patients who are pacer or ICD dependent as an ASA 3 or 4 physical status classification. Depending on the nature of the procedure and skill level of the sedation/anesthesia provider, the patient's physical status may simply preclude office-based treatment because of complexity, resources, and recommendations from expert consultation.
In terms of preoperative preparation and emergency response, the summary from the ASA and the Heart Rhythm Society recommends “having a magnet immediately available” if no reprogramming is performed to revert a CIED to asynchronous or nonsensing modes should life-threatening tachycardias be detected in the perioperative period.10 Figure 4 illustrates an example of the recommended 90-gauss doughnut-shaped magnet used for CIED inactivation. Equipment and medications for urgent cardioversion or defibrillation should be immediately available in the operatory. It should also be noted that magnets may not always deactivate the sensing capabilities of all CIEDs and may, in fact, be unaffected by magnet application depending on patient-specific programming or specific manufacturer. Close consultation with the CIED team may direct practitioners to the proper use of magnets in individual situations.
Figure 4. .

Typical 90-gauss “doughnut” magnet.
PERIOPERATIVE MANAGEMENT
By far, the major concern and most commonly reported complication in patients with CIED during surgery and anesthesia is with EMI. As technology progresses, advances in both surgical instrumentation and CIED filtering have marked reduced complications due to EMI.15
ELECTROSURGERY
Extensive studies investigating the use of monopolar electrosurgery cautery have demonstrated a low risk of initiating EMI detrimental to CIEDs. Nevertheless, most caution that bipolar electrosurgery be used instead of monopolar devices, and that electrosurgery dispersal electrode pads (“grounding pads”) be placed far away as possible from the CIED. Specific recommendations exist for each CIED model and manufacturer, and similarly, relevant information regarding the type of electrosurgery device must be investigated in the preoperative phase.
One manufacturer, for example, recommends limitations on using electrosurgery to prevent a defined path of the electrosurgical tip to the grounding pad from crossing the CIED.16 Other investigations into monopolar diathermy have recommended decreasing generator power, limiting duration of electrosurgery, using cut modes, draping the active electrode to avoid crossing the CIED, and maximizing the distance from the CIED and active electrode.17
Hospital-based radiofrequency rhizotomy of the trigeminal ganglia in the treatment of trigeminal neuralgia follows the same protocols stipulated with other forms of electrosurgery.18 Bursts of thermocoagulation should be kept to a minimal duration to decrease the likelihood of EMI.1
Recommendation: Avoid monopolar electrosurgery. Preferentially employ bipolar electrosurgery and keep exposure times short to minimize EMI. Place electrode dispersal pads as far away from CIED as possible.
ELECTRONIC AND PIEZOELECTRIC DENTAL SCALERS, APEX LOCATORS, LIGHT-CURING UNITS, AND OTHER COMMON DENTAL INSTRUMENTS
Recent investigations into potential EMI generated from piezoelectric devices have demonstrated no interactions with implantable cardioverter-defibrillators.19 Older studies and position papers suggest that electrosurgical units, ultrasonic bath cleaners, and magnetorestrictive ultrasonic scalers may interfere with pacer units up to a distance of 37.5 cm.20 However, there are no reports of pacer oversensing or unintended shock delivery. Recently, further investigations have found that electric motors found in dental handpieces, light-curing units (both battery powered and corded), endodontic heat carriers, apex locators, and electrosurgical units all generate some degree of EMI, yet only the electrosurgery units produce electromagnetic disturbances that may possibly adversely affect the function of ICDs by delivering an unintentional shock.21
Recommendation: Common dental devices and equipment, except electrosurgery, produce minimal EMI in dental practice. Exercise care and keep potential sources of EMI as far away from CIED as possible.
LASER SURGERY
As of this writing, no direct studies of dental lasers and their effects upon CIEDs exist. However, in a brief report published in 2013, investigators studied the electronic and magnetic field strengths surrounding various medical therapeutic laser systems, as an intense pulsed light system for EMI could possibly affect CIEDs. CO2 lasers and ruby lasers may have magnetic field strengths that exceed published exposure limits of Medtronic and St Jude CIEDs.22 Intense pulsed-light devices, often used in cosmetic dermatologic and epilation procedures, produce electromagnetic discharge too brief to be measured accurately in this study. Nevertheless, the units themselves have a small potential of producing EMI that is detectable, and the authors advocate keeping power-generating units and the laser/intense pulsed light as far away from the CIED as possible as a precautionary measure.
Similarly, in an investigation into ophthalmic lasers and their effects upon CIEDs, both excimer and neodymium-doped yttrium aluminum garnet lasers did not induce oversensing or inappropriate shock therapy in patients with CIED.23
Recommendation: Therapeutic lasers and intense pulsed-light devices generate little EMI to affect CIED function. Exercise care and keep potential sources of EMI as far away from CIED as possible.
RADIATION
Numerous studies have been conducted into radiation exposure and CIEDs. Most of these studies have investigated the use of therapeutic ionizing radiation therapy in oncologic therapy rather than diagnostic imaging used in contemporary dental practice. Reports of CIED failures are as high as 2.5% for patients with pacemakers and 6.8% for patients with ICDs when exposed to radiation therapy. Contemporary CIEDs are constructed with complementary metal dioxide semiconductor technology that is much more sensitive to the effects of ionizing radiation than older bipolar transistorized devices. Ionizing radiation exposure of 10 Gy can damage the random access memory of CIEDs used in programming and interrogation.24
Salerno et al24 recommend that, in general, ionizing radiation photonic energy be kept below 6 mV, and that therapeutic radiation to the head and neck region not exceed 2–10 Gy. In another study, 69 patients were studied for evidence of CIED interference or malfunction during radiation therapy. Of this study population, 58% received ionizing radiation less than 10 cm away from the device at varying levels of intensity and duration. No patient experienced adverse effects of CIED malfunction due to radiation therapy.25
However, in a robust 4-year study with a cohort of 34,706 patients, various interventions were required or performed for patients undergoing radiation therapy. Interventions included device reprogramming, relocation of the CIED out of radiation fields, magnet application, and device interrogation for a multitude of radiation therapy procedures. Device malfunction and failure was a rare event, and the investigation underscored a multidisciplinary approach to such patients prior to initiating radiation therapy.26
In regards to typical dental diagnostic radiographs, practitioners are also urged to consult with CIED teams for any directives and precautions with patients presenting with CIEDs. Dental radiographs, and digital dental radiography specifically, produce orders of magnitude less exposure to ionizing radiation than that seen in oncologic radiation therapy. For instance, comparing a typical orthodontic lateral cephalogram and cone-beam computed tomography lateral cephalogram to typical head and neck therapeutic radiation exposures, we find the dose to be extremely small. A traditional lateral cephalogram image exposes patients to 124.54 microsieverts, whereas head and neck ionizing radiation typically exposes patients to 6–10 Gy.27 To quantify this comparison, the amount of grays a patient is subject to from a lateral cephalogram would be 0.0001245 Gy. Nevertheless, typical shielding and radiation safeguards should be employed during the treatment of a patient equipped with CIED, including protective lead covering, thyroid protection, and minimal dose needed to obtain adequate imaging.
Of the relatively few imaging and diagnostic precautions related to CIED, magnetic resonance imaging (MRI) is contraindicated in patients with non–MRI-compatible CIED. High-rate pacing has been triggered by MRI field exposure that has led to fatalities.11
Recommendation: Common dental imaging studies using conventional and digital radiography contribute little to CIED interference. Traditional lead shielding and universal precautions further promote shielding of CIED from radiation exposure. MRI is contraindicated in patients with noncompatible CIED.
ANESTHESIA AND ADJUNCTIVE MEDICATIONS
Of particular concern to the clinician would be the use of sympathomimetic drugs and anesthetic agents commonly employed in office-based practice. Along with close consultation with the CIED team in terms of outlining anesthetic course and specific anesthetic or adjunctive agents to be administered, practitioners must judiciously consider the underlying cardiac condition of the patient in regards to tachycardia, tachyarrhythmias, bradycardia, and conductance stability.
Local anesthetics used in dentistry often contain varying amounts of epinephrine (0.005–0.02 mg/mL) that can promote tachyarrhythmias and initiate ICD events, particularly with higher doses or inadvertent intravascular administration. Myocardial ischemia, which is present in many patients with CIEDs, coupled with large doses of local anesthetics, which may interfere with cardiac electrophysiology, can increase electrophysiologic capture thresholds and increase the impedance (effective resistance) of CIED leads as well.28 General guidelines on anesthetic technique advise avoiding hyperventilation with resultant hypokalemia, acid-base balance shifts, intravascular volume overloading, and large volume blood transfusions.29
Adjunctive medications that have direct and indirect effects on cardiac function should be administered with caution to prevent precipitating CIED events perioperatively. Anticholinergics, beta blockers, and other vasoactive medications demand close attention to CIED response and may preclude treatment in an office-based or ambulatory setting.30
Additionally, vagal maneuvers and adjunctive medications used in anesthesia for reversal of neuromuscular blockade can potentially trigger CIED activity. Carotid sinus massage, Valsalva maneuvers, ocular tension, or the administration of adenosine or edrophonium may slow heart rate to trigger implanted pacemaker activity. Diagnostically, vagal maneuvers and rate-slowing dugs can be used to evaluate CIED function.31
Bispectral monitoring has not demonstrated EMI interference with CIEDs, but the opposite conditions have been observed. Early bispectral index monitors without filtering capability would sense pacer and CIED activity, which artificially raises the composite index of bispectral analysis.32 Recent innovations in bispectral monitoring offer filtering of the pacer signals.33
Recommendation: Thorough preoperative evaluation and consultation must be considered for any patient with CIED. Commonly used vasoactive medications may have considerable effects upon the sensing and function of CIED without prior modification to anesthetic techniques. Consider treatment of patient in a hospital setting or deferring treatment if the potential for cardiac events or irregular CIED activity is anticipated.
OTHER SOURCES OF ELECTROMAGNETIC INTERFERENCE
Emerging technologies will continue to proliferate in the dental setting as well as in the consumer market and should be closely scrutinized for possible EMI generation. Commonly used magnetic dental burr holders, dental bib clips, or other magnetically equipped devices in the dental operatory should be kept at distances away from CIEDs so as to not trigger asynchronous or nonsensing modes in CIEDs.
Increasing use of tablet devices for electronic medical record keeping or patient education has also come under scrutiny when these devices are placed near CIEDs. The commonly used iPad 2 wireless tablet device has been demonstrated to trigger magnetic modes in CIEDs when placed directly over the left pectoral region.34 Moreover, the tendency to use a supine or nearly supine dental patient's chest area for resting of instruments or monitors should be discouraged.
Of particular concern to the dental profession is the prevalence of modern dental chairs with magnets embedded into the cushioning and headrests. Because of the proximity of CIED-implanted patients while seated in the dental chair, clinicians must consider removing headrests or cushioning as possible sources of EMI—especially when magnetic field strength exceeds 10 gauss (1 milliTesla). Seating the patient in another chair not equipped with magnets is, of course, another option.35
Recommendation: Electronic devices and devices with magnetic fields all have the potential for producing EMI. Specific equipment and environments may require close consultation with CIED teams to determine relative risk of using unstudied devices. Avoid, if possible, extraneous use of devices and equipment with patients equipped with CIED. Avoid placing devices and equipment in close proximity to CIED.36
RESUSCITATION
In the event of inadequate pacer or ICD function, chest compressions and external defibrillation should be attempted in the event of a patient suffering from cardiac arrest. Rescuers performing chest compressions have reported experiencing very low-grade electrical shock while delivering chest compressions at the recommended rate and depth, but the shocks were not significant to cause interruption in compressions.
Failures of CIEDs in an office-based dental setting as well as in hospital operating rooms are, fortunately, rare. However, in the event of low perfusion states or cardiac arrest, advanced cardiac life support protocols must be initiated in standard fashion. Anticholinergics, such as atropine; catecholamines, such as epinephrine; and antiarrhythmics, such as amiodarone, remain viable resuscitative drugs in the event of failure of device failure or insufficiency. Cardioversion and transcutaneous pacing also can be utilized as per current algorithms.37 Again, it should be reiterated that patients with significant risks for developing arrhythmias despite CIED implantation may not be suitable candidates for office-based dental/oral and anesthesia.
Recommendation: Close consultation with the CIED team will provide the practitioner with resuscitation or complication directives. Although asynchronous or nonsensing modes can be activated with magnet application to the CIED, primary emphasis is placed upon prevention, recognition, and close monitoring of the patient. Should the patient become hemodynamically unstable, or if aberrations in rhythm or CIED function are detected, EMS should be summoned in office-based and ambulatory surgical settings while resuscitative measures are initiated. It is recommended that cardiac monitoring be employed throughout the procedure into the postoperative course, and that external defibrillation and cardioversion equipment be immediately available.
CONCLUSIONS
As more and more patients are living with CIEDs, it is imperative that the dentist be aware of the many issues that these patients and their devices present to them. There are many devices in the dental office that at least theoretically could interfere with these devices. Likewise, common medications used in dental/oral surgery as well as sedation and general anesthesia-related drugs may interfere with these devices. Consultation with the cardiology team is important for virtually all of these patients prior to dental treatment.
CONTINUING EDUCATION QUESTIONS
This continuing education (CE) program is designed for dentists who desire to advance their understanding of pain and anxiety control in clinical practice. After reading the designated article, the participant should be able to evaluate and utilize the information appropriately in providing patient care.
The American Dental Society of Anesthesiology (ADSA) is accredited by the American Dental Association and Academy of General Dentistry to sponsor CE for dentists and will award CE credit for each article completed. You must answer 3 of the 4 questions correctly to receive credit. Articles are eligible for CE credit for one year following the date of publication.
Submit your answers online at www.adsahome.org. Click on “On Demand CE.”
CE questions must be completed within three months and prior to the next issue.
-
1. Application of a magnet over an implanted pacemaker will
-
A.Cause the device to defibrillate automatically
-
B.Begin to recharge the lithium-ion battery source
-
C.Erase any and all device programming
-
D.Deactivate pacemaker sensing and activate asynchronous operation
-
A.
-
2. Which of the following cardiac conditions are treated by implanted cardioverter-defibrillators?
-
A.Hemodynamically unstable ventricular tachycardias
-
B.Hemodynamically stable ventricular tachycardias
-
C.Asymptomatic bradycardias
-
D.“Innocent” heart murmurs
-
A.
-
3. Dispersal electrode pads, or “grounding pads,” used in electrosurgery and electrocautery should be placed
-
A.Directly over the site of the cardiovascular implantable electronic device (CIED)
-
B.Under the patient's left shoulder
-
C.Directly under the site of the CIED
-
D.Under the patient's right shoulder
-
A.
-
4. Which of the following has the highest potential to trigger CIED activity?
-
A.Intraoral digital radiographs
-
B.Neodymium-doped yttrium aluminum garnet laser treatment
-
C.Carotid massage (vagal maneuver)
-
D.Bispectral monitoring
-
A.
REFERENCES
- 1. Healy JS, Merchant R, Simpson C, et al. Society position statement: Canadian Cardiovascular Society/Canadian Anesthesiologists' Society/Canadian Heart Rhythm Society joint position statement on the management of patients with implanted pacemakers, defibrillators, and neurostimulating devices. Can J Cardiol. 2012; 28: 141– 151. [DOI] [PubMed] [Google Scholar]
- 2. Knight J, Sarko J. Conduction disturbances and cardiac pacemakers. : JL Vincent, Abraham E, FA Moore, Kochanek PM, Fink MP. Textbook of Critical Care. 6th ed. Philadelphia, Pa: Elsevier-Saunders; 2011: 590– 591. [Google Scholar]
- 3. Rooke GA, Lombaard SA, Van Norman GA, et al. Initial experience of an anesthesiology-based service for perioperative management of pacemakers and implantable cardioverter defibrillators. Anesthesiology. 2015; 123: 1024– 1032. [DOI] [PubMed] [Google Scholar]
- 4. Schulman PM, Rozner MA. Use caution when applying magnets to pacemakers or defibrillators for surgery. Anesth Analg. 2013; 117: 422– 427. [DOI] [PubMed] [Google Scholar]
- 5. Cardall TY, Brady WJ, Chan TC, Perry JC, Vilke GM, Rosen P. Permanent cardiac pacemakers: Issues relevant to the emergency physician, part II. J Emerg Med. 1999; 17: 697– 709. [DOI] [PubMed] [Google Scholar]
- 6. Cismaru G, Gusetu G, Muresan L, et al. Recovery of ventriculo-atrial conduction after adrenaline in patients implanted with pacemakers. Pacing Clin Electrophysiol. 2015; 38: 857– 863. [DOI] [PubMed] [Google Scholar]
- 7. Karumbaiah K, Omar B. ST-elevation myocardial infarction in the presence of biventricular paced rhythm. J Emerg Med. 2013; 45: e35– e40. [DOI] [PubMed] [Google Scholar]
- 8. Konstantinou DM, Efthimiadis GK, Vassilikos V, et al. Implantable cardioverter defibrillators for primary prevention of sudden death in hypertrophic cardiomyopathy. J Cardiovasc Med . 2016. doi:10.2459/JCM.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 9. Paulin FL, Exner DV. Sudden cardiac death: implantable cardioverters-defibrillators. : JL Vincent, Abraham E, FA Moore, Kochanek PM, Fink MP. Textbook of Critical Care. 6th ed. Philadelphia, Pa: Elsevier-Saunders; 2011: 594– 603. [Google Scholar]
- 10. Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) expert consensus statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management: executive summary. Heart Rhythm. 2011; 8: e1– e18. [DOI] [PubMed] [Google Scholar]
- 11. McMullan J, Valento M, Attari M, Venkat A. Care of the pacemaker/implantable cardioverter defibrillator patient in the ED. Am J Emerg Med. 2007; 25: 812– 822. [DOI] [PubMed] [Google Scholar]
- 12. Schnickel AFL, Vriesendorp PA, Sijbrands EJG, Jordaens LJLM, ten Cate FJ, Michels M. Outcome and complications after implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy: systematic review and meta-analysis. Circ Heart Fail. 2012; 5: 552– 559. [DOI] [PubMed] [Google Scholar]
- 13. Conti S, Pala S, Biagioli V, et al. Electrical storm: a clinical and electrophysiological overview. World J Cardiol. 2015; 7: 555– 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Joshi GP. Perioperative management of outpatients with implantable cardioverter defibrillators. Curr Opin Anaesthesiol. 2009; 22: 701– 704. [DOI] [PubMed] [Google Scholar]
- 15. Gifford J, Larimer K, Thomas C, May P, Stanhope S, Gami A. Randomized controlled trial of perioperative ICD management: magnet application versus reprogramming. Pacing Clin Electrophysiol. 2014; 37: 1219– 1224. [DOI] [PubMed] [Google Scholar]
- 16. Bovie Medical Corporation. Bovie medical insights: electrosurgery and pacemakers: how to stay safe. 2012. Available at: http://blog.boviemed.com/blog-1/bid/130241/Electrosurgery-and-Pacemakers-How-to-Stay-Safe. Accessed February 21, 2016. [Google Scholar]
- 17. Robinson TN, Varosy PD, Guillame G, et al. Effect of radiofrequency energy emitted from the monopolar “Bovie” instruments on cardiac implantable electronic devices. J Am Coll Surg. 2014; 219: 399– 406. [DOI] [PubMed] [Google Scholar]
- 18. Udupi BP, Chouhan RS, Dash HH, Bithal PK, Prabhakar H. Comparatie evaluation of percutaneous retrogasserian glycerol rhizolysis and radiofrequency thermocoagulation techniques in the management of trigeminal neuralgia. Neurosurgery. 2012; 70: 407– 413. [DOI] [PubMed] [Google Scholar]
- 19. Maiorana C, Grossi GB, Garramone RA, Manfredini R, Santoro F. Do ultrasonic dental scalers interfere with implantable cardioverter defibrillators? An in vivo investigation. J Dent. 2013; 41: 955– 959. [DOI] [PubMed] [Google Scholar]
- 20. Miller CS, Leonelli FM, Latham E. Selective interference with pacemaker activity by electrical dental devices. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998; 85: 33– 36. [DOI] [PubMed] [Google Scholar]
- 21. Maheshwari KR, Nikdel K, Guillaume G, Letra AM, Silva RM, Dorn SO. Evaluating the effects of different dental devices on implantable cardioverter defibrillators. J Endod. 2015; 41: 692– 695 [DOI] [PubMed] [Google Scholar]
- 22. Lister T, Grant L, Lee SM, et al. Electromagnetic interference from lasers and intense light sources in the treatment of patients with artificial pacemakers and other implantable cardiac devices. Lasers Med Sci. 2015; 30: 1619– 1622 [DOI] [PubMed] [Google Scholar]
- 23. Sher NA, Golben MP, Kresge K, Sleznick L, Adabag S. An in vitro evaluation of electromagnetic interference between implantable cardiac devices and ophthalmic laser systems. Europace. 2011; 13: 583– 588. [DOI] [PubMed] [Google Scholar]
- 24. Salerno F, Gomellini S, Caruso C, et al. Management of radiation therapy patients with cardiac defibrillator or pacemaker. Ital Soc Med Radiol. 2015. doi:10.1007/s11547-015-0616-z. [DOI] [PubMed] [Google Scholar]
- 25. Makkar A, Prisciandaro J, Agarwal S, et al. Effect of radiation therapy on permanent pacemaker and implantable cardioverter-defibrillator function. Heart Rhythm. 2012; 9: 1964– 1968. [DOI] [PubMed] [Google Scholar]
- 26. Brambatti M, Mathew R, Strang B, et al. Management of patients with implantable cardioverter-defibrillators and pacemakers who require radiation therapy. Heart Rhythm. 2015; 12: 2148– 2154. [DOI] [PubMed] [Google Scholar]
- 27. Signorelli L, Patcas R, Peltomäki T, Schätzle M. Radiation dose of cone-beam computed tomography compared to conventional radiographs in orthodontics. J Orofac Orthop. 2016; 77: 9– 15. [DOI] [PubMed] [Google Scholar]
- 28. Stone ME, Apinis A. Current perioperative management of the patient with a cardiac rhythm management device. Semin Cardiothorac Vasc Anesth. 2009; 13: 31– 43. [DOI] [PubMed] [Google Scholar]
- 29. Stone ME, Salter B, Fischer A. Perioperative management of patients with cardiac implantable electronic devices. Br J Anaesth. 2011; 107 s1): i16– i26. [DOI] [PubMed] [Google Scholar]
- 30. Elhendy A, Windle J, Porter TR. Safety and feasibility of dobutamine stress echocardiography in patients with implantable cardioverter defibrillators. Am J Cardiol. 2003; 92: 475– 477. [DOI] [PubMed] [Google Scholar]
- 31. Cardall TY, Chan TC, Brady WJ, Perry JC, Vilke GM, Rosen P. Permanent cardiac pacemakers: issues relevant to the emergency physician, part I. J Emerg Med. 1999; 17: 479– 489. [DOI] [PubMed] [Google Scholar]
- 32. Gallagher JD. Pacer-induced artifact in the bispectral index during cardiac surgery. Anesthesiology. 1999; 90: 636. [DOI] [PubMed] [Google Scholar]
- 33. Dragoumanis C. Influence of an external pacemaker on bispectral index. Eur J Anaesthesiol. 2005; 22: 70– 71. [DOI] [PubMed] [Google Scholar]
- 34. Kozik TM, Chien G, Connolly TF, Grewal GS, Liang D. iPad2® use in patients with implantable cardioverter defibrillators causes electromagnetic interference: the EMIT study. J Am Heart Assoc. 2014; 3: e000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boston Scientific. Dental equipment and implantable pacemakers and defibrillators 2009. Available at: https://www.bostonscientific.com/content/dam/bostonscientific/quality/education-resources/english/ACL_Dental_Equipment_20090202.pdf. Accessed February 28, 2016. [Google Scholar]
- 36. Brand HS, Entjes ML, Amerongen AVN, van der Hoff EV, Schrama TAM. Interference of electrical dental equipment with implantable cardioverter-defibrillators. Br Dent J. 2007; 203: 577– 579. [DOI] [PubMed] [Google Scholar]
- 37. Lin JA, Borel CO, Wang WB, et al. Anesthetic management of an AAI pacemaker patient with paroxysmal atrial fibrillation during colorectal surgery. J Clin Anesth. 2006; 18: 372– 375. [DOI] [PubMed] [Google Scholar]


