Abstract
Exaggerated cardiovascular reactivity to behavioral challenges among otherwise healthy individuals has been associated with carotid atherosclerosis. We evaluated whether a similar relationship exists among hypertensives, who are at a heightened atherosclerotic risk. Untreated, hypertensive men (n=251; age range, 40 to 70 years; 197 white, 54 black) completed a standardized battery of behavioral challenges while their blood pressure responses to the battery were measured. Mean and maximum carotid intima-media thickness and the occurrence of carotid plaques were subsequently determined using B-mode ultrasonography. Although greater systolic and diastolic responses to the battery were associated with greater mean and maximum intima-media thickness in univariate analyses (P<0.01), only diastolic reactivity showed a unique association with mean and maximum carotid intima-media thickness after multivariate adjustment for age, race, socioeconomic status, smoking and alcohol use, body mass index, lipid profile, glucose and insulin concentrations, and resting blood pressure (P<0.05). Carotid plaque occurrence was associated with greater systolic reactivity (P=0.05) and was marginally associated with greater diastolic reactivity (P=0.07) in univariate analyses, but neither systolic nor diastolic reactivity was uniquely associated with the presence of carotid plaques after multivariate risk-factor adjustment. Among hypertensives, exaggerated behaviorally evoked cardiovascular reactivity appears to be uniquely associated with greater carotid intima-media thickness but not with carotid plaque occurrence.
Keywords: atherosclerosis, cardiovascular reactivity, carotid intima-media thickness, carotid plaque, stress
Two lines of research indicate that exaggerated cardiovascular reactivity to behavioral challenges is associated with atherosclerosis. First, cynomolgus macaques that display exaggerated heart rate (HR) reactivity to the threat of capture also display the most pronounced carotid and coronary atherosclerosis on necropsy.1,2 Second, humans who display exaggerated blood pressure reactivity to behavioral stressors are more likely to have increased carotid intima-media thickness (IMT),3–7 higher carotid plaque occurrence,3 greater carotid plaque height,4–6 and larger carotid plaque surface areas7 compared with levels for individuals who display attenuated blood pressure reactivity. Together, these 2 lines of research are consistent with the hypothesis that a disposition to respond to behavioral challenges with exaggerated cardiovascular reactions may enhance the risk for atherosclerosis.8 To our knowledge, however, no studies have examined whether exaggerated, behaviorally evoked cardiovascular reactivity is associated with atherosclerosis among individuals with hypertension, which is a leading risk factor for atherosclerosis.9–14 Indeed, the majority of research on cardiovascular reactivity and hypertension has focused on the extent to which exaggerated cardiovascular reactivity predicts the development of hypertension,15–17 or on whether hypertensives or those at risk for hypertension differ from controls in cardiovascular or autonomic nervous system reactivity to behavioral stressors.18,19 Consequently, it is unclear whether the relationship between cardiovascular reactivity and atherosclerosis, which has been documented in otherwise healthy individuals, generalizes to hypertensives.
The present study, therefore, examined (1) the cross-sectional univariate relationships between HR and blood pressure reactivity to a standardized battery of behavioral challenges and indices of carotid atherosclerosis (mean and maximum carotid IMT and carotid plaque occurrence) in a sample of untreated hypertensives, and (2) whether HR and blood pressure reactivity are associated with these indices of carotid atherosclerosis after statistically controlling for age, race, socioeconomic status (SES), smoking status, alcohol use, body mass index (BMI), serum lipids, fasting glucose and insulin concentrations, and resting blood pressure.
Methods
Participants
Participants were 40- to 70-year-old men (n=251; 197 white, 54 black) enrolled in the University of Pittsburgh Reactivity and Cardiovascular Risk Trial (REACT), which was designed to study the relationship between carotid atherosclerosis and behaviorally evoked cardiovascular reactivity among untreated hypertensives. Participants were recruited via mass mailings to Allegheny County residents in Pennsylvania. Exclusion criteria included cardiovascular medication use in the 2-month period before enrollment; hypertension treatment >1 year in the 5-year period before enrollment or lifetime hypertension treatment exceeding 2 years (85% of the participants had no history of hypertension treatment; the median treatment length of the remaining participants was 2 months); secondary hypertension; cerebrovascular accident or stroke; insulin treatment for diabetes or diabetic neuropathy; fasting serum glucose >200 mg/dL; obesity; cancer; serum creatinine level >2 mg/dL; hepatitis or cirrhosis; coronary artery disease; myocardial infarction or angioplasty in the past 12 months; angina pectoris; congestive heart failure; valvular heart disease; atrial fibrillation; pulmonary disease; alcoholism; psychiatric disorder or current use of psychotropic medication; and coronary bypass, carotid, or peripheral vascular surgery. Participants provided informed consent, and the University of Pittsburgh’s Institutional Review Board granted study approval.
Hypertension status was determined if the average of 2 seated resting blood pressure measurements fell between 140 and 180 mm Hg systolic blood pressure (SBP) and/or 90 and 110 mm Hg diastolic blood pressure (DBP) on each of 2 screening sessions.
Cardiovascular Reactivity Testing
Participants fasted and abstained from caffeinated beverages, tobacco products, and exercise for 3 hours before testing, and they refrained from drinking alcohol and taking nonessential medication for 12 hours before testing. A 20-minute rest period was initiated after participants were instrumented for physiological recording. After this period, participants completed a standardized reactivity battery20 while SBP, DBP, and HR responses were measured. The battery was comprised of six 6-minute tasks delivered via a computer and included a speeded reaction-time task, a mirror-image tracing task, a guided-path target task, a Stroop color-word interference task, and an impromptu speech preparation and speech delivery task. A 9-minute baseline period21 preceded each task.
Automated oscillometric measurements of SBP and DBP were obtained every 90 seconds using a Dinamap Vital Signs Monitor (Model 8100; Critikon), and HR was derived from a modified lead II ECG signal.22 Reactivity estimates were standardized for each task by dividing the task-minus-baseline change score by the standard deviation of that change score. A single HR, SBP, and DBP reactivity estimate was then derived by averaging these standardized reactivity values across the tasks.4
Ultrasound Assessment of Carotid IMT and Carotid Plaque Occurrence
Mean and maximum carotid IMT and carotid plaque occurrence were derived using B-mode ultrasonography. Trained sonographers imaged the right and left common carotid artery, carotid bifurcation, and the first centimeter of the internal carotid artery with a Toshiba SSA-270 scanner that was equipped with a 5-Mhz linear-array imaging probe. Mean and maximum IMT were then derived from digitized images of the lumen-intima and media-adventitia interface across each carotid segment, and were averaged across the near and far walls of the right and left distal common carotid artery (1 cm proximal to the carotid bulb), the far wall of the carotid bulb (starting at the point at which the near and far walls of the common carotid artery are no longer parallel and ending at the flow divider), and the far wall of the internal carotid artery (from the flow divider to the first cm distal to this point). Plaques were defined as focal areas projecting in to the vessel lumen with a ≥50% thickness than the bordering areas, and plaque occurrence (coded as 0=none and 1≥1) was determined for the proximal and distal common, bulb, internal, and external carotid segments.
In addition to the dichotomous measure of plaque occurrence, statistical analyses were also performed on a graded estimate of plaque severity, termed the plaque index.23 For this estimate, plaque severity was coded in the following manner: 0=no plaque; 1=a single plaque that was <30% of the vessel diameter, 2=a single plaque that was between 30% to 50% of the vessel diameter or multiple plaques that were <30% of the vessel diameter, and 3=a plaque that was >50% of the vessel diameter or multiple plaques with one that was between 30% to 50% of the vessel diameter. To derive the plaque index, these scores from the right and left carotid arteries were summed. Because the results of the statistical analyses using the plaque index as a dependent measure did not differ from those using the more basic, dichotomous measure of 0=no plaques and 1=≥one plaques, they are omitted.
Cardiovascular Risk Factor Assessment
The following cardiovascular risk factors were assessed: BMI, smoking status (coded as current smoker versus nonsmoker), frequency of alcohol use, total family income before taxes, the number of completed school years, race (coded as 1=white; 2=black), and SES, which was estimated by standardizing the number of years in school and average annual income and then taking the average of these 2 standardized values. Fasting levels of triglycerides, HDL and LDL, insulin, and glucose were also obtained (see Muldoon et al24 for details regarding blood assays).
Data Analysis
Relationships between carotid IMT and cardiovascular reactivity were evaluated using 2-level hierarchical regression analyses in which independent variables were entered in the following order: level 1 was age, race, SES, smoking status, alcohol use, BMI, HDL, LDL, natural-log triglyceride values, glucose, insulin, and screening and baseline values of the target cardiovascular parameter; level 2 was the cardiovascular reactivity estimate (HR, SBP, or DBP reactivity). We evaluated the proportion of variance in carotid IMT accounted for by the set of covariates in level 1 (R2), and the increment in the proportion of variance accounted for by the reactivity estimate in Level 2 (ΔR2). Unique correlations between each independent variable and IMT measure were evaluated using the partial correlation coefficient (rp). Associations between plaque occurrence and cardiovascular risk factors were evaluated using logistic regression analyses from which we evaluated odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for each independent variable in the model.
Results
Descriptive Characteristics
Table 1 contains descriptive characteristics of the sample, along with univariate correlations between atherosclerosis risk factors and carotid IMT and carotid plaque occurrence. Mean plasma lipid levels, glucose, insulin, and BMI were all within normal ranges. There were relatively few current smokers (13%) in the present sample, and the majority of individuals (72%) reported consuming ≤2 alcoholic beverages per week. The average number of years that the participants reported attending school was 14.8±2.8 years. The percentages of participants reporting total family incomes before taxes were as follows: 6.1% earning >$10 000, 12.6% earning between $10 000 and $19 999, 20.2% earning between $20 000 and $34 999, 19.8% earning between $35 000 and $50 000, and 41.3% reported earning >$50 000. Average SBP, DBP, and HR reactivity were comparatively higher than those reported in other studies using the same laboratory challenges.20 Consistent with previous findings, average mean (0.92 mm) and maximum (1.20 mm) carotid IMT levels were moderately elevated in this hypertensive sample; in addition, 69% of the present sample had ≥1 focal carotid plaques.
TABLE 1.
Sample Characteristics and Univariate Correlations Between Cardiovascular Risk Factors and Carotid Ultrasound Measures
| Characteristic | Value | Mean IMT | Maximum IMT | Plaque |
|---|---|---|---|---|
| r | r | rpb | ||
| Age, y | 56.1 (9.1) | 0.48* | 0.46* | 0.25* |
| Race, % white | 79.1% | −0.02 | −0.04 | 0.03 |
| SES, standardized value | 0.03 (1.74) | −0.20* | −0.19* | −0.06 |
| Smoking status, % current smokers | 13.0% | −0.07 | −0.06 | −0.06 |
| Alcohol use, median no. drinks/week | 1–2 | −0.06 | −0.05 | −0.04 |
| BMI, kg/m2 | 28.1 (3.1) | −0.01 | −0.02 | −0.02 |
| HDL, mg/dL | 50.1 (13.1) | −0.02 | −0.002 | 0.03 |
| LDL, mg/dL | 134.3 (34.6) | 0.26* | 0.23* | 0.21* |
| Triglycerides, mg/dL | 145.2 (85.2) | 0.02 | 0.03 | 0.11 |
| Glucose, mg/dL | 94.9 (15.9) | 0.20* | 0.19* | 0.10 |
| Insulin, mg/dL | 12.1 (6.4) | −0.08 | −0.12 | −0.09 |
| Screening SBP, mm Hg | 148.7 (10.6) | 0.33* | 0.31* | 0.16† |
| Screening DBP, mm Hg | 92.7 (7.3) | −0.36* | −0.36* | −0.18* |
| Screening HR, bpm | 73.4 (9.8) | −0.09 | −0.07 | −0.003 |
| Baseline SBP, mm Hg | 144.7 (13.9) | 0.32* | 0.28* | 0.08 |
| Baseline DBP, mm Hg | 87.8 (8.5) | −0.21* | −0.21* | −0.15† |
| Baseline HR, bpm | 70.2 (10.1) | −0.10 | −0.08 | −0.02 |
| SBP reactivity, mm Hg | 17.0 (8.5) | 0.20* | 0.19* | 0.14† |
| DBP reactivity, mm Hg | 9.6 (4.4) | 0.22* | 0.21* | 0.12 |
| HR reactivity, bpm | 5.7 (4.6) | 0.05 | 0.03 | −0.05 |
| Mean IMT, mm | 0.92 (0.16) | 0.96* | 0.49* | |
| Maximum IMT, mm | 1.20 (0.24) | 0.54* |
IMT, indicates intima-media thickness; SES, socioeconomic status (calculated as standardized average of annual income and years in school); BMI, body mass index; HDL, high density lipoproteins, LDL, low density lipoproteins, SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate. Carotid plaque was calculated as 0=none, 1=1 or more; race was coded as 1=white, 2=black; smoking status was coded as 1=nonsmoker, 2=current smoker. Values in parentheses indicate standard deviations.
P<0.01;
P<0.05.
Univariate Associations Between Cardiovascular Risk Factors and Carotid Atherosclerosis
Univariate correlation analyses showed that greater mean and maximum carotid IMT were associated with advanced age, lower SES, higher LDL and glucose concentrations, increased screening and baseline SBP, and lower screening and baseline DBP (all, P<0.01; Table 1). In addition, greater SBP and DBP, but not HR, reactivity to the test battery were associated with greater mean and maximum IMT (P<0.01; Table 1).
As shown in Table 1, the presence of carotid plaque was associated with increased age, higher LDL, higher screening SBP, lower screening and baseline DBP, greater mean and maximum carotid IMT, and greater SBP reactivity (P<0.05). Having ≥1 carotid plaques was also marginally associated with greater DBP reactivity (P=0.067). None of the remaining variables showed a univariate association with carotid plaque occurrence.
Unique Associations Between Behaviorally Evoked Cardiovascular Reactivity and Carotid Atherosclerosis
Across all hierarchical regression analyses for each cardiovascular reactivity parameter, age, SES, LDL, and screening blood pressure accounted for a unique percentage of the variance in mean and maximum IMT after adjusting for other variables in the model (P<0.05; Table 2 for results from DBP regression analyses). In contrast with the univariate results, however, glucose and baseline SBP and DBP at the time of reactivity testing did not show statistically independent relationships with carotid IMT after covariate adjustment.
TABLE 2.
Hierarchical Regression Analysis Predicting Mean and Maximum Carotid Intima-Media Thickness (IMT) from Behaviorally Evoked Diastolic Blood Pressure Reactivity
| Variable | Mean IMT | Maximum IMT |
|---|---|---|
| rp | rp | |
| Level 1 | ||
| Age | 0.33* | 0.30* |
| Race | 0.05 | 0.01 |
| SES | −0.17† | −0.16† |
| Smoking status | 0.03 | 0.04 |
| Alcohol use | −0.10 | −0.11 |
| BMI | 0.04 | 0.04 |
| LDL | 0.26* | 0.21* |
| HDL | 0.03 | 0.06 |
| Triglycerides | 0.04 | 0.07 |
| Glucose | 0.06 | 0.08 |
| Insulin | −0.13 | −0.16* |
| Screening DBP | −0.22* | −0.20* |
| Baseline DBP | 0.13 | 0.10 |
| Level 2 | ||
| DBP reactivity | 0.14† | 0.14† |
For mean IMT, Level 1 R2=0.364, P<0.001; Level 2 ΔR2=0.013, P<0.05. For maximum IMT Level 1 R2=0.328, P<0.001; Level 2 ΔR2=0.013, P<0.05. rp represents the partial correlation between each independent variable and carotid IMT after adjusting both the independent variable and IMT for other independent variables in the model.
P<0.01;
P<0.05.
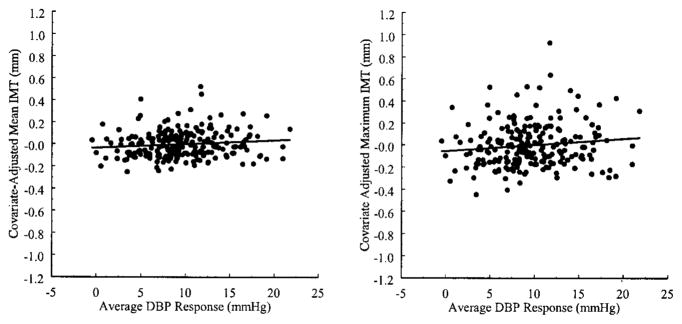
In addition, DBP reactivity to the battery showed a unique and positive association with mean and maximum carotid IMT after covariate adjustment. The Figure displays covariate-adjusted mean and maximum carotid IMT as a function of DBP reactivity. The unstandardized, covariate-adjusted regression coefficient relating mean IMT to the average DBP response to the battery was 0.005 mm per 1 mm Hg change in DBP (P=0.036); the same coefficient for maximum IMT was 0.008 mm per 1 mm Hg change in DBP (P=0.028). These results indicate that an additional 0.005 and 0.008 mm of mean and maximum carotid IMT, respectively, was associated with an additional 1 mm Hg increment in DBP reactivity to the battery after statistically controlling for age, race, SES, smoking and alcohol use, BMI, plasma lipid levels, glucose and insulin concentrations, and 2 measures of resting DBP. In contrast, neither SBP nor HR reactivity accounted for a significant proportion of the variance in IMT after covariate adjustment (SBP reactivity second step: ΔR2 for mean IMT=0.005, P=0.20; for maximum IMT: ΔR2=0.005, P=0.22; HR reactivity second step: ΔR2 for mean IMT <0.001, P=0.92; for maximum IMT: ΔR2=0.001, P=0.69).
Figure.
Adjusted mean and maximum carotid IMT as a function of DBP reactivity to a standardized battery of behavioral challenges. IMT measures were adjusted for age, race, SES, smoking and alcohol use, BMI, HDL, LDL, triglycerides, glucose, insulin, screening DBP, and resting DBP at the time of reactivity testing.
The occurrence of ≥1 carotid plaques was not independently associated with greater SBP (OR=0.995; 95% CI=0.97, 1.02), DBP (OR=1.28; 95% CI=0.80, 2.05), or HR (OR=0.70; 95% CI=0.46, 1.07) reactivity to the test battery when the remaining risk factors were also entered into the logistic regression models. Carotid plaque occurrence was, however, uniquely associated with increased age (OR=1.06; 95% CI=1.02, 1.10) and LDL (OR=1.01; 95% CI=1.01, 1.03) and triglyceride levels (OR=2.33; 95% CI=1.20, 4.56) when cardiovascular reactivity estimates and the remaining risk factors were covaried.
Analyses of carotid plaque occurrence were also conducted using regression models that covaried only those variables showing a significant univariate correlation with carotid plaque (age, LDL, screening SBP and DBP, and baseline SBP and DBP; Table 1). The results of these reduced-model analyses, however, yielded the same findings as those from the full-covariate models: no measure of cardiovascular reactivity was associated with carotid plaque after covariate adjustment.
Discussion
In this cross-sectional study, we evaluated whether greater behaviorally evoked cardiovascular reactivity is related to ultrasound measures of carotid atherosclerosis among hypertensive men. Participants in the present study were untreated for hypertension at the time of study (85% had never received treatment, and the remaining participants had a median lifetime treatment length of 2 months), which controlled for the possible confounding effects of antihypertensive treatment on cardiovascular reactivity and other study variables.
Overall, we found that greater blood pressure reactivity to a standardized battery of behavioral challenges was related to increased mean and maximum carotid IMT in univariate analyses; however, only exaggerated DBP reactivity was associated uniquely with increased mean and maximum carotid IMT after multivariate risk-factor adjustment. This association between exaggerated DBP reactivity and increased carotid IMT accords with recent reports3–7 that indicated that greater behaviorally evoked cardiovascular reactivity is similarly associated with increased carotid IMT among individuals without hypertension. Differing from the carotid IMT findings, however, were those regarding carotid plaque occurrence. Specifically, although greater SBP reactivity was associated significantly, and DBP reactivity marginally, with having ≥1 carotid plaques in univariate analyses, neither of these 2 estimates of cardiovascular reactivity was related to the presence of carotid plaques after adjusting for other cardiovascular risk factors. These particular findings contrast with those of prior studies that indicated that behaviorally evoked cardiovascular reactivity is associated positively with measures of carotid plaque among individuals without hypertension.3,4,7 Finally, HR reactivity was not related to any measure of carotid atherosclerosis in this hypertensive sample, which replicates findings from prior studies among normotensive individuals.3,4,7
An individual’s tendency to show exaggerated cardiovascular reactivity to behavioral challenges has been hypothesized8 to increase the risk of developing atherosclerosis by at least 2 possible mechanisms: exaggerated or sustained cardiovascular responses may promote atherosclerosis by sympathetically mediated lipid mobilization and platelet aggregation25 or by pressor-induced blood flow disruptions that lead to endothelial injury.26–28 To the extent that measures of carotid IMT reflect levels of preclinical atherosclerosis,29–32 then the current results indicate that exaggerated behaviorally evoked DBP reactivity does account for a unique percentage of the variance in preclinical carotid atherosclerosis among untreated hypertensives. Furthermore, although DBP reactivity accounted for a relatively modest percentage of unique variance in carotid IMT indices after risk-factor adjustment (≈1%), this percentage was comparable to what has been reported previously in nonhypertensive samples4–7 and was also comparable to or greater than the variance in carotid IMT that was accounted for by other recognized cardiovascular risk factors (eg, SES, smoking and alcohol use, BMI, glucose, and insulin; Table 2). The magnitude of the univariate and multivariate relationships between reactivity estimates and carotid ultrasound measures, however, was appreciably lower than that associated with age and LDL levels, both of which showed the strongest and most consistent relationships with carotid IMT and plaque occurrence.
Mean and maximum carotid IMT are widely accepted as valid markers of preclinical atherosclerosis because they correlate strongly with established risk factors for atherosclerosis,29 are reduced with treatment for atherosclerosis,30 are moderately correlated with indices of atherosclerosis in other vascular regions,31,32 and predict cardiac mortality and morbidity.33–35 Criticisms, however, of mean and maximum carotid IMT as exclusive markers of atherosclerosis have been voiced.36 In addition, at least 1 limitation of carotid IMT use in this study bears direct relevance to the interpretation of the present findings; namely, carotid IMT may reflect medial hypertrophy among hypertensives.37 Given that B-Mode ultrasound measures do not distinguish between processes reflecting medial hypertrophy or intimal atherosclerosis, it is not possible to determine whether increased carotid IMT reflects preclinical atherosclerosis, adaptive medial thickening, or a combination of both processes among hypertensives. Thus, in the context of the present results, inferences regarding the relationship between exaggerated cardiovascular reactivity and carotid atherosclerosis (as reflected by increased carotid IMT) are necessarily limited. In addition, the lack of a statistically significant, unique association between measures of cardiovascular reactivity and carotid plaque occurrence in the present study casts further uncertainty on the interpretation of the findings for carotid IMT.
More specifically, if the presence of carotid plaque reflects a more clearly interpretable atherosclerotic endpoint compared with increased mean or maximum carotid IMT, then the correlation between exaggerated DBP reactivity and increased carotid IMT may represent a relationship that reflects atherosclerotic processes, hypertensive vascular remodeling, or possibly both. Moreover, although greater DBP reactivity was uniquely associated with increased mean and maximum carotid IMT, the present cross-sectional data do not exclude the possibility that processes related to increased carotid IMT (eg, vascular remodeling) may increase blood pressure reactivity.8 Thus, future prospective studies should determine the temporal relationship between variations in behaviorally evoked cardiovascular reactivity and carotid IMT among hypertensives.
Perspectives
This is the first study, to our knowledge, to evaluate the relationship between behaviorally evoked cardiovascular reactivity and indices of carotid atherosclerosis among untreated hypertensives. Although increased DBP reactivity to a battery of behavioral challenges was associated with increased mean and maximum carotid IMT after statistically controlling for other cardiovascular risk factors, this finding may not necessarily indicate that cardiovascular reactivity is associated with carotid atherosclerosis among hypertensives. Indeed, the greater carotid IMT that was accounted for by enhanced blood pressure reactivity may have reflected processes that are related to an altered vascular morphology (eg, medial hypertrophy) among our hypertensive sample rather than carotid atherosclerosis per se. Further, behaviorally evoked cardiovascular reactivity was not uniquely associated with a more specific atherosclerotic endpoint, carotid plaque occurrence. These results may suggest that behaviorally evoked cardiovascular reactivity is not likely to be a primary determinant of carotid atherosclerosis among hypertensives. On the other hand, however, our sampling strategy may have affected the strength of the statistical association between carotid atherosclerosis and behaviorally evoked cardiovascular reactivity. Specifically, the self-selected nature of our study participants and our eligibility criteria may have yielded a sample of hypertensives that was relatively healthier (as reflected by low smoking and alcohol consumption rates, a relatively high SES, and normal ranges of glucose and cholesterol levels) than are the hypertensive samples that are typically studied. Thus, it is possible that a stronger relationship between carotid atherosclerosis and behaviorally evoked cardiovascular reactivity could exist in a more representative sample of hypertensive individuals.
Acknowledgments
This research was supported by National Institutes of Health grants HL 40962 (S.B.M.) and HL 07560 (University of Pittsburgh). We thank Thomas W. Kamarck and Natasha Tokowicz for their comments on an earlier draft of this manuscript.
References
- 1.Clarkson TB, Kaplan JR, Adams MR, Manuck SB. Psychosocial influences on the pathogenesis of atherosclerosis among nonhuman primates. Circulation. 1987;76:I-29–I-40. [PubMed] [Google Scholar]
- 2.Kaplan JR, Pettersson K, Manuck SB, Olsson G. Role of sympathoadrenal medullary activation in the initiation and progression of atherosclerosis. Circulation. 1991;84:VI-23–VI-32. [PubMed] [Google Scholar]
- 3.Matthews KA, Owens JF, Kuller LH, Sutton-Tyrrell K, Lassila HC, Wolfson SK. Stress-induced pulse pressure change predicts women’s carotid atherosclerosis. Stroke. 1998;29:1525–1530. doi: 10.1161/01.str.29.8.1525. [DOI] [PubMed] [Google Scholar]
- 4.Kamarck TW, Everson SA, Kaplan GA, Manuck SB, Jennings JR, Salonen R, Salonen JT. Exaggerated blood pressure responses during mental stress are associated with enhanced carotid atherosclerosis in middle-aged Finnish men: findings from the Kuopio Ischemic Heart Disease Study. Circulation. 1997;96:3842–3848. doi: 10.1161/01.cir.96.11.3842. [DOI] [PubMed] [Google Scholar]
- 5.Everson SA, Lynch JW, Chesney MA, Kaplan GA, Goldberg DE, Shade SB, Cohen RD, Salonen R, Salonen JT. Interaction of workplace demands and cardiovascular reactivity in progression of carotid atherosclerosis: population based study. BMJ. 1997;314:553–558. doi: 10.1136/bmj.314.7080.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch JW, Everson SA, Kaplan GA, Salonen R, Salonen JT. Does low socioeconomic status potentiate the effects of heightened cardiovascular responses to stress on the progression of carotid atherosclerosis? Am J Public Health. 1998;88:389–394. doi: 10.2105/ajph.88.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnett PA, Spence JD, Manuck SB, Jennings JR. Psychological stress and the progression of carotid artery disease. J Hypertens. 1997;15:49–55. doi: 10.1097/00004872-199715010-00004. [DOI] [PubMed] [Google Scholar]
- 8.Krantz DS, Manuck SB. Acute psychophysiologic reactivity and risk of cardiovascular disease: a review and methodologic critique. Psychol Bull. 1984;96:435–464. [PubMed] [Google Scholar]
- 9.Su TC, Jeng JS, Chien KL, Sung FC, Hsu HC, Lee YT. Hypertension status is the major determinant of carotid atherosclerosis: a community-based study in Taiwan. Stroke. 2001;32:2265–2271. [PubMed] [Google Scholar]
- 10.Zanchetti A. Prevalence of carotid atherosclerosis in hypertension: preliminary baseline data from the European Lacidipine Study on Atherosclerosis (ELSA) Blood Press Suppl. 1996;4:30–35. [PubMed] [Google Scholar]
- 11.Lakka TA, Salonen R, Kaplan GA, Salonen JT. Blood pressure and the progression of carotid atherosclerosis in middle-aged men. Hypertension. 1999;34:51–56. doi: 10.1161/01.hyp.34.1.51. [DOI] [PubMed] [Google Scholar]
- 12.Pauletto P, Palatini P, Da Ros S, Pagliara V, Santipolo N, Baccillieri S, Casiglia E, Mormino P, Pessina AC. Factors underlying the increase in carotid intima-media thickness in borderline hypertensives. Arterioscler Thromb Vasc Biol. 1999;19:1231–1237. doi: 10.1161/01.atv.19.5.1231. [DOI] [PubMed] [Google Scholar]
- 13.Zanchetti A, Bond MG, Hennig M, Neiss A, Mancia G, Dal Palu C, Hansson L, Magnani B, Rahn KH, Reid J, Rodicio J, Safar M, Eckes L, Ravinetto R. Risk factors associated with alterations in carotid intima-media thickness in hypertension: baseline data from the European Lacidipine Study on Atherosclerosis. J Hypertens. 1998;16:949–961. doi: 10.1097/00004872-199816070-00008. [DOI] [PubMed] [Google Scholar]
- 14.Lemne C, Jogestrand T, de Faire U. Carotid intima-media thickness and plaque in borderline hypertension. Stroke. 1995;26:34–39. doi: 10.1161/01.str.26.1.34. [DOI] [PubMed] [Google Scholar]
- 15.Carroll D, Smith GD, Shipley MJ, Steptoe A, Brunner EJ, Marmot MG. Blood pressure reactions to acute psychological stress and future blood pressure status: a 10-year follow-up of men in the Whitehall II study. Psychosom Med. 2001;63:737–743. doi: 10.1097/00006842-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Light KC, Girdler SS, Sherwood A, Bragdon EE, Brownley KA, West SG, Hinderliter AL. High stress responsivity predicts later blood pressure only in combination with positive family history and high life stress. Hypertension. 1999;33:1458–1464. doi: 10.1161/01.hyp.33.6.1458. [DOI] [PubMed] [Google Scholar]
- 17.Matthews KA, Woodall KL, Allen MT. Cardiovascular reactivity to stress predicts future blood pressure status. Hypertension. 1993;22:479–485. doi: 10.1161/01.hyp.22.4.479. [DOI] [PubMed] [Google Scholar]
- 18.Fredrikson M, Matthews KA. Cardiovascular responses to behavioral stress and hypertension: a meta-analytic review. Ann Behav Med. 1990;12:30–39. [Google Scholar]
- 19.Manuck SB, Polefrone JM, Terrell DF, Muldoon MF, Kasprowicz AL, Waldstein SR, Jennings RJ, Malkoff SB, Marsland A, Graham RE. Absence of enhanced sympathoadrenal activity and behaviorally evoked cardiovascular reactivity among offspring of hypertensives. Am J Hypertens. 1996;9:248–255. doi: 10.1016/0895-7061(95)00303-7. [DOI] [PubMed] [Google Scholar]
- 20.Kamarck TW, Jennings JR, Debski TT, Glickman-Weiss E, Johnson PS, Eddy MJ, Manuck SB. Reliable measures of behaviorally evoked cardiovascular reactivity from a PC-based test battery: results from student and community samples. Psychophysiology. 1992;29:17–28. doi: 10.1111/j.1469-8986.1992.tb02006.x. [DOI] [PubMed] [Google Scholar]
- 21.Jennings JR, Kamarck T, Stewart C, Eddy M, Johnson P. Alternate cardiovascular baseline assessment techniques: vanilla or resting baseline. Psychophysiology. 1992;29:742–750. doi: 10.1111/j.1469-8986.1992.tb02052.x. [DOI] [PubMed] [Google Scholar]
- 22.Debski TT, Kamarck TW, Jennings JR, Young LW, Eddy MJ, Zhang YX. A computerized test battery for the assessment of cardiovascular reactivity. Int J Biomed Comput. 1991;27:277–289. doi: 10.1016/0020-7101(91)90068-p. [DOI] [PubMed] [Google Scholar]
- 23.Thompson T, Sutton-Tyrrell K, Wildman R. Continuous quality assessment programs can improve carotid duplex scan quality. J Vasc Tech. 2001;25:33–39. [Google Scholar]
- 24.Muldoon MF, Nazzaro P, Sutton-Tyrrell K, Manuck SB. White-coat hypertension and carotid artery atherosclerosis: a matching study. Arch Intern Med. 2000;160:1507–1512. doi: 10.1001/archinte.160.10.1507. [DOI] [PubMed] [Google Scholar]
- 25.Wallen NH, Held C, Rehnqvist N, Hjemdahl P. Effects of mental and physical stress on platelet function in patients with stable angina pectoris and healthy controls. Eur Heart J. 1997;18:807–815. doi: 10.1093/oxfordjournals.eurheartj.a015346. [DOI] [PubMed] [Google Scholar]
- 26.Manuck SB, Kaplan JR, Clarkson TB. Behaviorally induced heart rate reactivity and atherosclerosis in cynomolgus monkeys. Psychosom Med. 1983;45:95–108. doi: 10.1097/00006842-198305000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Gordon D, Guyton JR, Darnovsky MJ. Intimal alterations in rat aorta induced by stressful stimuli. Lab Invest. 1981;45:14–27. [PubMed] [Google Scholar]
- 28.Hirsch EZ, Maksem JA, Gagen D. Effects of stress and propranolol on the aortic intima of rates. Artereosclerosis. 1984;4:526. Abstract. [Google Scholar]
- 29.Heiss G, Sharrett R, Barnes R, Chambless LE, Szklo M, Alzola C for the ARIC Investigators. Carotid atherosclerosis measured by B-mode ultrasound in populations: associations with cardiovascular risk factors in the ARIC study. Am J Epidem. 1991;134:250–256. doi: 10.1093/oxfordjournals.aje.a116078. [DOI] [PubMed] [Google Scholar]
- 30.Blankenhorn DH, Hodis HN. Arterial imaging and atherosclerosis reversal. Aterioscler Thromb. 1994;14:177–192. doi: 10.1161/01.atv.14.2.177. [DOI] [PubMed] [Google Scholar]
- 31.Craven TE, Ryu JE, Espeland MA, Kahl FR, McKinney WM, Toole JF, McMahan MR, Thompson CJ, Heiss G, Crouse JR. Evaluation of the associations between carotid artery atherosclerosis and coronary artery stenosis: a case-control study. Circulation. 1990;82:1230–1242. doi: 10.1161/01.cir.82.4.1230. [DOI] [PubMed] [Google Scholar]
- 32.Hulthe J, Wikstrand J, Emanuelsson H, Wiklund O, de Feyter PJ, Wendelhag I. Atherosclerotic changes in the carotid artery bulb as measures by B-mode ultrasound are associated with the extent of coronary atherosclerosis. Stroke. 1997;28:1189–1194. doi: 10.1161/01.str.28.6.1189. [DOI] [PubMed] [Google Scholar]
- 33.Aronow WS, Ahn C, Schoenfeld MR, Gutstein H. Association of extracranial carotid arterial disease and chronic atrial fibrillation with the incidence of new thromboembolic stroke in 1846 older persons. Am J Cardiol. 1999;83:1403–1404. doi: 10.1016/s0002-9149(99)00107-1. [DOI] [PubMed] [Google Scholar]
- 34.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharret AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) study, 1987–1993. Am J Epidem. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 35.Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu C, Liu C, Azen SP. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–269. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 36.Bonithon-Kopp C, Touboul P-J, Berr C, Leroux C, Mainard F, Courbon D, Ducimetere P. Relation of intima-media thickness to atherosclerotic plaques in carotid arteries: the vascular aging (EVA) study. Arterioscler Thromb Vasc Biol. 1996;16:310–316. doi: 10.1161/01.atv.16.2.310. [DOI] [PubMed] [Google Scholar]
- 37.Lakka T, Salonen R, Kaplan GA, Salonen JT. Blood pressure and the progression of carotid atherosclerosis in middle-aged men. Hypertension. 1999;34:51–56. doi: 10.1161/01.hyp.34.1.51. [DOI] [PubMed] [Google Scholar]