Abstract
Roux-en-Y gastric bypass surgery (RYGB) decreases caloric intake in both human patients and rodent models. In long-term intake tests, rats decrease their preference for fat and/or sugar after RYGB, and patients may have similar changes in food selection. Here we evaluated the impact of RYGB on intake during a “cafeteria”-style presentation of foods to assess if rats would lower the percentage of calories taken from fat and/or sugar after RYGB in a more complex dietary context. Male Sprague-Dawley rats that underwent either RYGB or sham surgery (Sham) were presurgically and postsurgically given 8-days free access to four semisolid foods representative of different fat and sugar levels along with standard chow and water. Compared with Sham rats, RYGB rats took proportionally fewer calories from fat and more calories from carbohydrates; the latter was not attributable to an increase in sugar intake. The proportion of calories taken from protein after RYGB also increased slightly. Importantly, these postsurgical macronutrient caloric intake changes in the RYGB rats were progressive, making it unlikely that the surgery had an immediate impact on the hedonic evaluation of the foods and strongly suggesting that learning is influencing the food choices. Indeed, despite these dietary shifts, RYGB, as well as Sham, rats continued to select the majority of their calories from the high-fat/high-sugar option. Apparently after RYGB, rats can progressively regulate their intake and selection of complex foods to achieve a seemingly healthier macronutrient dietary composition.
Keywords: supermarket diet, bariatric surgery, diet-induced obesity, conditioned avoidance
roux-en-Y gastric bypass (RYGB) is an effective means by which to reduce excess body weight in the morbidly obese (e.g., 28). While many physiological changes induced by the operation may contribute to weight loss, there is strong evidence that patients eat less and have reduced appetite (e.g., 1, 13, 35). In some studies, RYGB patients report less frequent consumption of high-fat foods, like cheeses and red meats (e.g., 14); sweets, like soda and candy (e.g., 7, 11, 31); and other palatable mixtures of sugar and fat, such as ice cream (e.g., 2, 10, 30). While self-report measures in humans are vulnerable to inaccuracies (e.g., 9, 15, 23, 24, 33; also see 19), these results are mirrored in rat models of RYGB. After RYGB, rats lose body weight, consume fewer overall calories, and show blunted preferences for high-fat laboratory diets, as well as for sucrose solutions, the soybean oil emulsion Intralipid, and dietary supplements like Ensure that are high in fat and sugar (e.g., 3, 5, 14, 16, 22, 26, 27, 36, 37).
Although these data from rodent models of RYGB are compelling, the nutritive stimuli studied are not always representative of “real-world” choices from which patients will select their new diet. We addressed this in the current report by presenting rats before and after either RYGB or a sham operation with a “cafeteria” or “supermarket” diet, which consists of ad libitum access to many different palatable items (For reviews see 25, 34). In these types of studies, rats typically display preference for high-fat and/or high-sugar items and tend to exhibit hyperphagia and body weight gain when the foods are available. In the present study, the supplemental foods were selected based on their fat and sugar content to represent the categories of low fat/low sugar, low fat/high sugar, high fat/low sugar, and high fat/high sugar, while containing relatively similar proportional amounts of protein. We hypothesized that, after RYGB, rats would shift their preferences toward the foods with lower caloric densities and/or glycemic indexes (i.e., the extent to which foods impact blood glucose levels) and thus select proportionally fewer calories from fat and/or sugar.
MATERIALS AND METHODS
Animals.
A total of 30 adult male Sprague-Dawley rats (Charles River) were used across two study replicates. Each phase consisted of 15 rats that were 2.5 mo of age and ∼330 grams upon arrival. In each replicate, the rats were individually housed in polycarbonate tub cages lined with wood chip bedding (unless otherwise noted) in a temperature- and humidity-controlled vivarium with a 12-h automated light cycle. A stainless steel toy (Rattle A Round, Otto Environmental) that could not be ingested was provided at all times as environmental enrichment. The rats were provided ad libitum with standard rodent chow (PMI 5001) and deionized water unless otherwise noted. All procedures were approved by the Animal Care and Use Committee of Florida State University.
Diets.
In addition to standard chow presented ad libitum in hanging stainless steel hoppers, the rats were at times provided with access to refried beans (Winn-Dixie), low-fat vanilla yogurt (Winn-Dixie), creamy peanut butter (Winn-Dixie), and sugar-fat whip, which is a custom-made mixture of corn oil (Winn-Dixie), vegetable shortening (Winn-Dixie), powdered sugar (Winn-Dixie), and unflavored whey protein (Jarrow Formulas; see Table 1 for diet compositions). These foods were selected to represent food items prototypical of low-fat/low-sugar (beans), low-fat/high-sugar (yogurt), high-fat/low-sugar (peanut butter), and high-fat/high-sugar (sugar-fat whip) categories while also containing comparable proportions of protein. All foods were presented in 2- to 4-oz. glass jars suspended from a lip of the corner of the home cage by metal wire.
Table 1.
Caloric densities (kcal/g) and macronutrient proportions (g/kg and % kcal) of the diets used in the cafeteria diet choice provided to rats before and after Roux-en-Y gastric bypass or sham operations
| Diet | Caloric Densitya | Fat, g/kg;% kcal | Protein, g/kg;% kcal | Carbohydrates,b g/kg; % kcal | Sugar, g/kg;% kcal | Fiber, g/kg |
|---|---|---|---|---|---|---|
| Chow | 3.36 | 50 | 239 | 487 | 62 | 5.1 |
| 13.5 | 28 | 58 | 6.2 | |||
| Beans (Low fat/low sugar) | 1.0 | 18.7 | 59.7 | 156.7 | 7.5 | 5.2 |
| 16.2 | 23.1 | 60.6 | 2.9 | |||
| Yogurt (Low fat/high sugar) | 0.9 | 11.0 | 48.5 | 154.2 | 145.4 | 0 |
| 10.9 | 21.3 | 67.8 | 63.9 | |||
| Peanut Butter (High fat/low sugar) | 6.1 | 468.8 | 218.8 | 250 | 93.8 | 6.3 |
| 69.2 | 14.4 | 16.4 | 6.2 | |||
| Sugar-fat whipc (High fat/high sugar) | 5.8 | 369.6 | 196.3 | 250 | 399.0 | 0 |
| 57.3 | 13.5 | 29.2 | 27.5 |
Caloric density computed based on estimates of 9 kcal per fat gram, 4 kcal per carbohydrate and protein gram, and 0 kcal per fiber gram.
Includes sugar.
Sugar-fat whip is a wt/wt mixture of 17.4 g each of shortening and corn oil (9 kcal/g), 40.1 g powdered sugar (4 kcal/g), and 25.1 g whey protein (4.3 kcal/g).
Procedure.
After at least an 8-day acclimation period to the vivarium, the rats were given 4 days of access to a single preweighed jar of one of the 4 food items for a total of 16 consecutive days. During the first 4 days beans were offered, then yogurt for the next 4 days, then 4 days of peanut butter, and finally 4 days of sugar-fat whip. Chow and water remained present at all times. At the same time each morning when the vivarium lights were on, chow and the additional food item were weighed to measure intake and a new jar of the appropriate food was presented. On some occasions, the jars were weighed and refilled midday. The jars were rotated to each corner of the cage daily in a clockwise fashion to reduce location bias. These days of individually presented foods served as an acclimation period to reduce novelty.
On the 17th day after the start of procedures, the rats were offered jars of all of the food items simultaneously; chow and water were still available ad libitum. On each of 8 consecutive days, intake was determined by the difference between the onset and offset weights of each food and new jars with fresh food were presented. All of the jars were rotated around the cage as previously described. After the recovery period following surgery (described under Surgery; an average of 24 days after the first 8-day choice session), the rats were once again presented with all of the food items for 8 consecutive days.
Surgery.
Surgical groups were assigned such that total caloric intake and body weight did not differ between the groups across replicates. All of the rats underwent either RYGB (average presurgical body weight = 572 ± 6 g) or a sham operation (Sham; average presurgical body weight = 562 ± 9 g) as previously described (e.g., 3, 16, 17). Before surgical manipulations, the rats were acclimated for at least 24 h to stainless-steel mesh flooring; wood chip bedding was removed and replaced with cage board placed underneath the mesh flooring insert. The rats were also presurgically provided with the moist diets that were used during postoperative care to prevent complications. These diets consisted of wet mash, which is a ∼1:4 mixture of powdered chow and water, and a custom-made gel diet, which consisted of corn starch, whey powder, corn oil, gelatin, baby vitamins, and water (as per Ref. 16). Food was removed from the rats on the evening before surgery; a 10-g chow pellet ration was left to minimize postsurgical hunger, and water was available ad libitum.
On their day of surgery, each rat was anesthetized (2–5% isoflurane in 1 l/min oxygen), shaved and scrubbed from pelvis to sternum, and positioned on a heating pad under aseptic conditions. Midline incisions were made through the skin and abdominal muscles. For the RYGB, the upper jejunum was transected ∼7 cm from the ligament of Treitz and each end ligated, creating two stumps. The biliopancreatic limb was made via an anastomosis of the proximal stump and a portion of the distal jejunum ∼25 cm oral of the cecum. The alimentary limb and gastric pouch were constructed via an anastomosis of the distal stump and a ∼5-mm portion of stomach tissue that was separated from the stomach just aboral to the esophageal junction; the stomach remnant was sutured closed and remained continuous with the biliopancreatic limb. For the Sham rats, sutures were placed in all of the gastrointestinal areas where incisions and closures were made during the RYGB procedure. After either operation, the abdominal muscles and skin were individually closed, and saline (7 ml sc), antibiotic (Baytril; 2.5 mg/kg sc), and analgesic (carprofen; 5 mg/kg sc) were administered. Once each animal was fully mobile, it was returned to its home cage and provided with 5–10 g of the moist diets and water.
The appearance, body weights, and food and water intakes of the rats were monitored daily for at least 10 days after surgery. For each of 3 days after surgery, Baytril and carprofen (as above) were administered and saline was injected as needed. Sole provision of moist diets continued for at least 6 days in increasing amounts; solid chow pellets were reintroduced between days 7 and 11 after surgery. In total, Sham was performed on 9 rats, all of which survived, and RYGB was performed on 21, 8 of which were euthanized before the start of postsurgical testing. Further experimental procedures were not conducted until at least 18 days after surgery at which time the body weights of the RYGB rats had stabilized (average postsurgical body weight = 463 ± 11 g) and those of the Sham rats returned to presurgical values or higher (average postsurgical body weight = 575 ± 9 g). Two other RYGB rats suddenly lost body weight before the end of the postsurgical 8-day choice test and so they were excluded from the study since their health was in question.
Data analysis.
The percent change in body weight of the rats for 18 days after surgery was calculated based on their body weights on the day before surgery when they were ad libitum fed. Weight change progression and differences between the surgical groups (RYGB vs. Sham) were assessed via two-way mixed ANOVA with repeated measures on one factor.
The weights consumed from chow and each food item were measured to the nearest gram and converted to calories (see Table 1); caloric densities of the foods were calculated using the quantity of each macronutrient listed on the nutritional labels and the estimates of 9 kcal/g of fat, 4 kcal/g of carbohydrate (including sugar) and protein, and 0 kcal for fiber (see Table 1). Caloric density and macronutrient composition for chow was based on the physiological fuel value and percent values provided by LabDiets (12). The percentage of calories from each food item was calculated from the number of calories consumed of that food divided by the total caloric intake of each animal on each choice day. The percentage of total calories consumed from each macronutrient and sugar was calculated for each rat based on relative intake of the available food items and their respective macronutrient compositions (see Table 1).
A two-way mixed ANOVA with repeated measures on one factor was used to establish that there were no differences in caloric intake or percent caloric selections of each food item and macronutrient (plus sugar) between the planned surgical groups on days 7 and 8 of presurgical testing. Thus the average intake from days 7 and 8 of presurgical choice procedures of each rat was used as the baseline for that rat. The percent caloric intake of each food item and macronutrient during each postsurgical choice day for each rat was subtracted from its percent caloric intake during baseline to assess progressive postsurgical changes in food item and macronutrient selection. Thus each rat served as its own control, and all comparisons between surgical groups were based on the pre- to postsurgical changes of each individual rat. Differences in these values between the surgical groups (RYGB vs. Sham) and across postsurgical days for each of the five food item choices (chow, beans, yogurt, peanut butter, sugar-fat whip) or the three primary macronutrients (fat, protein, and carbohydrates), as well as sugar, were assessed individually via two-way mixed ANOVA with repeated measures on one factor. When necessary, one-way ANOVA with repeated measures were conducted to assess within-group differences across days. Independent t-tests were used to compare the surgical groups on individual days. Bonferroni-corrected values of P ≤ 0.05 were considered statistically significant, but uncorrected values are also provided in the figures.
RESULTS
Presurgical total caloric consumption and percent caloric distribution (baseline).
Presurgically, there were no differences between the planned surgical groups in terms of total (see Table 2; Fig. 1B) or percent calories taken from any of the individual foods (all P values ≥ 0.15) or from the macronutrients or sugar (all P values ≥ 0.35) during the baseline period.
Table 2.
Calories consumed of each food item and each macronutrient category (plus sugar individually) by the groups during presurgical baseline testing (i.e., the average of days 7 and 8) and the first and last days of postsurgical testing
| RYGB |
Sham |
|||||
|---|---|---|---|---|---|---|
| Pre-Sx BL | Post-Sx D1 | Post-Sx D8 | Pre-Sx BL | Post-Sx D1 | Post-Sx D8 | |
| Chow | 9.0 ± 1.3 | 0.3 ± 0.3 | 7.6 ± 3.3 | 9.3 ± 2.0 | 0.7 ± 0.7 | 12.3 ± 3.6 |
| Beans | 9.8 ± 0.9 | 17.7 ± 2.5 | 22.7 ± 2.8 | 9.6 ± 2.1 | 34.0 ± 2.6 | 11.0 ± 2.8 |
| Yogurt | 27.7 ± 2.7 | 14.5 ± 1.3 | 26.5 ± 5.4 | 28.3 ± 3.2 | 22.4 ± 1.8 | 27.1 ± 3.6 |
| Peanut butter | 35.2 ± 4.4 | 15.5 ± 3.3 | 16.1 ± 5.1 | 39.0 ± 6.4 | 43.4 ± 4.7 | 31.9 ± 5.1 |
| Sugar-fat whip | 48.0 ± 3.1 | 47.5 ± 4.3 | 32.7 ± 5.2 | 42.2 ± 4.9 | 63.8 ± 5.2 | 66.4 ± 8.2 |
| Fat | 57.7 ± 3.2 | 42.4 ± 3.6 | 37.5 ± 3.8 | 57.0 ± 5.9 | 67.5 ± 7.8 | 66.5 ± 4.1 |
| Protein | 22.2 ± 0.7 | 15.9 ± 1.1 | 19.7 ± 0.9 | 22.2 ± 1.7 | 27.7 ± 1.4 | 25.3 ± 1.8 |
| Carbohydrates | 49.7 ± 1.7 | 37.1 ± 2.4 | 48.4 ± 2.6 | 49.2 ± 3.9 | 62.0 ± 3.3 | 56.8 ± 4.5 |
| Sugar | 33.9 ± 1.5 | 23.8 ± 1.4 | 28.1 ± 2.8 | 33.0 ± 2.3 | 35.6 ± 2.1 | 38.6 ± 3.4 |
Values are means ± SE in kcalories. RYGB, Roux-en-Y gastric bypass; SX, surgery; BL, baseline. D1, day 1; D8, day 8.
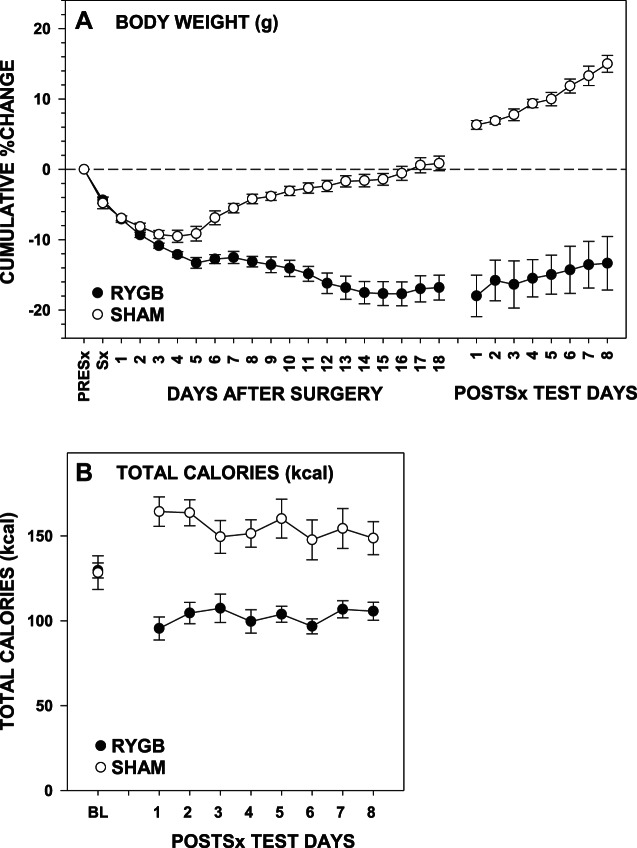
Fig. 1.
A: percent change in body weight (means ± SE) from the day before surgery (PreSx) for rats that received either Roux-en-Y gastric bypass surgery (RYGB) or a sham operation (Sham) across 18 postsurgical recovery days and across the 8 days during which postsurgical testing was conducted. B: total caloric intake (means ± SE) from presurgical baseline and daily during the 8-day postsurgical choice testing between the surgical groups.
Postsurgical body weight change and caloric intake.
By the end of 18 days of recovery from surgery, RYGB rats weighed significantly less than Sham rats [F(1,18) = 93.95, P < 0.001; Fig. 1A] and, across postsurgical testing, continued to weigh less [F(1,18) = 110.187, P < 0.001] and consumed fewer total calories than did Sham rats [F(1,18) = 44.20, P < 0.001; Fig. 1B].
Postsurgical change in intake from macronutrients.
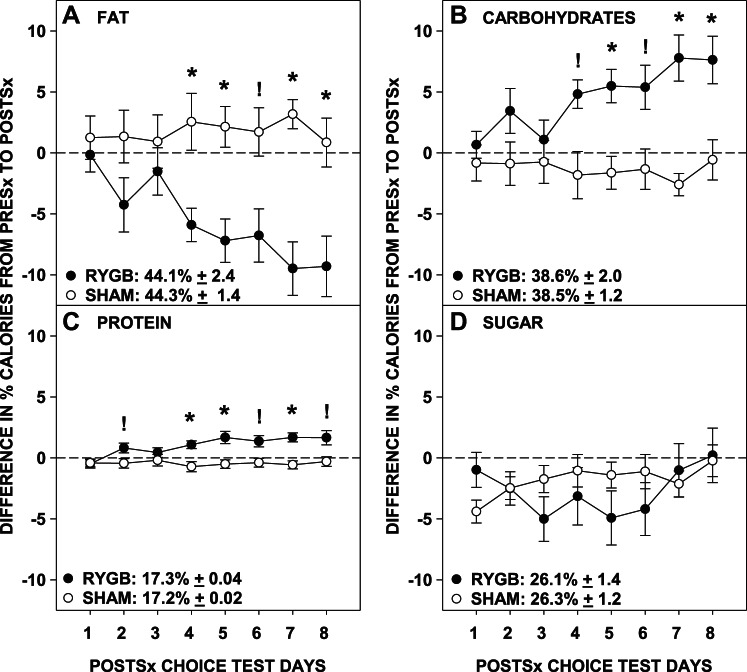
As hypothesized, RYGB rats postsurgically selected fewer proportional calories from fat than did Sham rats (Table 3; Fig. 2A). The proportional caloric intakes from fat decreased across days in the RYGB group [F(7,70) = 7.06, P < 0.001], but not in the Sham group [F(7,56) = 0.54, P = 0.80]. Interestingly, it was not until the fourth day of the postsurgical choice diet that the percent caloric intake from fat of the RYGB rats was lower than that of Sham rats (before days 4 and 6 Bonferroni-corrected P ≥ 0.72; days 4, 5, 7, and 8 Bonferroni-corrected P ≤ 0.05).
Table 3.
Results of the two-way ANOVAs demonstrating postsurgical changes in percent caloric intake from presurgical baseline of each macronutrient between the surgical groups (Sx Grp: Roux-en-Y gastric bypass,or sham operation) and across postsurgical days
| ANOVA Factor | Fat | Protein | Carbohydrates | Sugar |
|---|---|---|---|---|
| Sx Grp | F(1,18) = 10.19, P = 0.005 | F(1,18) = 9.36, P = 0.006 | F(1,18) = 9.71, P = 0.006 | F(1,18) = 0.21, P = 0.66 |
| Day | F(7,126) = 3.13, P = 0.003 | F(7,126) = 4.15, P < 0.001 | F(7,126) = 2.77, P = 0.01 | F(7,126) = 1.73, P = 0.11 |
| Day × Sx Grp | F(7,126) = 4.95, P < 0.001 | F(7,126) = 5.14, P < 0.001 | F(7,126) = 4.62, P < 0.001 | F(7,126) = 2.37, P = 0.028 |
Fig. 2.
Difference between the presurgical baseline (average of days 7 and 8; absolute values displayed in the legend) and the daily postsurgical percentage of calories (means ± SE) selected from fat (A), carbohydrates (B), protein (C), and sugar (D) by rats that received either Roux-en-Y gastric bypass surgery (RYGB) or a sham operation (Sham). *Signifies Bonferroni-corrected and ! signifies uncorrected P ≤ 0.05 of t-tests comparing the surgical groups on individual days following significant 2-way ANOVA interactions.
In almost the exact mirror image with the results with fat, RYGB rats selected higher proportions of calories from carbohydrates than Sham rats (Table 3; Fig. 2B). The proportional caloric intakes from carbohydrates by RYGB rats increased across days [F(7,70) = 6.52, P < 0.001], but the intakes by Sham rats remained stable across days [F(7,56) = 0.54, P = 0.80]. As with fat, differences did not emerge between surgical groups until day 5 (before day 5 and on day 6 Bonferroni-corrected P ≥ 0.88; days 5, 7, and 8 Bonferroni-corrected P ≤ 0.047). In contrast to the results assessing total carbohydrate selection, RYGB rats tended to have lower proportional caloric intakes from sugar compared with Sham rats (Table 3; Fig. 2D), but the proportional intake of neither group changed across days (both P values ≥ 0.13). There was, however, a significant group × day interaction (P = 0.028) for the proportional caloric intake from sugar, but analysis of between-group differences on each day failed to detect any significant effect, in part, due to the conservative Bonferroni-correction (all Bonferroni-corrected P ≥ 0.49).
After RYGB, rats also displayed higher proportional caloric intakes from protein than did Sham rats (Table 3; Fig. 2C). The proportional caloric intakes from protein changed across days only for RYGB rats [F(7,70) = 7.63, P < 0.001; Sham: F(7,56) = 0.58, P = 0.77] and was higher than Sham rats on days 4, 5, and 7 (Bonferroni-corrected P ≤ 0.023; other days Bonferroni-corrected P ≥ 0.32).
Postsurgical change in intake from diet types.
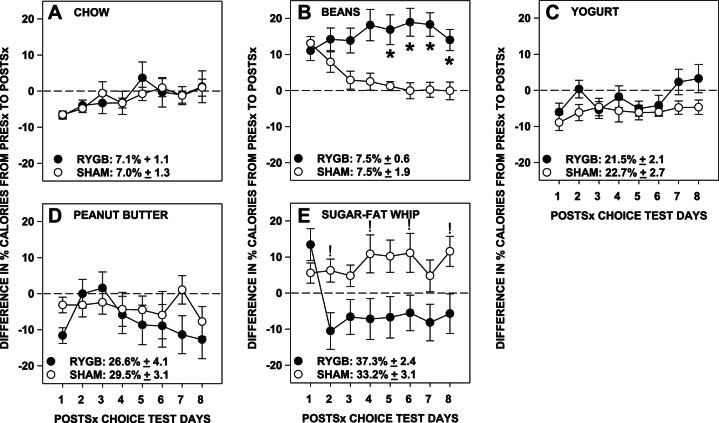
Compared with their presurgical baseline intakes, RYGB rats took proportionally fewer calories from sugar-fat whip postsurgically than did Sham rats (Table 4; Fig. 3E). Although daily differences between the change in percent intakes between RYGB and Sham rats did not pass Bonferroni correction (all P values ≥ 0.13), the proportional consumption of sugar-fat whip by the RYGB rats decreased across days [F(7,70) = 6.79, P < 0.001], whereas those of Sham rats remained stable [F(7,56) = 1.03, P = 0.42].
Table 4.
Results of the two-way ANOVAs demonstrating postsurgical changes in percent caloric intake from presurgical baseline of each diet between surgical groups (Sx Grp: Roux-en-Y gastric bypass or sham operation) and across postsurgical days
| ANOVA Factor | Chow | Beans | Yogurt | Peanut Butter | Sugar-Fat Whip |
|---|---|---|---|---|---|
| Sx Grp | F(1,18) = 0.003, P = 0.95 | F(1,18) = 10.87, P = 0.004 | F(1,18) = 2.20, P = 0.16 | F(1,18) = 0.44, P = 0.52 | F(1,18) = 5.08, P = 0.03 |
| Day | F(7,126) = 4.43, P < 0.001 | F(7,126) = 1.57, P = 0.15 | F(7,126) = 2.38, P = 0.03 | F(7,126) = 2.39, P = 0.03 | F(7,126) = 3.17, P = 0.003 |
| Day × Sx Grp | F(7,126) = 0.57, P = 0.78 | F(7,126) = 7.75, P < 0.001 | F(7,126) = 1.28, P = 0.26 | F(7,126) = 1.78, P = 0.10 | F(7,126) = 4.37, P < 0.001 |
Fig. 3.
Difference between the presurgical baseline (average of days 7 and 8; absolute values displayed in the legend) and the daily postsurgical percentage of calories (means ± SE) selected from chow (A), refried beans (B), low-fat vanilla yogurt (C), creamy peanut butter (D), and sugar-fat whip (E) by rats that received either Roux-en-Y gastric bypass surgery (RYGB) or a sham operation (Sham). *Signifies Bonferroni-corrected and ! signifies uncorrected P ≤ 0.05 of t-tests comparing the surgical groups on individual days following significant 2-way ANOVA interactions.
On the other hand, RYGB rats took proportionally more calories from beans than did Sham rats (Table 4; Fig. 3B). Further analysis showed that the proportional caloric intakes from beans by Sham rats decreased across days [F(7,56) = 12.91, P < 0.001], whereas that of RYGB rats remained relatively stable [F(7,70) = 1.93, P = 0.56]. Relative to their individual baselines, RYGB rats took more proportional calories from beans than Sham rats on days 5–8 (Bonferroni-corrected P ≤ 0.03; days 1-4: Bonferroni-corrected P ≥ 0.06).
The surgical groups did not differ in the proportion of calories taken postsurgically compared with their presurgical baseline for chow, yogurt, or peanut butter (Table 4; Fig. 3, A, C, and D).
DISCUSSION
We have shown here that, when provided with a “cafeteria diet,” not only do rats eat less after RYGB, but they also decrease the percentage of their daily calories taken from fat. At the same time, these rats conversely increase the percentage of calories they take from carbohydrate and, to a lesser extent, protein. The increase in relative caloric intake of carbohydrate could not be explained by an increase in the percent calories taken from sugars because, if anything, the latter tended to decrease. These changes in relative macronutrient caloric intake in RYGB rats were driven primarily by a significant decrease in the percent calories taken from sugar-fat whip (high-fat/high-sugar item) and a relatively stable (or trend toward increased) percent caloric intake taken from beans (low-fat/low-sugar item).
The present results are reminiscent of the decreased fat preferences seen when RYGB rats are presented with a normally preferred high-fat diet (typically a complete diet containing 60% fat such as Research Diets D12492; 14, 22, 26, 27, 37), a pure fat solid or fluid (such as corn oil or Intralipid; 14, 16, 17, 26, 36), or a full-nutrition sweet and fatty beverage (Ensure; 5, 16, 17, 26, 37) after surgery. These animals also choose to consume proportionally greater amounts of normal chow or other low-fat/low-sugar choices (e.g., water, V8 vegetable drink) than sham-operated rats. While these reports show that fat and/or sugar preferences by rats decrease after RYGB, the values never drop much below indifference and are often still above 50%. Here it is notable that despite decreased overall fat intakes by the RYGB rats, in all cases sugar-fat whip remained the most preferred way for rats in either surgical group to procure calories. The typical pattern by which rats preferentially consumed calories before surgery, and of Sham rats after surgery, was the following: sugar-fat whip > peanut butter > yogurt > beans > chow, whereas the postsurgical caloric preference of RYGB rats shifted to sugar-fat whip > beans > yogurt ≥ peanut butter > chow. Thus while the RYGB rats ate fewer proportional calories from fat, they by no means completely avoided the high-fat, high-sugar supplement and, numerically speaking, still continued to prefer getting calories from it compared with all the other options.
Importantly, these RYGB-induced changes in diet selection took time to develop. The decrease in proportional fat calories and increase in proportional carbohydrate and protein calories taken by RYGB rats was progressive. Indeed, RYGB rats consumed calorically comparable proportions of sugar-fat whip to that of Sham rats on the first day of its presentation. This is akin to a study by Saeidi et al. (22) in which there was an initial increase in high-fat diet consumption by RYGB rats in their first meal relative to Sham rats, but, by the end of 24 h, the high-fat diet preference of the RYGB rats had dropped to 51% that observed in Sham rats. We have also shown that RYGB rats will consume sucrose, Intralipid, or Ensure in quantities similar to that of Sham rats during the first meal of a 60-min session on the first postsurgical day of access but then show lower intakes for the remainder of the session and/or in subsequent sessions (17). Collectively, these results suggest that preference for and selection of both complete-nutrition and sole-macronutrient foods and fluids is altered by RYGB, but these changes require time to emerge, suggesting that they are learned. It should be pointed out that measures were taken more than 2 wk after surgery, at which point the body weights of the RYGB rats had stabilized (albeit at an expectedly lower weight than Sham rats), and different results may have been obtained if the five food items were presented sooner after surgery during the dynamic phase of weight loss.
It has been postulated that changes in the preference for certain foods and fluids after RYGB in rats or humans is due to altered taste sensibility; for example, sweet foods taste sweeter, even to the point of being cloying (e.g., 6, 29), and are thus consumed in smaller amounts. Indeed, the detectability of sugar is heightened after RYGB in humans, but this does not seem to translate to a reduced hedonic value of sweet foods and fluids (3, 4). Also, the results of brief-access tests or examinations of early-meal lick patterns, in which the postingestive consequences of nutritive stimuli are limited, are not always indicative of blunted avidity for sucrose or Intralipid by RYGB rats (14, 17, 18; though see 8, 27, 32). More to the point, the motivation to complete an operant work demand in a progressive ratio task is not decreased by RYGB in rats when sucrose or Intralipid are used as reinforcers, even after these rats displayed blunted and/or progressively decreasing preference for these stimuli in long-term tests (16).
Overall, we have shown that, after RYGB, rats will reduce the proportion of their daily intake that they select from fat, and they will instead select larger proportional amounts of carbohydrates and protein. Indeed, even when exposed to multiple complex food choices, the rats were able to adjust their overall intakes to limit fat consumption. However, they do not completely exclude fatty and/or sweet foods from their diet, even despite the many other choices available. In fact, RYGB rats, like Sham rats, still take the majority of their calories from the high-fat/high-sugar option, showing that RYGB rats still find this food item quite palatable. Of course, other factors may be influencing selection such as water and/or micronutrient content, as well as the textures of the diets, and these factors deserve further systematic examination. Furthermore, all the rats had preexposure to the various foods before surgery, thus it is not possible to determine whether food selection would have been different if the food items were presented for the first time after surgery. Previously, it has been shown that the patterns of acceptance of high-fat diet when presented for the first time after surgery are very different than if the rats had preexposure to the same high-fat diet (26). Regardless, the diet-selection changes seen here do not occur as soon as the diets are presented after surgery, but rather are progressive, which strongly suggests that RYGB rats are learning about the changed postingestive consequences of the diets, positive or negative, resulting from the gut remodeling associated with RYGB. Once the new learning is established, the RYGB rats alter their selections accordingly, but do not explicitly avoid palatable, or even calorically dense, foods. Thus, while learning is certainly implicated in the dietary choices made after RYGB in rats, it does not appear to be consistent with the development of a conditioned taste aversion.
Perspectives and Significance
The clear and systematic change in relative macronutrient intake over time after RYGB in rats in the face of complex diet choices raises the question of mechanism: how does this occur? The search for an answer, in any comprehensive way, requires at least two approaches.
First, the behavioral mechanism must be better understood. Toward this end, it will be important to evaluate how these intake outcomes are achieved behaviorally via the detailed monitoring of the feeding patterns of rats as they eat throughout the day. This will place the development of macronutrient intake changes in a language of behavior: the actual output that neural circuits generate. In addition, the application of complementary behavioral techniques will be needed to further evaluate whether the observed changes in food selection represent a fundamental conditioned change in the palatability of particular flavor cues, a characteristic feature of taste aversion learning. Although the profile of results, as noted above, does not obviously lend itself to such an interpretation, more systematic interrogation involving simpler and highly controlled presentations of sugar and fat stimuli and the measurement of oral motor acceptance and rejection responses (referred to as taste reactivity) that are widely accepted as a measure of taste stimulus palatability would shed significant light on this issue. If not conditioned taste aversion, then the type of learning demonstrated would likely be more akin to what is referred to as conditioned taste avoidance, in which animals decrease their intake of a taste stimulus via a learned association with the onset of discomfort, but without a fundamental change in the hedonic characteristics of the taste (see 21, 22). While true that both conditioned taste aversion and conditioned taste avoidance lead to decreased intake of a specific stimulus as a result of learning, they represent two distinct processes.
Second, the physiological mechanisms must be better understood: what are the consequences of the ingestion of specific food items and macronutrients after RYGB that lead to the changes in diet selection? As with the question of behavioral mechanism, there are various ways to investigate the physiology subserving the behavior. For example, it is well known that there is both a constituitive and postprandial increase in a variety of gut hormones, most notably including those implicated in satiation such as GLP-1 and PYY, and these might play a role. There is the possibility of neural signals, via the vagus or splanchnic routes, contributing as well. These are not mutually exclusive possibilities. Of course, how the brain integrates all of these signals along with the orosensory features of the food stimulus to ultimately effect a behavioral readout after RYGB is a key question.
Finally, it will be important to determine through direct measurement whether the basic phenomenon described here in rodents is evident in the clinic. A survey of the literature suggested that changes in relative macronutrient intake after RYGB are not common and are transient (19). However, virtually all of what is known about food choice after RYGB in humans is based on verbal report, such as dietary recall. While these techniques provide some insight, they are vulnerable to inaccuracy (e.g., 9, 15, 23, 24, 33). It would be instructive to measure food choice and intake directly before and after RYGB and track this behavior over time to determine the extent that changes might exist and to evaluate the role of experience.
GRANTS
This work was supported by NIH R21-DC012751 to A. C. Spector; C. W. le Roux is supported by Science Foundation Ireland ref 12/YI/B2480.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.M.M., C.W.l.R., and A.C.S. conception and design of research; C.M.M., C.L., and G.D.B. performed experiments; C.M.M. and G.D.B. analyzed data; C.M.M., C.W.l.R., and A.C.S. interpreted results of experiments; C.M.M. prepared figures; C.M.M. and A.C.S. drafted manuscript; C.M.M., G.D.B., C.W.l.R., and A.C.S. edited and revised manuscript; C.M.M., C.L., G.D.B., C.W.l.R., and A.C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
C. M. Mathes's current affiliation is the Department of Psychology and Program in Neuroscience, Baldwin Wallace University, Berea, OH.
REFERENCES
- 1.Borg CM, le Roux CW, Ghatei MA, Bllom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. B J Surg 93: 210–215, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Brolin RE, Robertson LB, Kenler HA, Cody RP. Weight loss and dietary intake after vertical banded gastroplasty and roux-en-y gastric bypass. Ann Surg 220: 782–790, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bueter M, Miras AD, Chichger H, Fenske W, Ghatei MA, Bloom SR, Unwin RJ, Lutz TA, Spector AC, le Roux CW. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol Behav 104: 709–721, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Burge JC, Schaumburg JZ, Choban PS, DiSilvestro RA, Flancbaum L. Changes in patients' taste acuity after Roux-en-Y gastric bypass for clinically severe obesity. J Am Diet Assoc 95: 666–670, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Chelikani PK, Shah IH, Taqi E, Sigalet DL, Koopmans HH. Comparison of the effects of Rpoux-en-Y gastric bypass and ileal transposition surgeries on food intake, body weight, and circulating peptide YY concentrations in rats. Obes Surg 20: 1281–1288, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Elliot K. Nutritional considerations after bariatric surgery. Crit Care Nurs Q 26: 133–138, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Ernst B, Thurnheer M, Wilms B, Schultes B. Differential changes in dietary habits after gastric bypass versus gastric banding operations. Obes Surg 19: 274–280, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Hajnal A, Kovacs P, Ahmed T, Meirelles K, Lynch CJ, Cooney RN. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol Gastrointest Liver Physiol 299: G967–G979, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hills RJ, Davies PS. The validity of self-reported energy intake as determined using the doubly labeled water technique. Br J Nutr 85: 415–430, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Kenler HA, Brolin RE, Cody RP. Changes in eating behavior after horizontal gastroplasty and Roux-en-Y gastric bypass. Am J Clin Nutr 52: 87–92, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Kruseman M, Leimgruber A, Zumbach F, Golay A. Dietary, weight, and psychological changes among patients with obesity, 8 years after gastric bypass. J Am Diet Assoc 110: 527–534, 2010. [DOI] [PubMed] [Google Scholar]
- 12.LabDiet. Laboratory Rodent Diet http://www.labdiet.com/cs/groups/lolweb/@labdiet/documents/web_content/mdrf/mdi4/∼edisp/ducm04_028021.pdf 2014. December 18, p. 1.
- 13.Laurenius A, Larsson I, Bueter M, Melanson KJ, Bosaeus I, Forslund HB, Lonroth H, Fandriks L, Olbers T. Changes in eating behavior and meal pattern following Roux-en-Y gastric bypass. Int J Obes 36: 348–355, 2012. [DOI] [PubMed] [Google Scholar]
- 14.le Roux CW, Bueter M, Theis N, Werling M, Ashradian H, Lowenstein C, Athanasiou T, Bloom SR, Spector AC, Olbers T, Lutz TA. Gastric bypass reduces fat intake and preference. Am J Physiol Regul Integr Comp Physiol 301: R1057–R1066, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livingstone MB, Black AE. Markers of the validity of reported energy intake. J Nutr 133: S895–S920, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Mathes CM, Bohnenkamp RA, Cortevillie C, Bueter M, Lutz TA, le Roux CW, Spector AC. Gastric bypass in rats does not decrease appetitive behavior towards fatty fluids despite at times blunting preferential intake of fat. Physiol Behav 142: 179–188, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathes CM, Bohnenkamp RA, le Roux CW, Spector AC. Reduced sweet and fatty fluid intake after Roux-en-Y gastric bypass in rats is dependent on experience without change in stimulus motivational potency. Am J Physiol Regul Integr Comp Physiol 309: R864–R874, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathes CM, Bueter M, Smith KR, Lutz TA, le Roux CW, Spector AC. Roux-en-Y gastric bypass in rats increases sucrose taste-related motivated behavior independent of pharmacological GLP-1 receptor modulation. Am J Physiol Regul Integr Comp Physiol 302: R751–R767, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathes CM, Spector AC. Food selection and taste changes in humans after Roux-en-Y gastric bypass: a direct-measures approach. Physiol Behav 107: 476–483, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Parker LA. Taste avoidance and taste aversion: evidence for two different processes. Learn Behav 31: 165–172, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Pelchat ML, Grill HJ, Rozin P, Jacobs J. Quality of acquired responses to tastes by Rattus norvegicus depends on type of associated discomfort. J Comp Psychol 97: 140–153, 1983. [PubMed] [Google Scholar]
- 22.Saeidi N, Nestoridi E, Kucharczyk J, Uygun MK, Yarmush ML, Stylopoulos N. Sleeve gastrectomy and Roux-en-Y gastric bypass exhibit differential effects on food preferences, nutrient absorption and energy expenditure in rats. Int J Obes 36: 1396–1402, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoeller DA. Limitations in the assessment of dietary energy intake by self-report. Metabolism 44: 18–22, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Schoeller DA. Validation of habitual energy intake. Public Health Nutr 5: 883–888, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Sclafani A. Psychobiology of food preferences. Int J Obes Relat Disord 25: S13–S16, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Seyfried F, Miras AD, Bueter M, Prechtl CG, Spector AC, le Roux CW. Effects of preoperative exposure to a high-fat versus a low-fat diet on ingestive behavior after gastric bypass surgery in rats. Surg Endos 27: 4192–4201, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin AC, Zheng H, Pistell PJ, Berthoud HR. Roux-en-Y gastric bypass surgery changes food reward in rats. Int J Obes (Lond) 35: 642–651, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial-a prospective controlled intervention study of bariatric surgery. J Intern Med 273: 219–234, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Sugerman HJ, Starkey JV, Birkenhauser R. A randomized prospective trial of gastric bypass versus vertical banded gastroplasty for morbid obesity and their effects on sweets versus non-sweets eaters. Ann Surg 205: 613–622, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas JR, Gizis F, Marcus E. Food selections of Roux-en-Y gastric bypass patients up to 2.5 years postsurgery. J Am Diet Assoc 110: 608–612, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Thomas JR, Marcus E. High and low fat food selection with reported frequency intolerance flowing Roux-en-Y gastric bypass. Obes Surg 18: 282–287, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Tichansky DS, Glatt AR, Madan AK, Harper J, Tokita K, Boughter JD. Decrease in sweet taste in rats after gastric bypass surgery. Surg Endosc 25: 1176–1181, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Trabulsi J, Schoeller DA. Evaluation of dietary assessment instruments against doubly labeled water, a biomarker of habitual energy intake. Am J Physiol Endocrinol Metab 281: E891–E899, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Warwick ZS, Schiffman SS. Role of dietary fat in calorie intake and weight gain. Neurosci Biobehav Rev 16: 585–596, 1992. [DOI] [PubMed] [Google Scholar]
- 35.Werling M, Fandriks L, Bjorklund P, Maleckas A, Brandberg J, Lonroth H, le Roux CW, Olbers T. Long-term results of a randomized clinical trial comparing Roux-en-Y gastric bypass with vertical banded gastroplasty. Br J Surg 100: 222–230, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Wilson-Perez HE, Chambers AP, Sandoval DA, Stefater MA, Woods SC, Benoit SC, Seeley RJ. The effect of vertical sleeve gastrectomy on food choice in rats. Int J Obes (Lond) 37: 288–295, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL, Berthoud HR. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol 297: R1273–R1282, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]