Abstract
Rising temperatures resulting from climate change will increase the incidence of heat stress, negatively impacting the labor force and food animal production. Heat stress elevates circulating β-OH butyrate, which induces vasodilation through GPR109a. Interestingly, both heat stress and intraperitoneal β-OH butyrate administration induce hypophagia. Thus, we aimed to investigate the role of β-OH butyrate in heat stress hypophagia in mice. We found that niacin, a β-OH butyrate mimetic that cannot be oxidized to generate ATP, also reduces food intake. Interestingly, the depression in food intake as a result of 8-h intraperitoneal niacin or 48-h heat exposure did not result from changes in hypothalamic expression of orexigenic or anorexigenic signals (AgRP, NPY, or POMC). Genetically eliminating GPR109a expression did not prevent the hypophagic response to heat exposure, intraperitoneal β-OH butyrate (5.7 mmol/kg), or niacin (0.8 mmol/kg). Hepatic vagotomy eliminated the hypophagic response to β-OH butyrate and niacin but did not affect the hypophagic response to heat exposure. We subsequently hypothesized that the hypophagic response to heat stress may depend on direct effects of β-OH butyrate at the central nervous system: β-OH butyrate induced hormonal changes (hyperinsulinemia, hypercorticosteronemia, and hyperleptinemia), or gene expression changes. To test these possibilities, we blocked expression of hepatic hydroxyl methyl glutaryl CoA synthase II (HMGCS2) to prevent hepatic β-OH butyrate synthesis. Mice that lack HMGCS2 maintain a hypophagic response to heat stress. Herein, we establish that the hypophagia of heat stress is independent of GPR109a, the hepatic vagus afferent nerve, and hepatic ketone body synthesis.
Keywords: heat stress, hyperketonemia, β-OH butyrate, niacin, GPR109a
rising temperatures during summer months have reduced labor capacity ∼10% in just three decades (9). The heat-related decrease in labor capacity is expected to reach 20% in 2050 (9). In the United States, heat stress is directly related to 400 deaths/year and increases the severity of another 1,800 illnesses per year (2, 6). An increase in average temperature by 2.8°C has been estimated to increase heat-related deaths by 2,000 (4). The 2-wk 2006 heat wave resulted in more than 16,000 heat-related emergency room visits in California alone (20). Given the decreased productivity of the work force and increased mortality associated with rising temperatures, it is essential to understand heat stress-mediated physiological adaptations. Currently, the primary means to prevent heat-related incidents is environmental control (8).
Heat stress also limits profitability of U.S. animal agriculture, costing approximately $2.4 billion each year (38). Heat abatement strategies, only applicable in intensive production systems, have decreased the losses associated with heat stress, but have not successfully eliminated the annual losses of $897 million, $369 million, $299 million, and $128 million for the U.S. dairy, beef, swine, and poultry industries, respectively (38). Most of these losses are associated with decreases in feed intake. Heat stress-induced hypophagia decreases growth in cattle, swine, and poultry (13, 15, 29). In cattle and swine, this decrease in growth is fully attributed to hypophagia (29, 36).
A better understanding of physiological changes that accompany heat stress may allow for pharmacological interventions that limit the severity of heat-related illnesses and animal production losses. Heat stress is characterized by hypophagia, increased energy expenditure (expressed per unit of body mass), and hyperketonemia (1, 26, 34, 42). By acting at GPR109a, β-OH butyrate induces vasodilation and inhibits adipose tissue lipolysis (30, 40). Thus, the hyperketonemia during heat stress may be an adaptive response to encourage heat dissipation through the vasodilation that accompanies β-OH butyrate agonism of GPR109a.
Because intraperitoneal β-OH butyrate induces hypophagia (23), we hypothesized that the hypophagia of heat stress was also mediated by β-OH butyrate. Because the liver is the primary source of β-OH butyrate production, and the hepatic vagus nerve mediates the hypophagic response to β-OH butyrate, we hypothesized that the hypophagic effects of heat stress are initiated at the liver and communicated through the vagal nerve to the central nervous system. To completely test the potential role of β-OH butyrate in the hypophagia of heat stress, we tested the response to heat stress in GPR109a-null mice, hepatic vagotomized mice, and mice that are unable to mount a ketogenic response.
MATERIALS AND METHODS
Animals.
Twelve- to fourteen-week-old male C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were acclimated to the new environment for at least 1 wk prior to any food intake studies being initiated. GPR109a-null mice were kindly provided by Dr. Klaus Pfeffer at the Institute of Medical Microbiology, Immunology and Hygiene at Heinrich Heine University (41). GPR109a +/+ and −/− siblings were derived by crossing GPR109a +/− mice. Until study initiation, mice were housed at three or four mice per cage in a 14:10-h light-dark cycle and given ad libitum access to NIH-31 chow (Harlan Laboratories, Indianapolis, IN) and water. All studies were approved by the Institutional Animal Use and Care Committee at the University of Arizona.
Hepatic vagotomy surgery.
Surgeries were performed in 12-wk-old male C57BL/6J mice under isoflurane anesthesia. A ventral midline incision through the skin and peritoneum allowed us to isolate the hepatic vagus nerve as it branched from the esophagus. In vagotomized mice, we severed the hepatic vagal nerve, while it remained intact in sham-operated mice. The peritoneum was sutured with absorbable polyglactin 910 suture, and the skin was closed with nylon suture. Mice were given a single postoperative dose of slow-release formulated buprenorphine analgesic (1.2 mg/kg sc slow release). We monitored food intake and body weight daily and removed sutures 7 days postoperation.
Hypophagic response to β-OH butyrate and niacin.
Twelve- to twenty-week-old male mice were singly housed with ad libitum chow and water. To acclimatize the mice to the handling procedures, we injected mice intraperitoneally with sterile PBS for 3 days before the study and monitored food intake and body weight. To encourage hyperphagia, we removed food immediately prior to lights off to initiate a 16-h fast that ended with refeeding at 4 h after lights on. Studies on the hypophagic effect of niacin included intraperitoneal injections of PBS (0.1 ml/10 g body wt) or niacin (0.8 mmol/kg; 0.1 ml/10 g body wt; Sigma Aldrich cat. no. PHR1276) every 2 h for the last 8 h of the fast. This dose has been shown to effectively inhibit lipolysis through GPR109a in the mouse (35). Injections every 2 h for 8 h allowed us to maintain elevated serum niacin levels throughout the 8-h duration, as the half-life for niacin is estimated to be 28–40 min. By maintaining elevated niacin over 8 h, we were able to study central nervous system gene expression changes that may mediate the hypophagic response. In separate studies, we did confirm that a single niacin injection induced similar hypophagic responses (data not shown). To study the hypophagic effect of β-OH butyrate, we intraperitoneally injected PBS (0.1 ml/10 g body wt) or β-OH butyrate (5.7 mmol/kg; 0.1 ml/10 g body wt) immediately before refeeding. This dose of β-OH butyrate has been shown to suppress food intake dependent on the hepatic vagal afferent nerve (23). One hour of food intake was measured. These studies were first conducted in wild-type (WT) mice. Subsequent studies investigated the effect of GPR109a status and hepatic vagotomy on the response to niacin or β-OH butyrate.
To allow for mice to serve as their own control and to confirm that mice that lacked sensitivity to β-OH butyrate also lacked the hypophagic response to niacin, we performed these studies using two back-to-back cross over studies. Studies were conducted at least 4 days apart to allow the mice to return to prefast body weight and resume normal food intake. At the onset of the study, mice were treated with PBS, niacin, or β-OH butyrate. Mice that received PBS as the first injection, subsequently received β-OH butyrate or niacin, and mice treated with either β-OH butyrate or niacin were treated with PBS. Next, the cross over study was repeated in the same mice using the opposing GPR109a agonist (β-OH butyrate or niacin). Thus, each mouse was injected with PBS on two occasions. Food intake did not differ on the 2 days in which PBS was injected and was not affected by previous treatment (P > 0.05). Therefore, the response to PBS in Figs. 2A, 4A, and 5B are indicative of the average value for each mouse on the two PBS injection days.
Fig. 2.
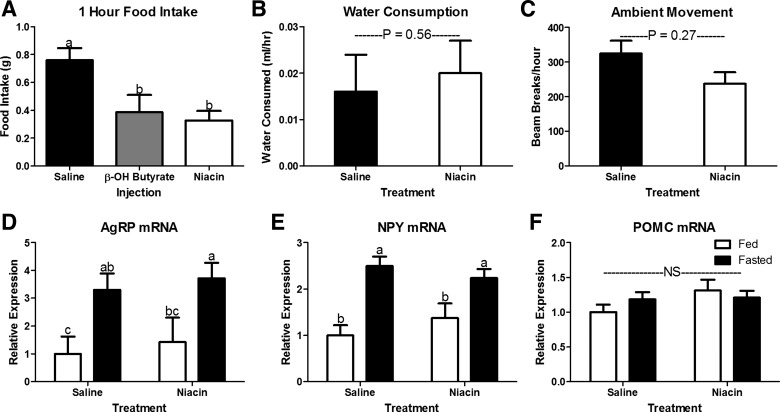
Phagic, behavioral, and neuroendocrine response to intraperitoneal β-OH butyrate and niacin injections. Intraperitoneal injection of β-OH butyrate (5.7 mmol/kg) and niacin (0.8 mmol/kg) decreased 1 h food intake in 16-h fasted mice (A; n = 8–13) but did not affect either water consumption (B; n = 4) or ambient movement (C; n = 4). Hypothalamic expression of mRNA encoding orexigenic [Agouti-related peptide (AgRP), D; neuropeptide Y (NPY), E] and anorexigenic [proopiomelanocortin (POMC), F] peptides in fed or fasted (16 h) mice injected with saline or niacin (0.8 mmol/kg) every 2 h for the last 8 h of the fast. NS, no significant difference. a,bBars that do not share a common letter differ significantly (P < 0.05; n = 6 or 7).
Fig. 4.
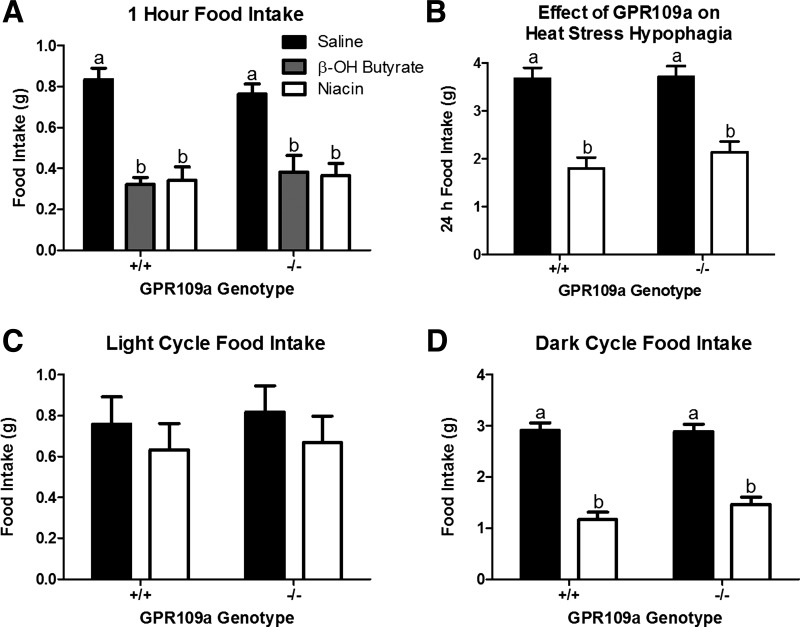
The role of GPR109a in the hypophagic response to β-OH butyrate, niacin, and heat exposure. β-OH butyrate (5.7 mmol/kg) and niacin (0.8 mmol/kg) depress food intake in GPR109a wildtype (+/+) and null (−/−) sibling mice (A; n = 6). 24 h (B), light cycle (C), and dark cycle (D) food intake in response to heat exposure (35○C) in GPR109a +/+ and −/− sibling mice. a,bBars that do not share a common letter differ significantly (P < 0.05; n = 6–9).
Fig. 5.
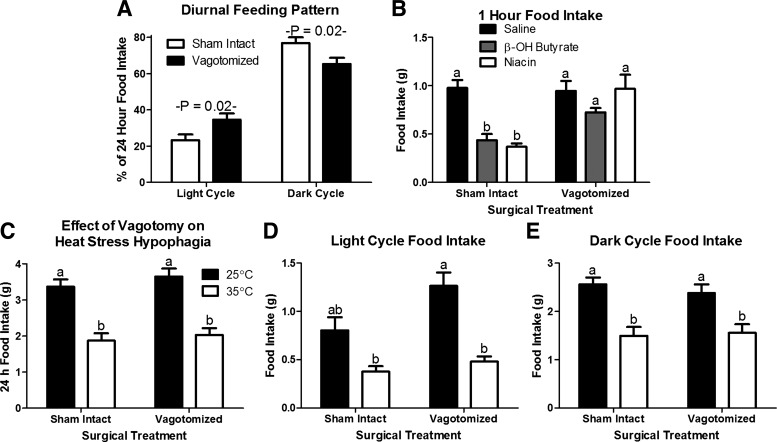
The role of the vagus nerve in the hypophagic response to β-OH butyrate, niacin, and heat exposure. A: hepatic vagotomy increases food intake in the light cycle and decreases food intake in the dark cycle, blunting the diurnal variation in food consumption at standard housing temperature (25○C; n = 8 or 9). B: hepatic vagotomy blocks the hypophagic response to β-OH butyrate (5.7 mmol/kg) and niacin (0.8 mmol/kg; n = 8 or 9). There is no effect of hepatic vagotomy on the 24 h (C), light cycle (D) or dark cycle (E) hypophagic response to heat exposure (35○C; n = 8 or 9). a,bBars that do not share a common letter differ significantly (P < 0.05; n = 8 or 9).
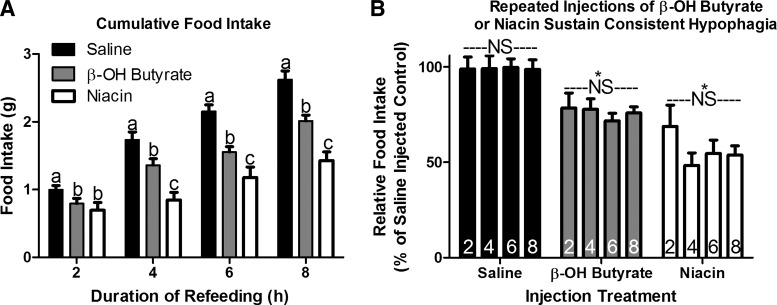
Separate studies were conducted to assess the potential for niacin or β-OH butyrate to sustain a long-term hypophagic response over 8 h. Again, we used a 16-h fast to stimulate hyperphagia. The intraperitoneal doses of niacin and β-OH butyrate were the same as described previously. Injections occurred every 2 h, beginning 4 h prior to refeeding with the last injection given 6 h after food provision. Food was weighed every 2 h at injection times to assess food intake.
Water consumption and ambient movement.
Mice were put into individual chambers in an Oxymax Lab Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH). Mice were housed in this system for 36 h prior to the initiation of monitoring. Access to feed was removed immediately prior to lights off. Eight hours after the initiation of the fast, mice were given intraperitoneal injections of PBS (0.1 ml/10 g body wt) or niacin (0.8 mmol/kg; 0.1 ml/10 g body wt) every 2 h for the last 8 h of the fast. During this time, water consumption and ambient movement (measured by laser beam breaks) were monitored by the CLAM system.
3-hydroxy-3-methylglutaryl-CoA synthase II knockdown.
Twelve-week-old male mice were given intraperitoneal injections twice weekly with 25 mg/kg of murine Hmgcs2-targeted antisense oligonucleotides (ASO). The HMGCS2 ASO, (ISIS 191229; 5′-CTGTTTGTCACTGCTGGATG-3′), a second-generation oligonucleotide that incorporates several chemical modifications to improve potency, duration of action, and tolerability. All of the internucleotide phosphates are chemically modified with a phosphorothioate substitution, in which one of the nonbridging oxygen atoms is substituted with sulfur. Additionally, the compound incorporates five 2′-O-(2-methoxyethyl) (2′-MOE)-modified ribonucleosides at the 3′ and 5′ ends with ten 2′-O-deoxyribonucleosides in between to support RNaseH-1-mediated target mRNA degradation. These modifications improve the binding affinity for target mRNA, as well as stability against exonuclease-mediated degradation. A control oligonucleotide, (ISIS 141923; 5′-CCTTCCCTGAAGGTTCCTCC-3′), containing the same chemical modifications, with no complementarity to known genes was employed to demonstrate the specificity of target reduction. Treatment with the HMGCS2-targeted ASO using this regimen has been shown to completely eliminate hepatic expression of HMGCS2 in adult mice (7).
Heat exposure.
Twelve- to twenty-week-old male mice were singly housed with ad libitum access to chow and water. All mice were individually housed for at least 1 wk prior to study initiation. Mice remained at ambient room temperature and humidity (22°C; 50% humidity; Temperature Humidity Index, THI = 67.2) or were housed in a controlled environment chamber (Coy humidity box; Coy Laboratory Products, Grass Lake, MI) set at 35°C (50% humidity; THI = 84.5). Food intake and body weight were measured over the subsequent 48 h at lights on and lights off.
Central regulation of food intake.
To investigate the effect of niacin treatment on the orexigenic and anorexigenic peptides in the hypothalamus, 12–14-wk-old male WT mice were allowed ad libitum access to food or fasted for 16 h ending at 4 h after lights on. As previously described, mice were intraperitoneally injected with sterile PBS or niacin (0.8 mmol/kg; 0.1 ml/10 g body wt) every 2 h for the last 8 h of the fast. At 4 h after lights were turned on, we anesthetized mice using bell jar exposure to isoflurane and killed mice by decapitation. This coincides with the timing of food provision in the food intake studies. The whole hypothalamus was collected, immediately frozen on dry ice, and stored at −80°C.
We examined the effect of 48 h of heat exposure (35°C) on hypothalamic mRNA expression of canonical orexigenic (AgRP and NPY) and anorexigenic neuropeptides (POMC-mRNA; α-MSH-peptide). At 4 h after lights were turned on, 48 h after the onset of differential temperature exposure (25°C or 35°C), mice were killed and hypothalami were collected, as described previously.
RNA expression analyses.
We used TRI Reagent (Life Technologies, Grand Island, NY) to extract hypothalamic and hepatic mRNA, performed reverse transcription using Verso cDNA synthesis kit (Thermo Scientific, Waltham, MA), and real-time PCR using SYBR 2× mastermix (Bio-Rad Laboratories, Hercules, CA) and the Bio-Rad iQ5 iCycler (Bio-Rad Laboratories). Before initiating the reverse transcription reaction, RNA was cleared of any phenol contamination using a water-saturated butanol and ether method (21). The primers used to analyze expression of β-actin, neuropeptide Y (NPY), Agouti-related peptide (AgRP), proopiomelanocortin (POMC), and 3-hydroxy-3-methylglutaryl-CoA synthase II (HMGCS2) are listed in Table 1. Raw amplification data were imported into linreg PCR analysis software (32), and output data were converted to fold change in expression using the ΔΔCt method and with β-actin as the housekeeping gene (25).
Table 1.
Real-time PCR primer sequences
| Target | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| β-actin | TCGGTGACATCAAAGAGAAG | GATGCCACAGGATTCCATA |
| AgRP | GTACGGCCACGAACCTCTGT | TCCCCTGCCTTTGCCCAA |
| NPY | CTCGTGTGTTTGGGCATTCT | CTTGCCATATCTCTGTCTGGTG |
| POMC | GGTGAAGGTGTACCCCAACGT | GACCTGGCTCCAAGCCTAATGG |
| HMGCS2 | GAAGAGAGCGATGCAGGAAAC | GTCCACATATTGGGCTGGAAA |
AgRP, Agouti-related peptide; NPY, neuropeptide Y; POMC, proopiomelanocortin; HMGCS2, 3-hydroxy-3-methylglutaryl-CoA synthase 2 (mitochondrial).
Statistical analysis.
Data were analyzed using mixed models in SAS (SAS Institute, Cary, NC). Across studies, the applicable independent variables [injection (saline, niacin, or β-OH butyrate), temperature (22°C or 35°C), genotype (GPR109a+/+ or −/−), surgery (vagotomized or sham), and oligonucleotide target (scramble control or HMGCS2)], and all interactions were included as main effects. Crossover studies were analyzed using a repeated-measures ANOVA. Independent variables were identified as classification variables in all models. Probabilities of differences between means were determined using Tukey adjustment for multiple comparisons. All data were plotted as means ± SE in GraphPad PRISM version 5.04 for Windows (GraphPad Software, San Diego CA, www.graphpad.com).
RESULTS
Studies comparing the response to β-OH butyrate and niacin were designed to understand the role of ATP generation in the hypophagic response to monocarboxylates. Niacin and β-OH butyrate injections given every 2 h suppress food intake following a fast (Fig. 1A). Interestingly, the hypophagia induced by each compound was steady across time with β-OH butyrate and niacin causing an approximately 25% and 45% depression in food intake at each time point (Fig. 1B). One-hour fasting-induced hyperphagia was similarly muted by β-OH butyrate and niacin (Fig. 2A), suggesting that monocarboxylates suppress food intake independent of ATP generation.
Fig. 1.
Sustained hypophagia in response to repeated intraperitoneal administration of β-OH butyrate and niacin. Intraperitoneal injection of β-OH butyrate (5.7 mmol/kg) and niacin (0.8 mmol/kg) decreased 2, 4, 6, and 8 h food intake in hyperphagic 16-h fasted mice (A; n = 6 or 7). When expressed as a percentage of saline-injected controls, we can see that the relative hypophagia induced by β-OH butyrate and niacin was similar throughout the refeeding duration (B; n = 6 or 7). a,b,cBars that do not share a common letter differ significantly (P < 0.05). *Significant decrease in relative food intake for all time points compared with saline (P < 0.05). NS, no significant difference in relative food intake within an injection treatment at any time point (P > 0.1).
Niacin injections every 2 h do not affect water consumption or ambulatory movement (Fig. 2, B and C; P > 0.1). Thus, this suppression in food intake cannot be attributed to a general malaise induced by niacin. We gave niacin injections every 2 h for 8 h to understand whether the niacin-induced hypophagia depended on changes in orexigenic or anorexigenic hypothalamic signals. Fasting increases the hypothalamic expression of the orexigenic neuropeptides, NPY and AgRP, and decreases the anorexigenic neuropeptide POMC (27, 33). Here, we show that niacin did not blunt the fast-induced upregulation of AgRP and NPY mRNA expression (Fig. 2, D and E) or increase expression of the anorexigenic POMC mRNA (Fig. 2F).
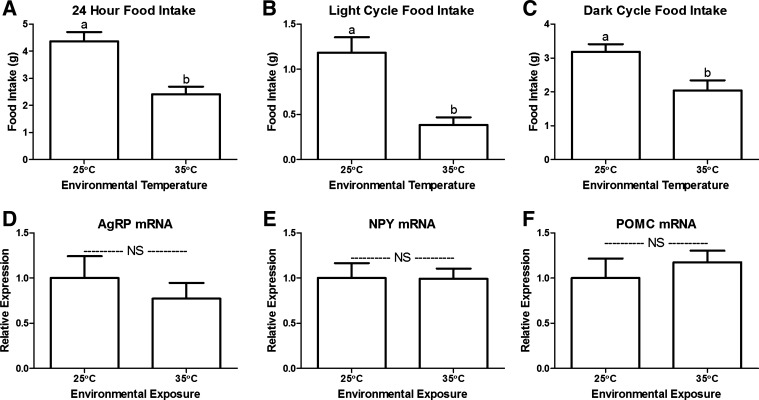
We further confirmed that heat exposure suppresses food intake in wild-type mice (Fig. 3A). The suppression of food intake occurs during both the hypophagic light cycle and the hyperphagic dark cycle (Fig. 3, B and C). Interestingly, despite decreasing food intake, heat exposure did not alter hypothalamic expression of NPY, AgRP, or POMC (Fig. 3, D–F, respectively). Thus, the hypophagic response to both niacin and heat exposure is independent of this canonical AgRP/NPY/POMC feeding circuit (10). Admittedly, we are not able to eliminate the potential effects on neuronal activity. Yet, it is well established that mRNA expression of these peptides does change as expected in response to orexigenic and anorexigenic stimuli (Fig. 2, D–F; Refs. 5 and 33).
Fig. 3.
Phagic and neuroendocrine response to heat stress. Heat exposure (35○C) decreased 24 h (A), light cycle (B), and dark cycle food intake (C; n = 6–9). Hypothalamic expression of mRNA encoding orexigenic (AgRP, D; NPY, E) and anorexigenic (POMC, F) peptides after 48-h temperature exposure (25○C or 35○C). NS, no significant difference. a,bBars that do not share a common letter differ significantly (P < 0.05; n = 5).
As both β-OH butyrate and niacin act as agonists of GPR109a, we tested the response to these monocarboxylates in GPR109a null (−/−) mice. The hypophagic responses to β-OH butyrate and niacin were independent of GPR109a expression (Fig. 4A). Likewise, genetic deletion of GPR109a did not alter the hypophagic response to heat exposure (Fig. 4, B–D).
The hepatic vagus is known to mediate the hypophagic response to IP β-OH butyrate. Accordingly, we tested the hypophagic response to niacin and heat stress in hepatic vagotomized mice. To confirm the efficacy of hepatic vagotomy, we examined the diurnal rhythm of food intake. As previously shown, hepatic vagotomy increased the percentage of daily food intake consumed during the light cycle and decreased the proportion of daily food intake consumed at night (Ref. 11; Fig. 5A). Hepatic vagotomy did not affect the hyperphagic response to fasting (Fig. 5B). Yet, it did eliminate the hypophagic response to both niacin and β-OH butyrate injection (Fig. 5B). Surprisingly, hepatic vagotomy did not alter the hypophagic response to heat exposure (Fig. 5, C–E). Thus, the vagal nerve is essential to the hypophagic response to acute monocarboxylate injection, but it is not required for the hypophagia of heat exposure.
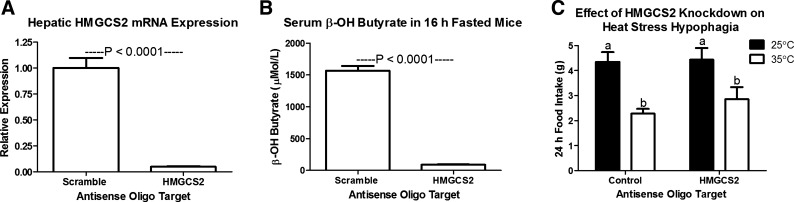
To eliminate a potential role of β-OH butyrate in heat stress hypophagia, we muted expression of HMGCS2, an enzyme in the ketogenic pathway. Four weeks of biweekly HMGCS2-targeted ASO injection suppressed HMGCS2 mRNA expression to 5% of control expression and eliminated the increase in serum β-OH butyrate that results from a 16-h fast (Fig. 6, A and B; P < 0.0001). The hypophagic response to heat exposure was not altered in these mice that lack HMGCS2 and the ability to mount an increase in serum β-OH butyrate.
Fig. 6.
Four weeks of biweekly 3-hydroxy-3-methylglutaryl CoA synthase II (HMGCS2) targeted antisense oligonucleotide injection (25 mg/kg) eliminates the hepatic expression of HMGCS2 mRNA (A) and the fast induced rise in serum β-OH butyrate (B), but does not affect the hypophagic response to heat exposure (C; 35○C; n = 6). a,bBars that do not share a common letter differ significantly (P < 0.05).
DISCUSSION
An increase in average yearly and summertime temperatures is expected to have a profound impact on health, labor productivity, and food animal production (9, 18, 19, 38). Further enhancing our understanding of the physiological adaptations to heat exposure will allow for pharmacological interventions that can reduce the impact of heat. Heat stress is accompanied by an increase in serum β-OH butyrate, which is known to induce hypophagia through actions at the hepatic vagal afferent nerve. Accordingly, we conducted studies that focused on understanding the role of β-OH butyrate in the hypophagia of heat stress. To assess a potential role of cellular energy status in the hypophagia induced by β-OH butyrate, we first evaluated the hypophagic response to niacin, a GPR109a agonist that cannot be oxidized to produce ATP. Understanding that the hypophagic response to β-OH butyrate was dependent on an intact vagal afferent nerve, we examined the hypophagic response to niacin and heat stress in vagotomized mice. We concurrently determined whether the hypophagic response to β-OH butyrate, niacin, or heat stress was dependent on expression of GPR109a. Finally, we assessed the hypophagic response to heat stress in mice that were unable to mount a ketogenic response to heat exposure.
To understand whether niacin, a monocarboxylate and GPR109a agonist that cannot be oxidized to generate ATP, can mimic the hypophagic effects of β-OH butyrate, we compared the hypophagic response to β-OH butyrate and niacin in wild-type, GPR109a-null, and hepatic vagotomized mice (12, 14, 17, 37, 39, 41). Here, we showed that both niacin and β-OH butyrate suppress food intake (Figs. 1A and 2A). By assessing mRNA expression of neuropeptides that regulate food intake, we found that the hypophagia resulting from niacin injection is not a result of changes in the arcuate nucleus NPY/AgRP/POMC signaling (10) (Fig. 1). Similarly, we showed that heat exposure did not alter expression of these hormonal signals that control food intake (Fig. 3, D–F). Thus, heat stress hypophagia and the hypophagia of monocarboxylates appear to be independent of this canonical pathway known to regulate food intake and energy expenditure.
The vasodilatory effects of β-OH butyrate and niacin are mediated by GPR109a (30). In fact, the hyperketonemia during heat exposure may be an adaptive response to cause GPR109a-mediated vasodilation. The adipose tissue antilipolytic response to niacin is also dependent on GPR109a expression (40). Surprisingly, the antilipidemic effects of niacin are independent of this antilipolytic response and GPR109a (24). Herein, we show that the hypophagic response to niacin or β-OH butyrate is also independent of GPR109a (Fig. 4). We further show that the hypophagic response to heat exposure was not dependent on GPR109a expression.
Monocarboxylates induce hypophagia dependent on an intact hepatic vagal nerve (Ref. 23; Fig. 5B). We hypothesized that the hypophagia of heat stress may depend on the paracrine action of hepatic produced β-OH butyrate on dendritic terminals of the hepatic vagus nerve to affect afferent activity to the CNS and the resulting changes in phagic drive. This would allow for an acute increase in β-OH butyrate production to be peripherally sensed and transmitted to central nervous system appetite centers. Here, we show that hepatic vagotomized mice lack the hypophagic response to β-OH butyrate and niacin but maintain the hypophagia of heat stress (Fig. 5, C–E).
Despite showing that the hypophagia of heat stress was not dependent on an intact vagus nerve, these studies did not discount a possible role of chronically elevated β-OH butyrate. In fact, we hypothesized that heat stress hypophagia may depend on direct hypophagic effects of β-OH butyrate at the central nervous system or changes in gene expression mediated through the β-OH butyrate response element. Alternatively, heat stress hypophagia may be secondary to hormonal changes resulting from elevated β-OH butyrate, such as elevated insulin or leptin (3, 16, 22, 28, 31). Using a recently published model that eliminated hepatic production of β-OH butyrate, we show here that the hypophagic response to heat stress is independent of hepatic β-OH butyrate production (Ref. 7; Fig. 6).
Perspectives and Significance
Together, these studies have established that the hypophagia of heat stress is independent of GPR109a, the hepatic vagus nerve, and hyperketonemia. Thus, the hyperketonemia of heat stress decreases thermal load by increasing peripheral vasodilation, but not by suppressing phagic drive. We propose that future work focus on understanding the relative roles of peripheral vasodilation and visceral vasoconstriction in thermoregulation during heat exposure. Further, we propose studies assessing the potential for visceral vasoconstriction to induce the hypophagia of heat stress.
GRANTS
This material is based upon work that is supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number 2015-06367 (B. J. Renquist).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.H., C.E.F., M.R.H., and B.J.R. performed experiments; C.H., C.E.F., and B.J.R. analyzed data; C.H. and B.J.R. interpreted results of experiments; C.H. and B.J.R. prepared figures; C.H. drafted manuscript; C.H., C.E.F., M.R.H., and B.J.R. edited and revised manuscript; C.H., C.E.F., M.R.H., and B.J.R. approved final version of manuscript; C.E.F. and B.J.R. conception and design of research.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Klaus Pfeffer at the Institute of Medical Microbiology, Immunology and Hygiene at Heinrich Heine University for providing a founding pair of GPR109a−/− mice. We would also like to thank Mark Graham and Isis Pharmaceuticals (Carlsbad, CA) for kindly providing both the HMGCS2 and control antisense oligonucleotides.
Present address for C. Hepler: Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX 75235.
REFERENCES
- 1.Abeni F, Calamari L, Stefanini L. Metabolic conditions of lactating Friesian cows during the hot season in the Po valley. 1. Blood indicators of heat stress. Int J Biometeorol 52: 87–96, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Allen A, Segal-Gidan F. Heat-related illness in the elderly. Clin Geriatr 15: 37–45, 2007. [Google Scholar]
- 3.Biden TJ, Taylor KW. Effects of ketone bodies on insulin release and islet-cell metabolism in the rat. Biochem J 212: 371–377, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobb JF, Peng RD, Bell ML, Dominici F. Heat-related mortality and adaptation to heat in the United States. Environ Health Perspect 122: A220, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology 151: 4745–4755, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Carlson AE. Heat waves, global warming, and mitigation. UCLA J Environ Law Policy 26: 169–216, 2008. [Google Scholar]
- 7.Cotter DG, Ercal B, Huang X, Leid JM, d'Avignon DA, Graham MJ, Dietzen DJ, Brunt EM, Patti GJ, Crawford PA. Ketogenesis prevents diet-induced fatty liver injury and hyperglycemia. J Clin Invest 124: 5175–5190, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dukes-Dobos FN. Hazards of heat exposure. A review. Scan J Work Environ Health 7: 73–83, 1981. [DOI] [PubMed] [Google Scholar]
- 9.Dunne JP, Stouffer RJ, John JG. Reductions in labour capacity from heat stress under climate warming. Nat Clim Change 3: 563–566, 2013. [Google Scholar]
- 10.Ellacott KL, Cone RD. The central melanocortin system and the integration of short- and long-term regulators of energy homeostasis. Recent Prog Horm Res 59: 395–408, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Friedman MI, Sawchenko PE. Evidence for hepatic involvement in control of ad libitum food intake in rats. Am J Physiol Regul Integr Comp Physiol 247: R106–R113, 1984. [DOI] [PubMed] [Google Scholar]
- 12.Ganapathy V, Thangaraju M, Gopal E, Martin PM, Itagaki S, Miyauchi S, Prasad PD. Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J 10: 193–199, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geraert PA, Padilha JC, Guillaumin S. Metabolic and endocrine changes induced by chronic heat exposure in broiler chickens: growth performance, body composition and energy retention. Br J Nutr 75: 195–204, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Gopal E, Fei YJ, Miyauchi S, Zhuang L, Prasad PD, Ganapathy V. Sodium-coupled and electrogenic transport of B-complex vitamin nicotinic acid by slc5a8, a member of the Na/glucose co-transporter gene family. Biochem J 388: 309–316, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyun Y, Ellis M, Riskowski G, Johnson RW. Growth performance of pigs subjected to multiple concurrent environmental stressors. J Anim Sci 76: 721–727, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda T, Yoshida T, Ito Y, Murakami I, Mokuda O, Tominaga M, Mashiba H. Effect of β-hydroxybutyrate and acetoacetate on insulin and glucagon secretion from perfused rat pancreas. Arch Biochem Biophys 257: 140–143, 1987. [DOI] [PubMed] [Google Scholar]
- 17.Jackson VN, Halestrap AP. The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver cells determined using the fluorescent intracellular pH indicator, 2′,7′-bis(carboxyethyl)-5(6)-carboxyfluorescein. J Biol Chem 271: 861–868, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Kjellstrom T, Holmer I, Lemke B. Workplace heat stress, health and productivity - an increasing challenge for low and middle-income countries during climate change. Global Health Action 2, 10.3402/gha.v2i0.2047, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjellstrom T, Kovats RS, Lloyd SJ, Holt T, Tol RS. The direct impact of climate change on regional labor productivity. Arch Environ Occup Health 64: 217–227, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Knowlton K, Rotkin-Ellman M, King G, Margolis HG, Smith D, Solomon G, Trent R, English P. The 2006 California heat wave: impacts on hospitalizations and emergency department visits. Environ Health Perspect 117: 61–67, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krebs S, Fischaleck M, Blum H. A simple and loss-free method to remove TRIzol contaminations from minute RNA samples. Anal Biochem 387: 136–138, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Lallemand F, Courilleau D, Sabbah M, Redeuilh G, Mester J. Direct inhibition of the expression of cyclin D1 gene by sodium butyrate. Biochem Biophys Res Commun 229: 163–169, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Langhans W, Egli G, Scharrer E. Selective hepatic vagotomy eliminates the hypophagic effect of different metabolites. J Auton Nerv Syst 13: 255–262, 1985. [DOI] [PubMed] [Google Scholar]
- 24.Lauring B, Taggart AK, Tata JR, Dunbar R, Caro L, Cheng K, Chin J, Colletti SL, Cote J, Khalilieh S, Liu J, Luo WL, Maclean AA, Peterson LB, Polis AB, Sirah W, Wu TJ, Liu X, Jin L, Wu K, Boatman PD, Semple G, Behan DP, Connolly DT, Lai E, Wagner JA, Wright SD, Cuffie C, Mitchel YB, Rader DJ, Paolini JF, Waters MG, Plump A. Niacin lipid efficacy is independent of both the niacin receptor GPR109A and free fatty acid suppression. Sci Transl Med 4: 148ra115, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 26.McDowell RE, Moody EG, Van Soest PJ, Lehmann RP, Ford GL. Effect of heat stress on energy and water utilization of lactating cows. J Dairy Sci 52: 188–194, 1969. [DOI] [PubMed] [Google Scholar]
- 27.Mizuno TM, Makimura H, Silverstein J, Roberts JL, Lopingco T, Mobbs CV. Fasting regulates hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocortin in diabetic mice independent of changes in leptin or insulin. Endocrinology 140: 4551–4557, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Morera P, Basirico L, Hosoda K, Bernabucci U. Chronic heat stress up-regulates leptin and adiponectin secretion and expression and improves leptin, adiponectin and insulin sensitivity in mice. J Mol Endocrinol 48: 129–138, 2012. [DOI] [PubMed] [Google Scholar]
- 29.O'Brien MD, Rhoads RP, Sanders SR, Duff GC, Baumgard LH. Metabolic adaptations to heat stress in growing cattle. Domest Anim Endocrinol 38: 86–94, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Offermanns S. The nicotinic acid receptor GPR109A (HM74A or PUMA-G) as a new therapeutic target. Trends Pharmacol Sci 27: 384–390, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Park S, Kim da S, Daily JW. Central infusion of ketone bodies modulates body weight and hepatic insulin sensitivity by modifying hypothalamic leptin and insulin signaling pathways in type 2 diabetic rats. Brain Res 1401: 95–103, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett 339: 62–66, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Renquist BJ, Murphy JG, Larson EA, Olsen D, Klein RF, Ellacott KL, Cone RD. Melanocortin-3 receptor regulates the normal fasting response. Proc Natl Acad Sci USA 109: E1489–E1498, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhoads ML, Rhoads RP, VanBaale MJ, Collier RJ, Sanders SR, Weber WJ, Crooker BA, Baumgard LH. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J Dairy Sci 92: 1986–1997, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Richman JG, Kanemitsu-Parks M, Gaidarov I, Cameron JS, Griffin P, Zheng H, Guerra NC, Cham L, Maciejewski-Lenoir D, Behan DP, Boatman D, Chen R, Skinner P, Ornelas P, Waters MG, Wright SD, Semple G, Connolly DT. Nicotinic acid receptor agonists differentially activate downstream effectors. J Biol Chem 282: 18,028–18,036, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Safranski TJ, Harris DL, Nienaber JA, Hahn GL, Korthals RL. Heat stress and limit feeding pigs: effects on growth and composition. In: Proceedings of the Fifth International Livestock Environment Symposium. Bloomington, MN: ASAE, 1997, p. 772–780. [Google Scholar]
- 37.Shimada A, Nakagawa Y, Morishige H, Yamamoto A, Fujita T. Functional characteristics of H+-dependent nicotinate transport in primary cultures of astrocytes from rat cerebral cortex. Neurosci Lett 392: 207–212, 2006. [DOI] [PubMed] [Google Scholar]
- 38.St-Pierre NR, Cobanov B, Schnitkey G. Economic losses from heat stress by US livestock industries. J Dairy Sci 86: E52–E77, 2003. [Google Scholar]
- 39.Tachikawa M, Murakami K, Martin PM, Hosoya K, Ganapathy V. Retinal transfer of nicotinate by H+-monocarboxylate transporter at the inner blood-retinal barrier. Microvasc Res 82: 385–390, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, Ren N, Kaplan R, Wu K, Wu TJ, Jin L, Liaw C, Chen R, Richman J, Connolly D, Offermanns S, Wright SD, Waters MG. d-β-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem 280: 26,649–26,652, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, Offermanns S. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med 9: 352–355, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Whittow GC, Findlay JD. Oxygen cost of thermal panting. Am J Physiol 214: 94–99, 1968. [DOI] [PubMed] [Google Scholar]